Hydrolytic enzyme production (cellulases, laminarinases and xylanases) was studied in cultures of Lentinula edodes on sterilized coffee pulp. Samples of substrate colonized by mycelia were taken after 7, 14, 21, 28 and 35 days of incubation at 25°C (W1 to W5) and during the fruiting period at different stages: formation of primordia (PF), first harvest (H) and one week after the first harvest (PH). The enzymatic activity was lower during the early mycelial growth and showed higher levels during the formation and development of fruiting bodies. During the reproductive stage of the fungus, the samples were subjected to a soaking treatment; however, it was not possible to relate this soaking treatment to the increase in enzyme production. The levels of enzymatic activity suggest that secretion of the studied enzymes does not influence the adaptability of the strains to the substrate.
Se estudió la producción de enzimas hidrolíticas (celulasas, laminarinasas y xilanasas) en cultivos de Lentinula edodes en pulpa de café estéril. Se tomaron muestras de sustrato colonizado por el micelio después de 7, 14, 21, 28 y 35 días de incubación a 25°C (W1 a W5) y durante el período de fructificación en diferentes etapas: formación de primordios (PF), primera cosecha (H) y una semana después de la primera cosecha (PH). La actividad enzimática fue menor al inicio del crecimiento micelial y mostró mayores niveles en la formación y el desarrollo de basidiomas. Durante la etapa reproductiva del hongo, las muestras se sometieron a un tratamiento de remojo. Sin embargo, no fue posible relacionar este tratamiento con el aumento de la producción de enzimas. Los niveles de actividad enzimática sugieren que la secreción de las enzimas estudiadas no influye en la capacidad de adaptación de las cepas al sustrato.
The mushroom commonly known as shiitake (Lentinula edodes [Berk.] Pegler) is widely appreciated for both its delicate flavor and medicinal properties. Its cultivation began in China around the year 1100 A.D., currently being the second most commercially produced species, exceeded only by the white button mushroom (Agaricus bisporus [Lange] Imbach)1. The improvement in cultivation techniques has enabled the production of shiitake in substrates that are very different from those in its natural habitat. The modern cultivation of this species is mainly based on the use of different substrates that are rich in lignin and cellulose, which are subjected to different thermal disinfection treatments such as autoclave sterilization and steam pasteurization1,7.
Coffee pulp is an agroindustrial byproduct available in considerable quantities in tropical and subtropical regions5. Despite the fact that various studies have been conducted on coffee pulp for the cultivation of edible species10,15, this substrate is still not used intensively. The organic nature and composition of this material makes coffee pulp an ideal substrate for processing with microorganisms and for the generation of products of high aggregate value. The use of coffee pulp has been proposed in the generation of silage, biogas, vermicompost, fodder and ethanol, among other products5. Coffee pulp has been used in the experimental cultivation of shiitake; however, as with other species, there are no data regarding commercial cultivation on this agroindustrial byproduct.
Due to differences in the chemical composition of substrates utilized in the cultivation of shiitake, it is necessary to select genotypes that present suitable characteristics for growth and production of fruiting bodies in a given substrate. The capacity of a species to grow in a particular lignocellulosic substrate depends on its ability to utilize the majority of the components of the substrate as nutritive elements2. This is determined by the capacity of the fungus to synthesize the necessary hydrolytic and oxidative enzymes.
The enzymes produced for the degradation of lignin and cellulose, and for the transformation of these materials into low molecular weight compounds that are easily assimilated by the mushroom, are induced by the presence of different components in the substrates13. Enzyme production has been widely studied in shiitake, especially when cultivated in substrates based on, or derived from, wood3,14. In addition, the spectrum of enzymes obtained during the complete cycle of cultivation of shiitake on wheat straw has been studied4. However, there is still a lack of information regarding enzyme production during cultivation on coffee pulp. Therefore, the objective of this study was to quantify hydrolytic enzyme production during cultivation on this substrate and to determine its possible role in the degradation of the substrate and sporome production, with the aim of optimizing the use of this residue for shiitake cultivation.
The following commercial strains of L. edodes were studied: IE-40, IE-105, IE-124, IE-171, IE-242 (cross of IE-244×IE-245), IE-243, IE-244 IE-245 and IE-246. All these strains are deposited in the strain collection of the Instituto de Ecología (Xalapa, Mexico) and, for the purposes of this study, were sown in a potato dextrose agar medium (PDA, Bioxon, Becton Dickinson and Company, Queretaro, Mexico) and incubated at 25°C in darkness.
