Changes were made to the original formulation of the EMJH medium (Ellinghausen–McCullough–Johnson–Harris) enrichment and some aspects such as growth time of Leptospira and utilization in the microscopic agglutination test (MAT) were evaluated and compared to the original enrichment and to a commercially available enrichment (DIFCO™). Leptospira samples (24 antigens) that make up our panel of antigens used in MAT were used, among them, reference and autochthonous strains isolated in Brazil. The samples were grown individually in the EMJH medium under the three previously mentioned conditions (adapted enrichment, original enrichment and commercial enrichment). In addition, 89 blood serums from domestic and wild animals were analyzed by MAT using the antigens grown in these media. All samples tested grew efficiently with the adapted enrichment, and the MAT results were satisfactory. Therefore, other laboratories could also benefit from the use of this adapted enrichment when culturing the Leptospira strains applied in their MAT panels.
Realizamos cambios en la formulación original del enriquecimiento del medio Ellinghausen-McCullough-Johnson-Harris y evaluamos algunos aspectos, como el tiempo de crecimiento de Leptospira y la utilización en la prueba de aglutinación microscópica (MAT), comparándolos con el enriquecimiento original y un medio de enriquecimiento comercialmente disponible (DIFCO™). Se usaron muestras de Leptospira (24 antígenos) que componen nuestro panel de antígenos utilizados en la MAT, entre ellos, cepas autóctonas y de referencia aisladas en Brasil. Las muestras se cultivaron individualmente en medio Ellinghausen-McCullough-Johnson-Harris en las tres condiciones mencionadas (enriquecimiento adaptado, enriquecimiento original y enriquecimiento comercial). Adicionalmente, 89 sueros de sangre de animales domésticos y salvajes fueron analizados por MAT usando los antígenos cultivados en estos medios. Todas las muestras analizadas crecieron eficientemente con el enriquecimiento adaptado y los resultados de la MAT fueron satisfactorios. Por lo tanto, otros laboratorios también podrían beneficiarse del uso de este enriquecimiento adaptado al cultivar las cepas de Leptospira aplicadas en sus paneles MAT.
Leptospirosis is a bacterial disease that affects several species of animals and humans8. This neglected zoonosis has worldwide distribution; nonetheless, its occurrence in developing countries is higher, especially in tropical areas, where environmental conditions contribute to the endemic status of leptospirosis5.
The microscopic agglutination test (MAT) is the reference assay for the serological diagnosis of leptospirosis, detecting both IgM- and IgG-class antibodies11. The test is based on an antigen–antibody reaction and live samples of Leptospira spp. (serovars) are used as antigens8; for this purpose, the bacteria must be periodically seeded in a new medium for maintenance in the laboratory3. Although other serological automated or more modern techniques are available, such as ELISA (enzyme-linked immunosorbent assay) and LFA (lateral flow assay)7, MAT is still the most widely utilized tool.
The EMJH medium (Ellinghausen–McCullough–Johnson–Harris) is commonly used in the cultivation of leptospires for performing MAT, and consists of a base supplemented with an enrichment traditionally composed of bovine serum albumin (fraction V), Tween 80, chlorides, sulfides and vitamins. This enrichment can also be purchased commercially. However, due to the high cost of using this commercial enrichment routinely, many laboratories produce their own enrichment in accordance with the formulation described by Faine4. In our laboratory, we observed that some Leptospira strains did neither grow nor reach the ideal density for use in MAT (∼2×108leptospires/ml) when seeded in the EMJH medium with the enrichment produced by us using this formula. Thus, the purpose of this work was to adapt the original composition of the enrichment to provide the adequate growth of all strains used as antigens in our laboratory MAT panel.
The enrichments were prepared by weighing the ingredients in agreement with the desired volume, hydrating and sterilizing by filtration in a PES membrane (polyethersulfone, 0.22μm). The final pH of the enrichments was 7.4±0.1. The enrichment formulations are shown in Table 1, except for the commercial enrichment (DIFCO™), whose compounds are not disclosed by the manufacturer. The base of the EMJH medium (DIFCO™) was prepared following the manufacturer's instructions and divided into three media according to the enrichment used: 1 – adapted enrichment; 2 – original enrichment4; 3 – commercial enrichment (DIFCO™). This base was sterilized by autoclaving at 15psi pressure and 121°C for 15min and, after the medium reached a temperature of approximately 50–60°C, the enrichments were added in the proportion of 10% (v/v) to the base medium.
