Asymptomatic meningococcus carriers in hospitals is a risk factor for acquiring meningococcal disease. Meningococcal carrier (MC) frequency was investigated in oropharyngeal swab samples collected from 200 staff members at a teaching hospital from Brazil. MC prevalence was 9% (95% CI 5–13%). Risk factors associated with MC were: mean age of 26.5 years, male gender, bar attendance frequency and number of persons/house. Of 18 isolated meningococcal strains, 14 were non-groupable (NG), 3 corrresponded to serogroup B and 1 to serogroup 29E. The frequency of serotypes and serosubtypes was heterogenous, with a slight predominance of serotypes 4 and 7 and serosubtypes P1.7 and P1.5. Most strains (n=13) were susceptible to the antimicrobials tested. The ctrA gene (PCR) was identified in 9 (64.3%) of the 14 NG strains, suggesting virulence in most of the NG isolated strains. Therefore, a constant surveillance of these asymptomatic carriers is required.
Los portadores asintomáticos de meningococos en hospitales son un factor de riesgo (FR) para adquirir la enfermedad meningocócica. La frecuencia de portadores de meningococos fue determinada a través de colecta orofaríngea en personal de un hospital de Brasil (n = 200). La prevalencia de portadores fue del 9% (IC del 95%, 5-13%). Los FR asociados al estado de portador fueron los siguientes: edad promedio 26,5 años, sexo masculino, hábito de frecuentar bares y número de personas/casa. Entre las 18 cepas de meningococos aisladas, 14 eran no agrupables (NG), 3 correspondieron al serogrupo B y una al 29E. La frecuencia de los serotipos y serosubtipos fue heterogénea, con un ligero predominio de los serotipos 4 y 7 y de los serosubtipos P1.7 y P1.5. La mayoría de las cepas (n=13) fueron sensibles a los antimicrobianos estudiados. El gen ctrA fue identificado por PCR en 9 (64,3%) de las 14 cepas NG, lo que sugiere virulencia en la mayoría de las cepas NG aisladas. Por lo tanto, se requiere una vigilancia constante de estos portadores asintomáticos.
The sole ecological niche of Neisseria meningitidis is the mucosa of oropharynx in humans4. Asymptomatic meningococcus carriers are a risk for the dissemination of pathogenic strains, especially in hospitals. The state of asymptomatic carriers can be chronic, intermittent or transitory3,15 and this state, represents a successful relationship between the host and the bacterium, with the host experiencing no detectable pathology4.
The global prevalence of asymptomatic carriers of N. meningitidis in the oropharynx of adult individuals ranges from 10 to 35%8,12,15. Several factors influence the variability of these frequencies: age, recent contact with a sick person or N. meningitidis carrier, Influenza-like illness (ILI), smoking (active or passive), socio-economic conditions, geographical location, bacterial genetic polymorphisms, genetic polymorphism of the host immune system and crowded conditions8.
Encapsulated or unencapsulated strains can be present in the naso-or oropharynx of asymptomatic carriers. The meningococcal capsular operon (cps) is composed of a number of genes that are involved in capsular synthesis and transport. Differences in capsular synthesis genes may allow discrimination between meningococcal serogroups1,2,15.
The capsular transport gene ctrA occurs exclusively in N. meningitidis and is part of the capsular polysaccharide biosynthesis locus. It encodes a conserved meningococcal outer membrane protein involved in the transport of the capsular polysaccharide9. The search for the ctrA gene by PCR is frequently used for confirmation of non-culture cases of meningococcal disease, and can be used as a surrogate marker of capsular status. However, it is well described that, for reasons yet unknown, the carriage isolates rarely cause disease even harboring the capsule9. The capsule locus, including the ctrA gene, is subject to rearrangement and 16% or more of carrier meningococcal strains have been shown to lack the ctrA gene6. A new target gene, sodC, has been used for the diagnosis of meningococcal meningitis due to its mutational stability and its presence in all strains9.
Certain N. meningitidis strains have lost genes for the transport and synthesis of the capsule polysaccharide, and some regions are replaced by a stretch of DNA termed the capsule null locus (cnl). Carriage studies have found the prevalence of capsule null mutants to be ∼16 among nasopharyngeal meningococci in healthy children and young adults6. Cnl strains can cause meningococcal disease (MD), indicating that the capsule is not the only virulence factor involved in disease6.