The strain inoculum was produced with hydrated sorghum seeds (≈55% moisture). The seeds were placed in polypropylene bags for autoclave sterilization (121°C, 1h) (Aesa, Model 300, Mexico). Sterile seeds were inoculated with the mycelium of each of the cultures previously sown in PDA. The inoculated samples were incubated at 25°C in darkness.
The coffee pulp was collected from a local coffee bean processing plant, sundried (to ≈20% moisture) and stored at ambient temperature until use. For sowing the fungus, the substrate was rehydrated in water for 12h, and the excess water was drained until the substrate reached a moisture content of 65%. Substrate samples of 500g (dry weight) were prepared, placed in polypropylene bags and sterilized at 121°C for 1h. Twenty-five grams of inoculum (5% of the inoculation percentage) were added to each sample and distributed homogenously throughout the sample. Eight replicates were prepared per strain and were all incubated at 25±2°C in darkness.
In order to evaluate the enzymatic activity of the fungi during the reproductive stage, some strains were randomly selected. Following incubation, the plastic bags were removed and the substrate blocks containing mycelium were subjected to two watering treatments: (1) rewetting (R), which consisted in submerging the complete block in water at 10°C for 12h, and (2) no rewetting (NR). The blocks were then kept under suitable environmental conditions for fruiting body development: alternate 12h periods of light/darkness, temperature of 23±3°C and relative humidity of 85±5%. The number of days required for primordia formation and production of the first harvest as well as the fresh weight of the basidiomes collected were recorded. These data were used to determine the biological efficiency of the different strains.
Samples were taken after 7, 14, 21, 28 and 35 days of incubation (samples W1 toW5). During the fruiting period, samples were taken at the following stages: primordia formation (PF), first harvest (H) and one week after the first harvest (PH). The enzymatic extracts were prepared in 50ml flasks by placing 1g of substrate with mycelium in 10ml of sterile distilled water. The flasks were agitated at 45rpm for 30min in a roto torque shaker (Cole Parmer, Model 7637, USA). The solids were then removed by filtration through an inert nylon mesh and the filtrate was centrifuged twice at 10000×g for 15min at 4°C (Heraeus, Model Biofuge, USA). The obtained extract was immediately used for enzyme determination.
The activities of cellulases, laminarinases and xylanases were estimated through the liberation of reducing sugars using 3,5-dinitrosalicylic acid (DNS) (SIGMA, D-0550, USA)4, in reactions containing an 0.1M sodium acetate buffer (SIGMA, S-7670, USA) solution at pH 5 and the specific substrate: carboxymethyl cellulose at 2% (SIGMA, C-4888, USA), laminarin at 1% (SIGMA, L-9634, USA) and xylan at 0.5% (SIGMA, X-4252,USA)4. Standard curves were obtained with glucose (SIGMA, G-7528, USA) for the cellulases and laminarinases, while xylose (SIGMA, X-2126, USA) was used for the xylanases. The results of the enzymatic activities were expressed as units of activity per gram of substrate, defined as the quantity of enzyme necessary for the generation of 1μmol of product per minute per gram of substrate. The reported values correspond to the means of three replicates. The analysis of variance (ANOVA) was performed on enzymatic production data. Means were compared with Tukey's multiple range test (p<0.05).