Formulas of the tested enrichments.
| Ingredients | Volume/l |
|---|---|
| 1 – Adapted enrichment | |
| Bovine serum albumin (fraction V) | 10g |
| Magnesium chloride | 0.15mg |
| Calcium chloride | 0.15mg |
| Zinc sulfate | 0.04mg |
| Copper sulfate | 0.003mg |
| Manganese sulfate | 0.003mg |
| Ferrous sulfate | 0.05g |
| Sodium pyruvate | 0.1g |
| Sodium acetate | 0.1g |
| Cyanocobalamin (Vitamin B12)Sodium bicarbonateTween 80Ultrapure water | 0.004mg0.05g1.2ml100ml |
| 2 - Original enrichment4 | |
| Bovine serum albumin (fraction V) | 10g |
| Magnesium chloride | 0.15mg |
| Calcium chloride | 0.15mg |
| Zinc sulfate | 0.04mg |
| Ferrous sulfate | 0.05g |
| Sodium pyruvate | 1.0mg |
| Glycerol | 1.0mg |
| Cyanocobalamin (Vitamin B12) | 0.002mg |
| Tween 80 | 1.2ml |
| Ultrapure water | 100ml |
For the functional test, we used 24 samples of Leptospira spp., encompassing reference and autochthonous strains isolated in Brazil (Supplementary material 1) which compose our MAT panel applied in the diagnosis of leptospirosis in animals.
Initially, a first passage was made from the strains stocked in Fletcher's semi-solid medium. Each sample was seeded individually in tubes containing the EMJH medium supplemented with three different enrichments and incubated in a bacteriological incubator at 28°C for 6–7 days for a first adaptation to the liquid medium (EMJH). Consecutive weekly passages, always at a ratio of 1/10 (v/v), were carried out in new tubes containing the EMJH medium with the distinct enrichments and incubated as described above. These passages were followed up until the growth of all the strains was macroscopically visible (similar to white smoke). Each strain was individually examined through dark field microscopic observation for cell viability (motility), contamination and auto-agglutination. The cell density was determined by counting, using a Petroff-Hausser chamber4. Cultures should ideally reach ∼2×108leptospires/ml for subsequent use in the MAT4 and the number of days for this to occur was taken into account.