Limited published data describing carriage of N. meningitidis in Latin America are currently available. There are not many studies about carriers in specific populations such as health staff. This study aimed to investigate the prevalence of asymptomatic carriers of N. meningitidis in this population, and to investigate the risk factors associated with the carrier state. We characterized the sero/serosubtype, the presence of the ctrA gene and the sensitivity profile to antimicrobials of the isolated strains. A total of 200 individuals from Miguel Riet Correia University Hospital in Rio Grande do Sul State were enrolled in this study (100 students and 100 employees). These individuals were 20–60 years old. This sample was chosen taking into account a prevalence of 30%±7% and a 95% confidence level. The biological samples were collected between January and December 2011. All participants read and signed an informed consent form.
The following variables were studied: gender, age, time in the hospital, schooling, household size, number of people sharing the same bedroom, monthly family income, smoking status, Influenza-like illness (ILI), use of antibiotics within 15 days prior to collection, and frequency of pub or nightclub attendance.
Briefly, the material was collected from the posterior oropharyngeal area with sterile Rayon swabs and plated immediately in modified Thayer Martin (Difco) medium containing 5% defibrinated sterile horse blood, VCNT 1% (vancomycin, 300μg/ml; colistin, 750μg/ml; nystatin, 1250μg/ml; trimethoprim, 500μg/ml) (Laborclin). The plates were incubated for 48h at 37°C in a CO2 (5%) atmosphere10. Suspected colonies of N. meningitidis were identified by conventional methods (biochemical tests)10. After N. meningitidis species confirmation, a single colony was spread onto blood agar for storage at −70°C (10% glycerol, 10% lactose, 3% tryptic soy broth, agar 2.5%). The isolates identified as N. meningitidis were serogrouped using an agglutination test on slides with specific antisera for the A, B, C, E, W, X, Y and Z serogroups. Subsequently, the serotyping and serosubtyping of all N. meningitidis isolates was performed through dot-blotting using a whole-cell suspension10.
The gene amplification by polymerase reaction (PCR) was performed as previously described by Sadler et al.13, for the detection of the ctrA gene (capsule transport gene). The minimum inhibitory concentration (MIC) of antibiotics was determined using the microdilution technique10. The antimicrobials used were ciprofloxacin, ceftriaxone, chloramphenicol, penicillin G, ampicillin and rifampin.
All statistical analyses were performed in Stata 13.0 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP.). The descriptive analysis included calculation of mean and standard deviation for quantitative variables, and computation of proportions for categorical variables. Prevalence of carrier state and 95% confidential intervals (95% CI) were identified. Differences in the outcome between groups were analyzed by the Student's test or Kruskal–Wallis for numerical data and by the chi-square test for categorical data. In all statistical tests a p value <0.05 of a two tail test was considered significant.
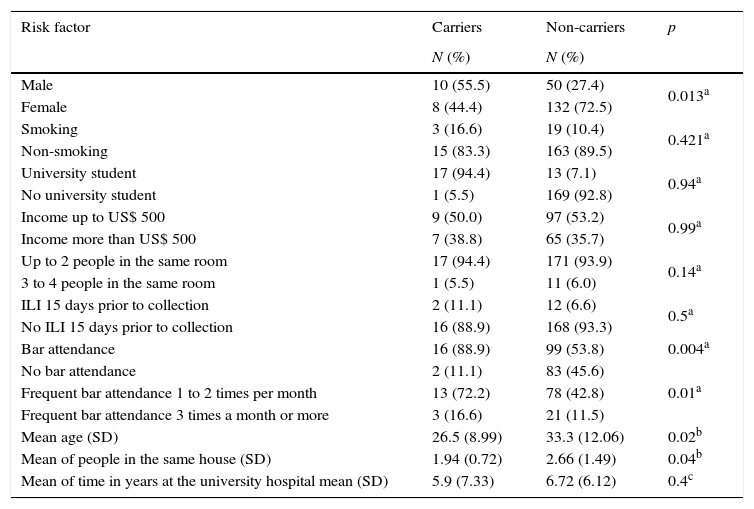
Of the 200 individuals participating in the study, 9% (18) were asymptomatic carriers of N. meningitidis. As shown in Table 1, the variables that influenced the carrier state were: gender male, age, bar attendance frequency and number of individuals living in the same house. Out of 18 carriers, 15 were isolated from students and 3 from hospital staff. Among students, 10 were men and 5 were women. All hospital staff carriers (n=3) were female. Overall, the prevalence of male carriers was 16.7% (10/60), while the prevalence of female carriers was 5.7% (8/140). These data show that the male gender was more frequently associated with the carrier state than women (p=0.013) in the group studied. The mean ages were 23.6 (18–38 years old) for students and 41.9 (20–63 years old) for hospital staff workers. Younger individuals (mean age 26.5 years old) were more frequently found to be carriers of N. meningitidis (p=0.02). With respect to bar attendance frequency, 88.9% (16) of the 18 carriers attended bars with a frequency of one to three times per month, against only 54.4% (99) of the 182 non-carriers (p=0.01). Surprisingly, there were more non-carriers living in the same house (mean of 2.66 individual per house) than carriers (mean of 1.94, p=0.04).