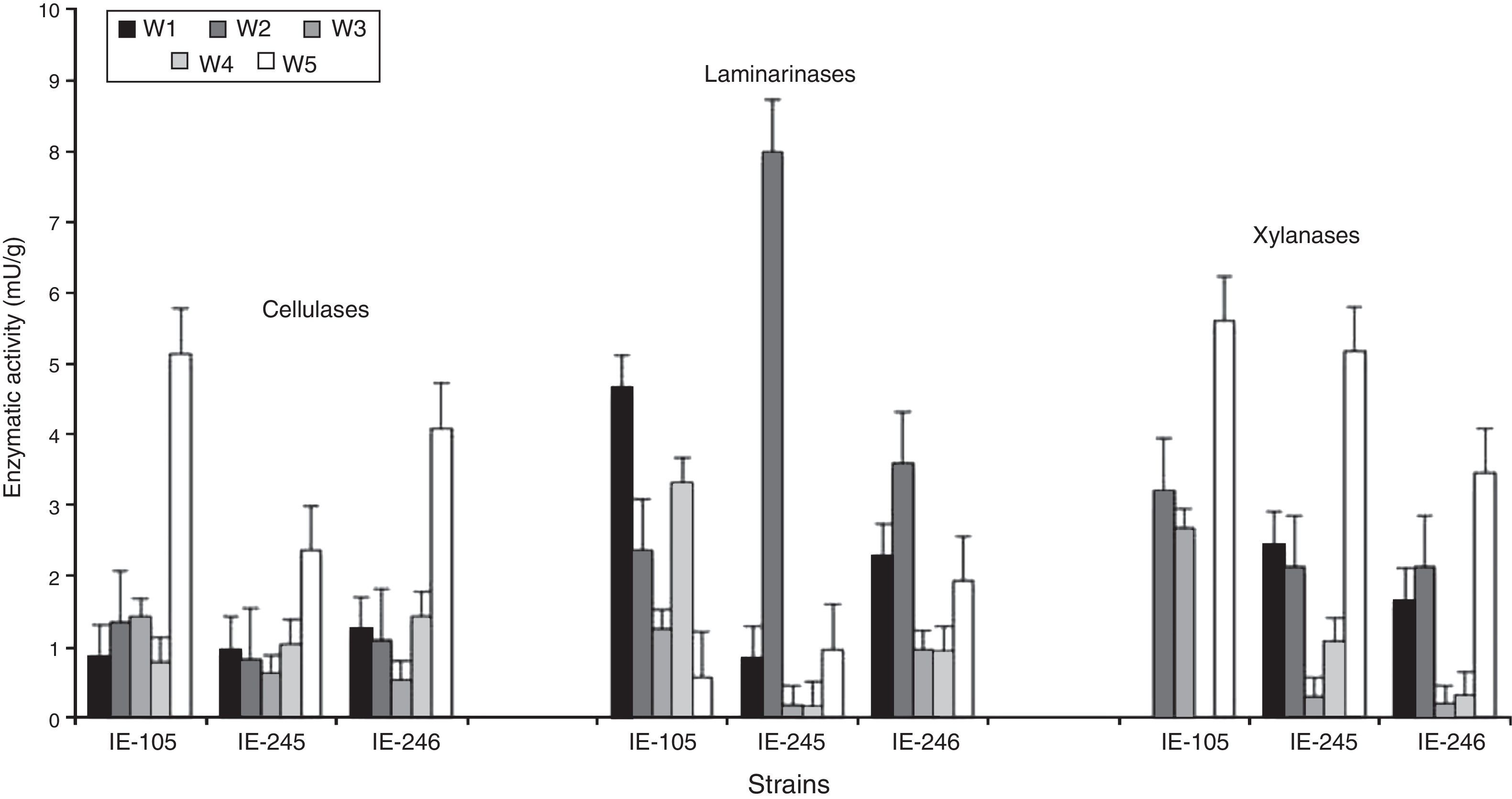
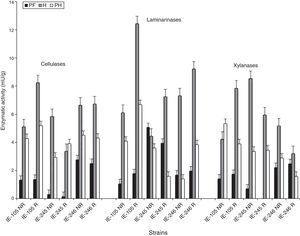
The results showed that all strains presented similar enzymatic profiles (data not shown), for that reason, three strains (IE-105, IE-245 and IE-246) were selected to describe the pattern of behavior during the incubation period and until the end of mushroom production in the first harvest. The strains presented enzymatic activity from the first week of incubation showing low activity during the incubation period that increased during the reproductive stage. During the first four weeks of incubation (W1 to W4), the activity of the cellulases detected among the strains was under 1.45mU/g. Maximum activity occurred during the fifth week of incubation, showing peaks of activity over 2.35mU/g (Fig. 1).