The MAT was performed according to Faine et al.3, using three panels with 24 antigens, each grown in EMJH with the respective enrichments tested. Eighty nine (89) blood serums from domestic and wild animals were randomly selected from the laboratory serum bank. They were collected from 12 horses, 15 buffaloes, 13 bovines, 11 dogs, 9 sheep, 15 pigs, 4 peccaries (Tayassu pecari), 4 crab-eating foxes (Cerdocyon thous) and 6 ocelots (Leopardus pardalis). The results obtained with the three MAT panels were compared by descriptive statistics using frequency measures. Furthermore, we sent the adapted enrichment to be tested by five other laboratories that study leptospires in Brazil.
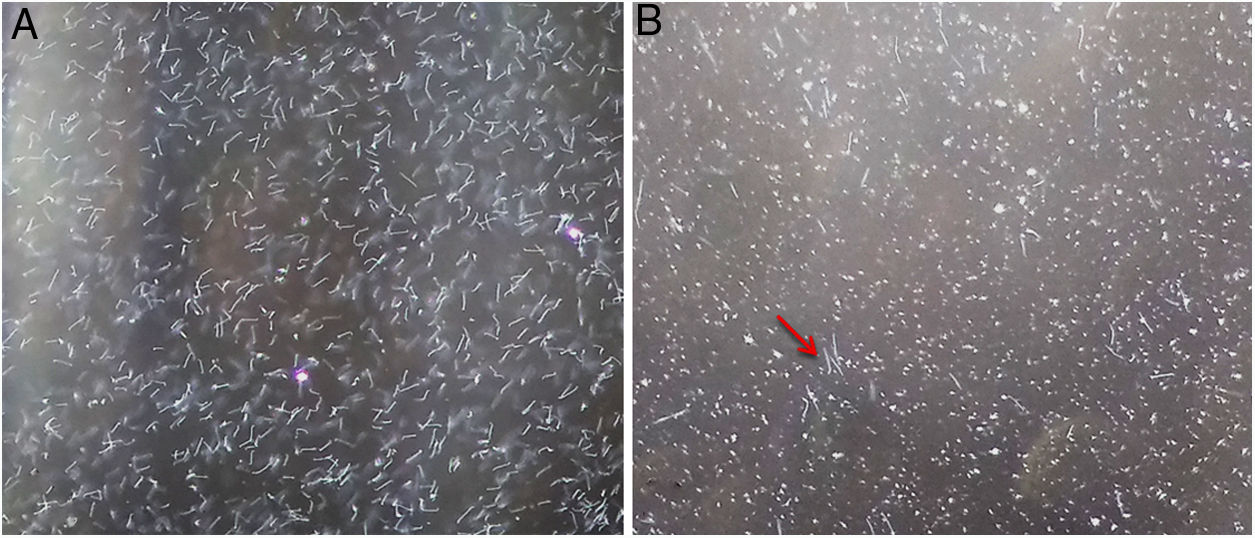
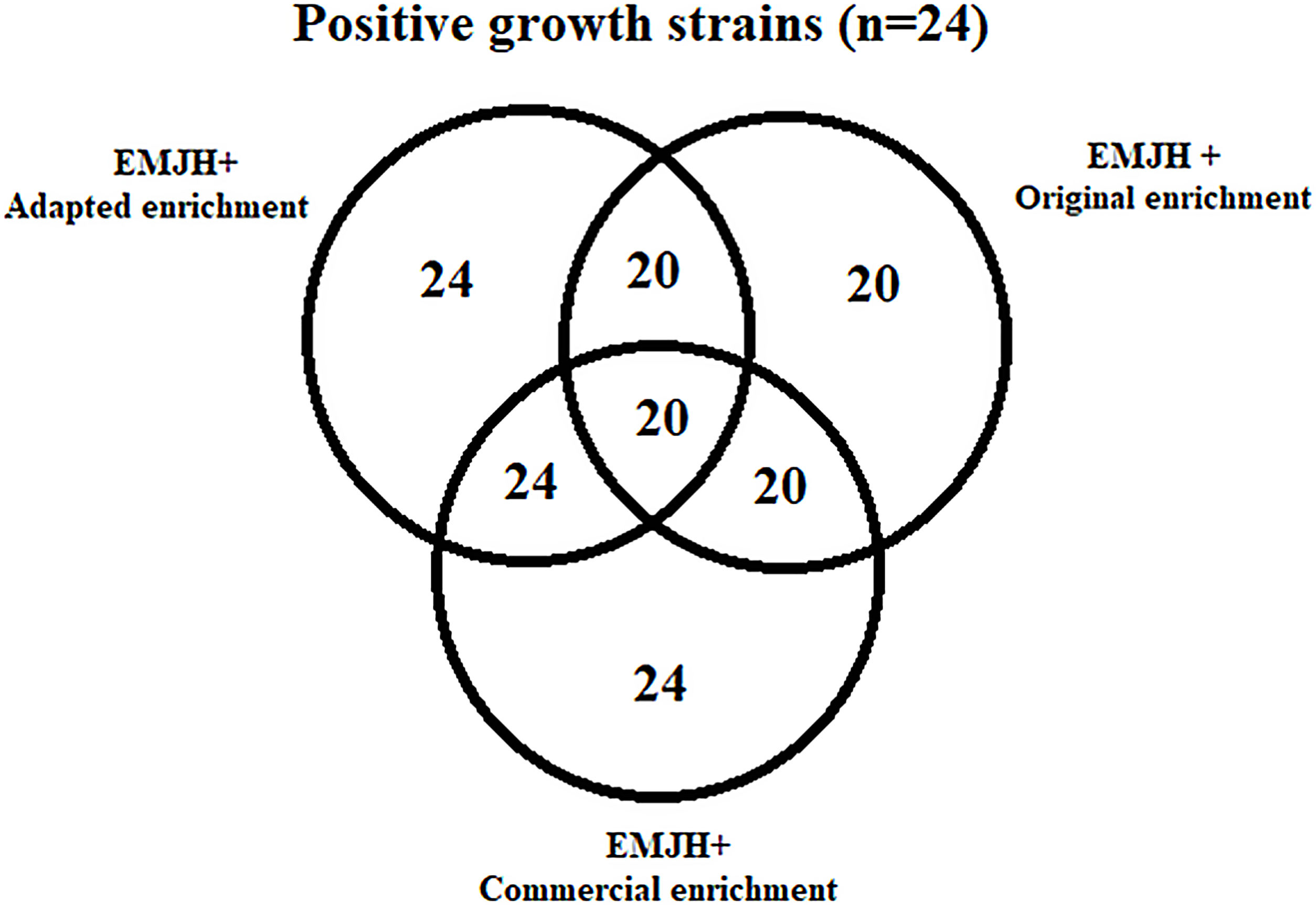
Using dark field microscopy, we did not detect any contamination or presence of auto-agglutination in all media used, and cell viability (motility) was preserved in all cultures. With regard to the growth of Leptospira strains, all of them grew in EMJH with the adapted and commercial enrichment; however, only 20 samples grew in EMJH with the original enrichment (Supplementary material 2). Two reference strains (Autumnalis and Panama) and two autochthonous strains (Guaricura and GR6) did not grow. This was one of the reasons that motivated the change in formulation of the original enrichment4, as we observed that glycerol appeared to be toxic for these samples, since microscopically the leptospires grown in this medium lost their spirochete shape, assumed a coccoid shape (Fig. 1), and died within days (data not shown). This was interesting, because glycerol was reported as a growth requirement for pathogenic Leptospira and used to decrease generation times for serovars Pomona and Canicola10.
