Anisakidosis is an infection caused by larval nematodes that belong to several genera within the family Anisakidae. Anisakidosis has about 20000 cases reported to date, the vast majority (90%) in Japan. Usually, human anisakiosis is more common than human pseudoterranovosis in Japan and Europe, although in North America Pseudoterranova spp. is the more frequent. Cases of human pseudoterranovosis have been reported from Chile and Peru. We here report one of the few cases of human infection by Pseudoterranova cattani by consumption of “ceviche” in Buenos Aires, Argentina.
La anisakidosis es una infección por larvas de nematodos que pertenecen a varios géneros dentro de la familia Anisakidae. Se han registrado aproximadamente 20.000 casos hasta la fecha, la mayoría (90%) en Japón. En Europa y Japón la anisakidosis es más frecuente en el humano que la pseudoterranovosis. En cambio, en América del Norte es más frecuente la infección humana por Pseudoterranova spp. También se han informado casos de pseudoterranovosis humana en Chile y en Perú. Informamos uno de los pocos casos de infección humana por Pseudoterranova cattani por consumo de ceviche en Buenos Aires, Argentina.
Anisakidosis is an infection caused by larval nematodes that belong to several genera within the family Anisakidae. The term anisakidosis refers to disease caused by any member of the family Anisakidae, whereas anisakiasis is caused by members of the genus Anisakis and pseudoterranovosis refers to disease caused by the genus Pseudoterranova5. The most common anisakids found in humans are: Anisakis simplex complex, Pseudoterranova decipiens complex and Pseudoterranova cattani. Other less common anisakids that are found in humans are Contracaecum spp. and Hysterothylacium spp.10,14. Anisakidosis has about 20000 cases reported to date, the vast majority (90%) in Japan3. Human anisakis infection is found particularly in Japan (sushi and sashimi), the Netherlands (green herring) and Latin America (ceviche). Other reports of human anisakiasis come from Belgium, Germany, Norway, Canada, Denmark, Thailand, New Zealand, Switzerland, Argentina and the United States1,11. In Chile 10 out of 13 reported cases of Anisakis worms were due to Pseudoterranova spp.12. Four human infections with P. cattani were diagnosed in Chile during 2012–201415. In Peru, 2 cases of Pseudoterranova decipiens have been reported in Lima, in patients who had ingested “ceviche”4. The source of human pseudoterranovosis is the consumption of raw or undercook fish (ceviche, sushi or sashimi), smoked fish, and pickled fish, containing third- or fourth-stage larvae14. Human infection may be found wherever raw, poorly cooked, pickled or salted fish or squid contaminated with third- or fourth-stage larvae are consumed. Larvae usually do not mature in humans1.
Clinical features depend on whether anisakid larvae only attach to the mucosa of the gastrointestinal tract, or invade tissues. Larvae sometimes migrate up the esophagus and attach to the throat, causing coughing or a tickling sensation. In that case, larvae may be expectorated or passed in the stool. When larvae penetrate the stomach wall they may provoke gastritis, with severe epigastric pain, diarrhea, nausea and vomiting. Symptoms usually develop within 48h of ingesting larvae. Sometimes anaphylactic reactions may occur2. The definitive diagnosis occurs when the entire larva is recovered through endoscopy, where it can be directly visualized and removed. Molecular biology analyses have epidemiological usefulness to determine the specific species within the genus. Microscopic diagnosis is hindered by the lack of distinguishing morphologic features in larval stages15. The surgical removal of invading larvae is curative. Corticosteroids may decrease the inflammatory response to larvae, but no effective anthelmintic drugs are available.