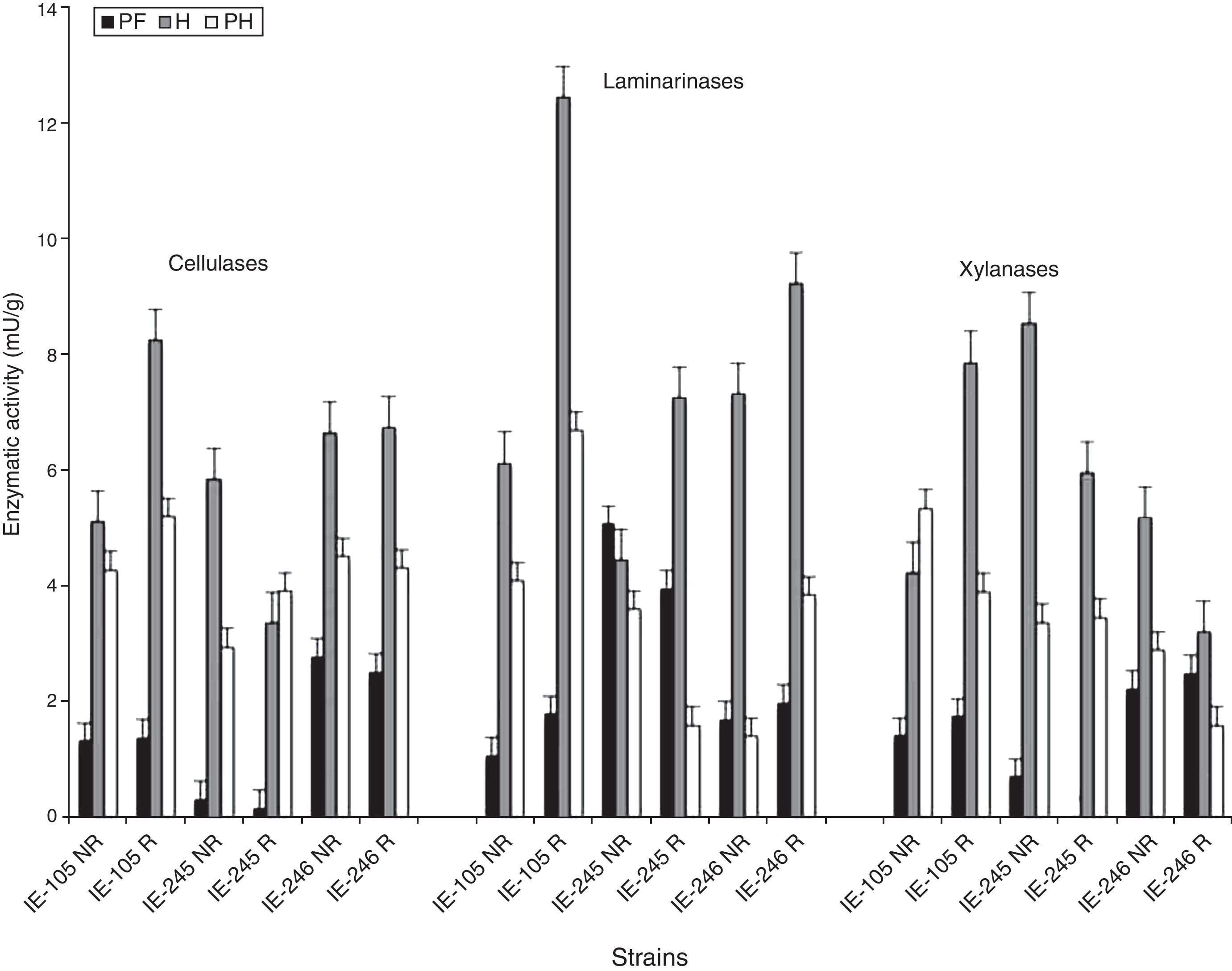
During primordia formation (stage PF), a decrease in cellulolytic activity was observed in the strains with respect to the previous stage (W5) before presenting peaks of activity linked to the development of the basidiomes (stage H). Noteworthy, the sample strains did not show a uniform effect: IE-105 presented maximum activity in the rewetted samples, IE-245 in the treatment with no rewetting, and IE-246 showed no effect at all. In the PH stage, a general decrease was observed in the activity of the cellulases, except in the samples that corresponded to strain IE-245 in the rewetting treatment (Fig. 2).
From the first week of incubation, the laminarinases presented fluctuations in activity over the five weeks of evaluation. Maximum activity was registered at the beginning of the incubation period (W1 or W2) reaching values of 8.00mU/g (IE-245 W2). During the following stage, enzyme activity declined in all strains (Fig. 1).
As with the cellulases, greater activity was detected in the laminarinases during the reproductive stage of the strains, specifically in the period of development and maturation of the basidiomes (H). The profiles observed showed a positive effect of the rewetting treatment in all the strains. One week after the first harvest (stage PH), the enzymatic activity declined with respect to the previous stage (Fig. 2).