Analysis of risk factors and their relationship with N. meningitidis according to carrier condition (n=200). Rio Grande, Brazil.
| Risk factor | Carriers | Non-carriers | p |
|---|---|---|---|
| N (%) | N (%) | ||
| Male | 10 (55.5) | 50 (27.4) | 0.013a |
| Female | 8 (44.4) | 132 (72.5) | |
| Smoking | 3 (16.6) | 19 (10.4) | 0.421a |
| Non-smoking | 15 (83.3) | 163 (89.5) | |
| University student | 17 (94.4) | 13 (7.1) | 0.94a |
| No university student | 1 (5.5) | 169 (92.8) | |
| Income up to US$ 500 | 9 (50.0) | 97 (53.2) | 0.99a |
| Income more than US$ 500 | 7 (38.8) | 65 (35.7) | |
| Up to 2 people in the same room | 17 (94.4) | 171 (93.9) | 0.14a |
| 3 to 4 people in the same room | 1 (5.5) | 11 (6.0) | |
| ILI 15 days prior to collection | 2 (11.1) | 12 (6.6) | 0.5a |
| No ILI 15 days prior to collection | 16 (88.9) | 168 (93.3) | |
| Bar attendance | 16 (88.9) | 99 (53.8) | 0.004a |
| No bar attendance | 2 (11.1) | 83 (45.6) | 0.01a |
| Frequent bar attendance 1 to 2 times per month | 13 (72.2) | 78 (42.8) | |
| Frequent bar attendance 3 times a month or more | 3 (16.6) | 21 (11.5) | |
| Mean age (SD) | 26.5 (8.99) | 33.3 (12.06) | 0.02b |
| Mean of people in the same house (SD) | 1.94 (0.72) | 2.66 (1.49) | 0.04b |
| Mean of time in years at the university hospital mean (SD) | 5.9 (7.33) | 6.72 (6.12) | 0.4c |
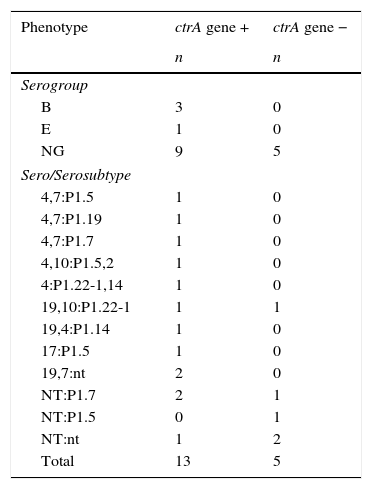
The 18 carrier strains isolated were identified phenotypically and characterized genotypically by the presence of the capsule transport gene (ctrA) (Table 2). The CtrA gene was identified in 13 of the 18 N. meningitidis strains. Most (n=14) of the meningococcal strains were non-serogroupable (NG) (Table 2). Three strains were serogroup B and 1 strain was serogroup E (Table 2). As expected, all ctrA- strains were NG.
Frequency of serogroup and sero/serosubtype of N. meningitidis (n=18) isolated from carriers classified according to the presence of the ctrA gene. Rio Grande, Brazil.
| Phenotype | ctrA gene + | ctrA gene − |
|---|---|---|
| n | n | |
| Serogroup | ||
| B | 3 | 0 |
| E | 1 | 0 |
| NG | 9 | 5 |
| Sero/Serosubtype | ||
| 4,7:P1.5 | 1 | 0 |
| 4,7:P1.19 | 1 | 0 |
| 4,7:P1.7 | 1 | 0 |
| 4,10:P1.5,2 | 1 | 0 |
| 4:P1.22-1,14 | 1 | 0 |
| 19,10:P1.22-1 | 1 | 1 |
| 19,4:P1.14 | 1 | 0 |
| 17:P1.5 | 1 | 0 |
| 19,7:nt | 2 | 0 |
| NT:P1.7 | 2 | 1 |
| NT:P1.5 | 0 | 1 |
| NT:nt | 1 | 2 |
| Total | 13 | 5 |
NG-non-grouped; NT – non-serotyped; nt – non-serosubtypeable.