Other reagents were added to the adapted enrichment formulation and we observed that antigens reached the ideal density for use in MAT faster in comparison to EMJH with the original enrichment (Table 1). Moreover, when the autochthonous strains were seeded in the medium with original enrichment without addition of glycerol, poor growth occurred, and they did not reach the recommended concentration for use in MAT. This situation was resolved the moment that these samples were seeded in EMJH with adapted enrichment.
Metals, minerals, and some metal traces, such as manganese and copper are important for the growth of leptospires and can be related to bacterial pathogenicity6. An increase in the concentration of sodium pyruvate (0.1g/l) proved to be useful in the cultivation of fastidious strains of leptospires belonging to the Sejroe serogroup1, in which the serovar Guaricura is inserted. Other reagents such as cyanocobalamin8, sodium acetate and sodium bicarbonate2 have been shown to be essential and enhancers in the cultivation of Leptospira spp.
MAT results using EMJH with the adapted and commercial enrichments were similar in both screening and titration (Table 2). Due to the fact that the MAT panel was not complete using EMJH with the original enrichment, as four strains did not grow, the technique was not performed. MAT panels can vary from laboratory to laboratory, as the insertion of strains representing most prevalent serogroups in the region is recommended11, with the consequent increase in the sensitivity of the test to detect leptospirosis-positive samples9.
Evaluation of the enrichments in relation to growth time and MAT results.
| EMJH supplemented with | Time (days) to reach density for use in MATa | MAT | ||||||
|---|---|---|---|---|---|---|---|---|
| Screeningb | Titrationc | |||||||
| Pos. | Neg. | 100 | 200 | 400 | 800 | 1.600 | ||
| 1 – Adapted enrichment | 4–6 | 20 | 69 | 33 | 16 | 3 | 0 | 1 |
| 3 – Original enrichment (Faine, 1982) | 5–8 | NT | NT | NT | NT | NT | NT | NT |
| 3 – Commercial enrichment (DIFCO™) | 3–5 | 21 | 68 | 35 | 15 | 3 | 0 | 1 |
NT: non-tested; Pos.: positive; Neg.: negative.
In this work we did not aim to assess the costs for preparing adapted enrichment. Nevertheless, the acquisition of commercial enrichment (DIFCO™ – 6 vials×100ml), in our reality (São Paulo, Brazil) can range from US$ 800.00 to US$ 1000.00, while the cost for the production of the same volume of adapted enrichment (600ml) is approximately US$ 300.00. It is also worth emphasizing that although the preparation of enrichment in the laboratory is laborious, it can be a valuable alternative for laboratories at this time of financial crisis in many countries, including Brazil.
Despite the fact that an exact shelf-life has not yet been defined after preparation, we observed in our routine that for up to three months after being produced and stored in refrigeration, the enrichment has given us satisfactory results (data not shown).
Finally, all five laboratories that received the adapted enrichment approved its use in their laboratories. In our laboratory we optimized this enrichment for more than a year, until we achieved this final formula that gave us satisfactory results, similar to the ones obtained with the commercial enrichment. Thus, other laboratories that manipulate autochthonous strains different from the reference strains could also benefit from the use of this improved enrichment in the cultivation of the antigens applied in their MAT panels.
FundingThis work was financed in part by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), Brasil – Finance Code [001] and by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) [420110/2018-6].
Conflicts of interestThe authors declare that they have no conflicts of interest.
MBH would like to thank CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for the fellowship (CNPq 309146/2017-8). IBG and JFPC are grateful to CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for the scholarship. We appreciate the following laboratories for testing the adapted enrichment: Laboratório de Doenças Bacterianas da Reprodução – Instituto Biológico de São Paulo; Laboratório de Zoonoses e Saúde Pública, Instituto de Medicina Veterinária – UFPA; Laboratório de Zoonoses, FMVZ – Unesp Botucatu; Laboratório Especial de Desenvolvimento de Vacinas – Instituto Butantan; Laboratório de Leptospirose – Instituto de Pesquisas Veterinárias Desidério Finamor.