Xylanolytic activity was detected as from the first week of incubation (W1), except in the IE-105 strain. Over the following weeks of incubation (W2 toW4), there was minimal enzymatic activity; however, it increased significantly during stage W5 (Fig. 1).
During primordia formation (PF), the pattern of behavior of the xylanases was similar to that observed in the cellulases and laminarinases, showing a decrease in the enzymatic activity of the strains with respect to the previous stage (W5) (Fig. 2). The maximum values of activity for this enzyme were detected during the harvest of fruiting bodies (stage H). Strain IE-105 presented its maximum activity in the rewetted samples, while strains IE-245 and IE-246 in the no rewetting treatment (Fig. 2).
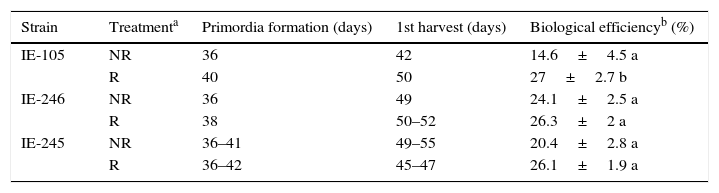
In terms of basidiome production, the rewetting treatment did not influence the time required for the formation of primordia and production of the first harvest. The biological efficiencies attained in the rewetting treatment were greater in the three randomly selected strains; however, only the values of strain IE-105 presented statistically significant differences (Table 1).
Times required for the formation and development of fruiting bodies in different strains of L. edodes and the productivity recorded in the first harvest
The results of the study show that each strain of L. edodes presented a similar pattern of behavior in terms of the secretion of hydrolytic enzymes, showing reduced activity during the early stages of adaptation to the substrate and increased activity during the formation and development of the fruiting bodies. The low enzymatic activity during the first days of colonization is likely to be related to the availability of soluble sugars in the coffee pulp, which could be utilized by the fungus during this early stage of adaptation to the substrate.
An increased activity of hydrolytic enzymes during the formation of carpophores has been previously reported in substrates such as wood and straw3,4,8, demonstrating that these increases favor the mobilization of nutrients for the formation of the reproductive structures. This pattern of behavior is similar to that reported for species of the genus Pleurotus cultivated in coffee pulp6,10,15.
Overall, low enzymatic activity was observed compared to other substrates used commercially for shiitake cultivation. Mata and Savoie4 cultivated L. edodes strains in barley straw and reported activities greater than 50, 30 and 10mU/g during the reproductive stage, for cellulases, laminarinases and xylanases, respectively. The low enzymatic productivity found in this study could be a result of the chemical composition of the substrate, since coffee pulp contains a lower quantity of cellulose and hemicellulose than wheat straw10.
In previous studies, this species has been considered a moderate producer of cellulases and hemicellulases8,9,11. It is well known that these enzymes are induced under certain cultivation conditions2; however, the results of this study show that coffee pulp does not provide suitable conditions for this induction. Moreover, the presence of phenolic compounds (caffeine and tannins) in this substrate can act as inhibitors of mycelial growth10 and could have affected the normal development of the shiitake fruiting bodies, since some primordia did not develop completely.
The rewetting treatment did not produce a uniform pattern in the enzymatic profiles of the strains. While at the industrial level this treatment is considered an important step in the process in order to stimulate the production of basidiomes, in this study productivity was significantly higher in only one strain (IE-105) as a result of this treatment.
Coffee pulp is a substrate that is rich in nutrients for the growth of fungi; however, in the case of shiitake, it does not appear to provide conditions that facilitate adaptation of the strains. In this study, the production of enzymes of the oxidase group was not studied, although they must surely play an important role in the degradation of phenolic compounds, particularly in the initiation of mycelial growth as in other species15. To achieve an increase in the biological efficiency of this substrate, it would be necessary to select strains presenting characteristics that are suited to development in coffee pulp.
Shiitake mushroom is well known for its medicinal properties, its potential in the food industry, its application in paper industrial processes such as biopulping and its capacity for the treatment of different industrial effluents12 and the enzymes produced by this mushroom play a very important role in that capacity.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare that they have no conflicts of interest.
The authors thank the authorities of the Instituto de Ecología, A.C. and the National Council of Science and Technology (CONACYT) for their support to their research.