Regarding the sero/serosubtype of carrier strains, we noticed a variety of phenotypes (Table 2). The most common serotypes were 4 and 7 (n=3) and 7 were non-serotypable (NT) either for crtA+ or crtA- strains. Serosubtypes P1.7, P1.5 and P1.22-1 were present in 4, 4 and 3 of all meningococcal strains, respectively. Two of the ctrA- strains were non-serosubtypable.
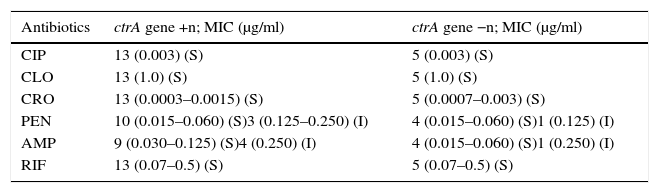
Of the 18 strains analyzed, 13 were sensitive to all the antibiotics tested. Table 3 shows the distribution of the antibiotic susceptibilities of the strains according to the MIC of the antibiotics (μg/ml) tested. Four of the strains showed intermediate susceptibility to PEN and two to AMP.
Distribution of meningococcal strains according to the MIC of the antibiotics
| Antibiotics | ctrA gene +n; MIC (μg/ml) | ctrA gene −n; MIC (μg/ml) |
|---|---|---|
| CIP | 13 (0.003) (S) | 5 (0.003) (S) |
| CLO | 13 (1.0) (S) | 5 (1.0) (S) |
| CRO | 13 (0.0003–0.0015) (S) | 5 (0.0007–0.003) (S) |
| PEN | 10 (0.015–0.060) (S)3 (0.125–0.250) (I) | 4 (0.015–0.060) (S)1 (0.125) (I) |
| AMP | 9 (0.030–0.125) (S)4 (0.250) (I) | 4 (0.015–0.060) (S)1 (0.250) (I) |
| RIF | 13 (0.07–0.5) (S) | 5 (0.07–0.5) (S) |
MIC, minimun inhibitory concentration; CIP, ciprofloxacin; CLO, chloramphenicol; CRO, ceftriaxone; PEN, penicillin; AMP, ampicillin; RIF, rifampin; S, Susceptible; I, Intermediate.
Studies of meningococci isolated from asymptomatic carriers are essential to improve knowledge of the epidemiology of meningococcal disease. The results of carriage studies, however, are highly dependent on the swabbing techniques and laboratory methods used. Swabbing of the posterior wall of the oropharynx followed by immediate cultivation on selective medium is the recommended procedure to detect asymptomatic meningococcal carriage in an individual10. Some real-time PCR methods have been attempted more recently, but in our experience a sodC RT-PCR has a sensitivity similar to the isolation of bacteria by culture9. In the present study using only bacterial culture to investigate the carrier state, we described a prevalence of 9% of meningococcal carriers in a University Hospital population. According to the literature, the prevalence of asymptomatic carriers can reach over 50%2 in local agglomerations. However, our data indicated a frequency of only 9%, suggesting that at this time, this particular hospital did not behave like an agglomeration site. Indeed, the prevalence of meningococcal carriers in our study was similar to that described for the general population (∼10%)4,12. Rodriguez et al., 2014 found a prevalence of 4%, n=500, age 18–24 years of age. In 2010, a prevalence around 20% was found among Brazilian employees of an oil refinery (n=500)14.
The present study identified four risk factors associated with the carrier state. These were: age (individuals with a mean age of 26.5 years), male gender, bar attendance frequency and number of individuals per household (p<0.05). Accordingly, it has been described that the number and quality of social contacts of young male individuals are factors associated with the carrier state8. Since most of the meningococcal carriers were students (83.3%), and they had a bar attendance frequency of one to three times per month, these data suggest a link between social behavior and the development of the carrier state. Alcohol is a substance that decreases the immune response, consequently increasing the susceptibility to N. meningitidis8. Alcohol consumption might also facilitate attachment of N. meningitidis to the pharyngeal mucosa by an irritating and/or drying effect. Bars are places where there is usually a high population density, which promotes person to person contact. All together, these factors may be related to the high frequency of meningococcal carriers among students5,8. The mean age of students was lower compared with that of hospital employees and was positively associated with the carrier state.
Although overcrowding has often been associated with higher meningococcal carriage frequency8, the present study did not reflect this association. Although students showed the highest carrier frequency, they presented a lower (p=0.004) occupancy rate (1.9 individuals per household) than non-carriers (2.7 individuals per household). Therefore, although agglomeration promotes carriership8, we must take into account not just the number of individuals who live in the same house, but also the age and social behavior of these individuals5,8. Smoking has been associated with the state of asymptomatic carriers of N. meningitidis because it decreases salivary IgA levels, affects oral flora and interferes with the action of respiratory mucosa ciliary cells8. However, in this study, the use of tobacco was not associated with the carrier state due to the low number of smokers among the individuals (n=22).