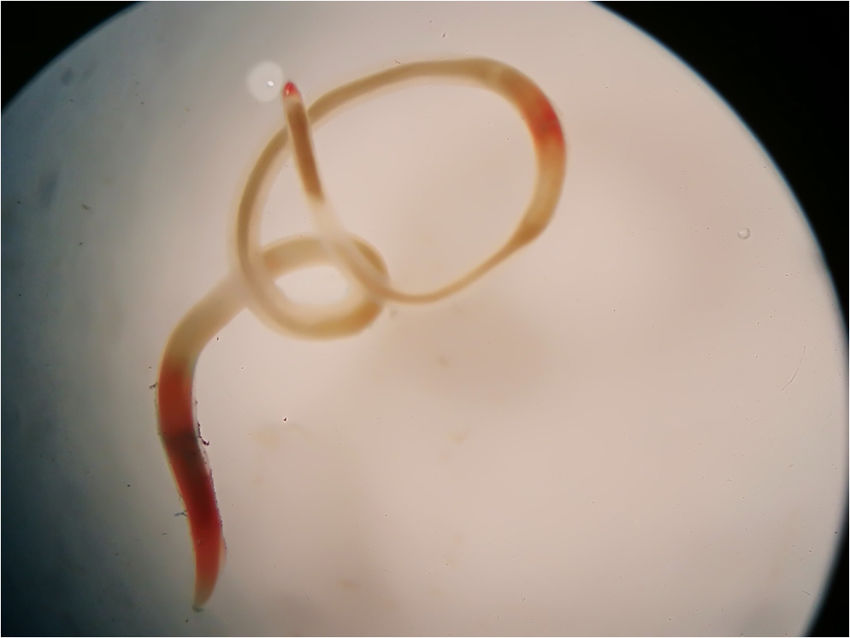
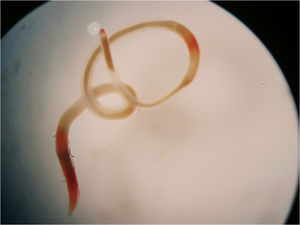
On August 11, 2018 a 44-year-old man living in the city of Buenos Aires, Argentina, was admitted to the emergency department carrying a whitish to reddish 5cm length worm (Fig. 1). He referred having expelled the larva during vomiting. He mentioned having eaten smoked salmon on August 4. He explained that he had prepared “ceviche” with grouper and sole bought in the same store. During that week, he experienced respiratory symptoms and nasal congestion, and later he expelled a larva by vomiting after feeling a “rare sensation” in the throat (“tingling throat syndrome”). He presented no allergic symptoms. The specimen was sent to the Parasitology Laboratory of the Hospital de Clínicas of Buenos Aires, where a presumptive diagnosis of anisakid was made. Later, it was derived to the Department of Parasitology, INEI, ANLIS “Dr. Carlos G. Malbrán” for a precise molecular identification. The anisakid larva was identified as P. cattani using PCR amplification of the internal transcribed spacer (ITS1) of nuclear ribosomal DNA followed by nucleotide sequencing. A 3-mm long piece from the mid-body region of the specimen was cut off. DNA was isolated according to the “Rapid isolation of Mammalian DNA” protocol13. The PCR mix was brought to a volume of 25μl, containing 0.5U of Taq polymerase (Invitrogen), 1× Taq buffer, 1.5mM MgCl2, 0.8μM of each dNTP, 0.25μM of each primer (Ani-9F: 5′-CCGCCTTAATCGCAGTGG-3′ and Ani-552R: 5′-CAATTCGCACTATTTATCGCAGC-3′) and 5ng of parasite DNA7. The cycling conditions were: 94°C for 3min, 40 cycles of 94°C for 1min, 60°C for 1min and 72°C for 1min, and a final extension at 72°C for 10min. Amplification was carried out in a Px2 Thermal Cycler/Electron Corporation. Double-distilled water was used as negative control. The amplified fragments were separated by electrophoresis on a 1.5% agarose gel, stained with GelRed® (Biotium) and compared to a 100-bp DNA ladder molecular weight marker (fermentas). PCR amplification fragments of the expected size were purified from the agarose gel using an AccuPrep Gel Purification Kit (Bioneer). Sequences were determined using an ABI 3500 Genetic Analyzer (Applied Biosystems). Chromatograms were viewed with Chromas Lite 2.01 and sequences were compared with those in the GenBank database using the BLASTn program (https://blast.ncbi.nlm.nih.gov). The DNA sequence obtained, deposited in the GenBank database under accession number MK174377, showed the highest identity (99–100%) to the species P. cattani6,7. After his examination in the emergency room, the patient did not return to hospital for a follow-up visit.
The consumption of sushi, sashimi, ceviche and other raw or uncooked delicacies lead to the acquisition of diseases such as anisakiosis and pseudoterranovosis. The risk of human infection can be reduced by the visual examination of fish, removal of the parasites, and confiscation of the parasitized fish. Larvae are killed by heating to temperatures of more than 60°C for a least 1min. The US Food and Drug Administration recommends that fish to be consumed raw should be kept frozen at −20°C for seven days or −35°C for 15h9. The best protection against anisakidosis is to provide public education about the hazards of eating raw fish and to recommend avoiding the consumption of smoked, marinated, or salted marine fish or squid8. This report represents one of the few cases of P. cattani diagnosed in the city of Buenos Aires, Argentina.
Este estudio fue aprobado por el Comité de Ética en Investigación del CEMIC.
Conflict of interestThe authors declare that they have no conflicts of interest.