Of note, since 2002, serogroup C prevalence has overtaken serogroup B cases in most regions of Brazil. For this reason, in 2010, the meningoccocal C conjugate vaccine was included in the Brazilian Immunization Program. However, only after 2013 N. meningitidis C (MenC) was prevalent in the southern region of the country11. The lethality rate of MD in Rio Grande do Sul (RS) State is high, reaching 7.1% among the cases with serogrouped strains11. No MenC was isolated among carriers in this work, probably because in 2011, the time when the throat samples were collected, we still had a low prevalence of N. meningitidis serogroup C in our city and consequently in the University Hospital.
According to Beathgen et al., 2008, the prevalent phenotypes in patients with MD in RS State were B:4,7:P1.19,15, B:15:P1.7,16 and B:NT:P1.3. In our study, the meningococcal strains of asymptomatic carriers showed a larger phenotypic diversity than the strains from patients with MD from RS, as has already been described in other studies2,4. Due to the small number of meningococcal strains isolated in this study we cannot draw any conclusions about the prevalent phenotype in carrier strains; however, if the sample numbers were larger we would have more isolates and then better conclusions. In agreement with previous studies in the literature we found a high proportion (77.7%) of NG strains among carriers3,8,12. The absence of capsule in meningococcal strains facilitates adherence to oropharyngeal mucosa4. Detecting capsule genes such as the ctrA gene in fluid helps in the diagnosis of MD, showing the importance of this gene, though it is not the only gene used for diagnostic purposes. All clones of hypervirulent strains have a capsule9,15. However, not only capsulated strains can cause DM. Capsule null strains (cnl) can cause disease in immunocompetent individuals, although much fewer cases than the encapsulated strains, showing that the capsule is not the only virulence factor6.
Various attempts have been made to identify the genetic elements that are associated with meningococcal invasion15. These bacteria possess great capacity for genetic variation, including the capsule locus, in order to colonize the mucosa and overcome the physical and immunological barriers of the host15. N. meningitidis that lack both a capsule and the ctrA gene can receive capsule genes from other encapsulated N. meningitidis strains through horizontal transference4. Our data showed that 72% (n=13) of the 18 strains of the carriers harbored the ctrA gene, indicating that these samples could express capsule some times. However, when or whether, the disease will develop depends on the individuals’ immune system (basically a lack of bactericidal antibodies or defects in the complement system), the circulating bacterial strain and not well understood factors4,15.
Penicillin G and ampicillin are the antibiotics indicated by the Brazilian Ministry of Health for the treatment of MD11. Most of the strains in this study were sensitive to the antimicrobials tested. Only 4 of the 18 strains showed intermediate susceptibility to ampicillin and/or penicillin G. Nonetheless, meningococcal strains with reduced susceptibility to penicillin isolated from Brazilian patients suffering from MD in the 2006 to 2008 period ranged from 10.3% to 15.1%7.
The detection of asymptomatic carriers in a hospital is of great importance because both healthy individuals as well as those with compromised immune systems represent a large part of this environment. Surveillance of these asymptomatic carriers is required. Once detected, it is advisable to vaccinate these individuals with the conjugate vaccine since this vaccine can eradicate the carrier state and prevent the spread of bacteria in the hospital. Surveillance of antibiotic susceptibility to meningococcal strains is recommended, since the emergence of strains with intermediate resistance to penicillin has been described in several countries, with frequencies ranging from 4% in the U.S., to 23% in Sweden and 30% in France7.
We conclude that studies of the meningococcal carrier state are a very important measure for the control of this severe disease. More studies to be conducted in healthcare staff in other places will be important to compare the behavior of the meningococcus. New research studies involving the genes responsible for bacterial colonization and transmission are needed to elucidate the intriguing behavior of this bacterium.
Ethical disclosuresProtection of human and animal subjectsThe project was approved by the Ethics Committee of the Federal University of Rio Grande do Sul under the number 102/2011.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflict of interestThe authors declare they have no conflict of interest.
This study received support from CNPq (National Council for Scientific and Technological Development, Brazil) and CAPES (Coordination for the Improvement of Higher Education Personnel, Brazil). The authors would like to thank the Laboratory of Phytoplankton Ecology and Marine Microorganisms of the Federal University of Rio Grande do Sul, RS, Brazil.