Cystic fibrosis patients with Burkholderia cepacia complex pulmonary infections have high morbidity and mortality. Worldwide, this disease is undergoing substantial epidemiological changes. Advances in the diagnosis and treatment have conditioned an increase in child survival as well as in the proportion of affected adults. In order to know our reality, we refer to an epidemiological study in 64 CF patients during 11 years of surveillance, focusing on infections caused by Burkholderia species. Conventional and automated phenotypic tests, restriction fragment length polymorphism-recA, recA gene sequencing, and matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry were applied. Bacterial isolates were also tested for antimicrobial susceptibility patterns. The prevalence of Burkholderia cepacia complex was 9.4%. Based on recA gene sequencing, the most common species identified were Burkholderia cenocepacia (67.3%) and Burkholderia vietnamiensis (20.3%). Ceftazidime and meropenem were the most active, inhibiting 53% and 46% of isolates, respectively. This report represents the first systematic study of Burkholderia infections in our CF population since beginning of monitoring and treatment and highlights the importance of continued longitudinal studies.
Los pacientes con fibrosis quística (FQ) con infecciones pulmonares causadas por especies del complejo Burkholderia cepacia tienen una alta morbimortalidad. En todo el mundo, esta enfermedad está experimentando cambios epidemiológicos sustanciales. Los avances en el diagnóstico y el tratamiento han condicionado un aumento en la supervivencia infantil, así como en la proporción de adultos afectados. Para conocer nuestra realidad, nos referimos a un estudio epidemiológico en 64 pacientes con FQ durante 11 años de vigilancia, focalizando las infecciones causadas por especies del género Burkholderia. Se aplicaron pruebas fenotípicas convencionales y automatizadas, polimorfismo de longitud de fragmentos de restricción-recA, secuenciación del gen recA y espectrometría de masa MALDI-TOF. Los aislados bacterianos también se analizaron para determinar los patrones de susceptibilidad antimicrobiana. La prevalencia de complejo B. cepacia fue del 9,4%. Con base en la secuenciación del gen recA, las especies más comunes identificadas fueron Burkholderia cenocepacia (67,3%) y Burkholderia vietnamiensis (20,3%). Ceftazidima y meropenem fueron los antibióticos más activos e inhibieron el 53 y el 46% de los aislamientos, respectivamente. Este informe representa el primer estudio sistemático de las infecciones por Burkholderia en nuestra población desde el comienzo de la monitorización y el tratamiento, y resalta la importancia de continuar los estudios de vigilancia longitudinales.
The Burkholderia cepacia complex (Bcc) bacteria are important opportunistic human pathogens and represent the most feared of all infections in cystic fibrosis (CF) patients6. CF is an inherited chronic disease with a mean incidence value of about 1/7000 live births in Argentina1. The rate of Bcc bacterial infection in CF ranges from 0.1% to 36%, depending on the hospital center12. Bacteria of the Bcc are highly problematic pathogens for multiple reasons, among more relevant high antibiotic resistance9,13,19; potential for patient-to-patient transmission7,10,13; ability to cause septicemic and fatal necrotizing pneumonia known as “cepacia syndrome”7.
The relevance of the epidemiology of Bcc in CF has been highlighted by several observations. The potential for patient-to-patient spread has been further underlined by the detection of numerous outbreaks among patients with CF attending in the same hospital center5,7,10,12. Early studies of transmissibility identified Burkholderia cenocepacia lineage, known as ET-12, as the most prevalent CF species in North America and Europe10. Bcc is a group with a dynamic taxonomy that currently includes 23 species; however, new species have been identified or reclassified over the years14,18,21. Despite advances in taxonomy identification of Bcc species predicting prognosis after their infection in CF is challenging. Accurate identification of Bcc species is essential for treating CF patients because of interspecies variation in antimicrobial susceptibility patterns9,13, and potential for patient-to-patient transmission7,10.
Bcc species comprise phenotypically indistinguishable microorganisms with high genetic similarity20. Phenotypic identification methods such as VITEK2, Phoenix, protein signature identification methods like VITEK MS and Bruker Biotyper (MALDI-TOF MS) were reported with varying levels of discrimination to identify Bcc15,17,20. Accurate identification of Bcc at species-level requires the use of molecular assays such as polymerase chain reaction (PCR)-based assays, PCR-restriction fragment length polymorphism (RFLP-PCR), DNA sequencing, while that multilocus sequence typing (MLST) is required for discrimination at the strain level 2,20.
Here, we present an analysis based on 11 years of epidemiological surveillance and clinical outcome of Burkholderia infection in the CF Treatment Center of “Dr. Fernando Barreyro” Pediatric Hospital, in Posadas.
Materials and methodsPatient populationThis CF population comprises a total of 64 patients, of which 91% were children under 18 years old and 9% were adults. In the pediatric population 49% were females, while in the adult population females represented the 40%. Bcc bacteria have been isolated from 6 of these patients, belonging to both the adult (n=1; male) and pediatric (n=5; female) populations. The age at the time of the first Bcc isolation varied between 5.7 and 18.8 years old, with an estimated mean age of 12.3 years.
Bacteria collectionIn total 653 isolates were recovery from 64 CF patients between January 2007 and July 2017. Sputum samples were obtained from CF patients every 3–4 months during periodic consultations to monitor their clinical status or inpatient and more often for patients showing clinical deterioration.
Species determinationThe clinical samples were plated onto MacConkey agar, Sheep blood agar, Mannitol salt agar and Burkholderiacepacia selective agar (BCSA) medium (Britania Labs, Argentina) for 2 days at 35°C, followed by 3 days of incubation at room temperature. The microorganisms were characterized at the genus and species level by polyphasic approach of combined phenotypic methods8. In order to confirm and identify the distinct Bcc species, a 1040bp PCR product corresponding to the recA gene was amplified by PCR17. Aliquots of the amplified products were subjected to restriction fragment length polymorphism (RFLP) analysis with the restriction enzyme HaeIII (Promega, Inc.). Restriction PCR patterns obtained were analyzed and compared with those reported in the literature. The amplified products were purified with a QIAquick PCR purification kit (Qiagen Inc., CA) and sequenced with two additional primers, BCR3 and BCR, as previously described2,12. MALDI-TOF analysis was performed as previously described15; species identification was obtained when the scores were ≥2.0 as recommended by the manufacturer.
Antimicrobial susceptibility testingAntimicrobial susceptibility testing was performed by the disk diffusion method according to recommendations of Clinical and Laboratory Standards Institute4. Due to the periodic lack of laboratory supplies, all antibiotics were not evaluated in all isolates. Isolates were tested against 4 antimicrobial agents: ceftazidime – CAZ (30μg), meropenem – MEM (10μg), minocycline – MIN (30μg) and trimethoprim/sulfametoxazol – TMS (38/2μg) (Britania Lab., Argentina). Additionally, we tested others antimicrobials included as supplementary material (Table S1), which could be used during treatment in our center: cefepime – FEP (30μg), doripenem – DOR (30μg), imipenem –IMP (10μg), amikacin – AKN (30μg), gentamicin – GEN (10μg), ciprofloxacin – CIP (5μg) and levofloxacin – LVX (5μg). Because these drugs do not count with defined breakpoints for Burkholderia spp. in current standards, breakpoints for Pseudomonas aeruginosa or Enterobacteriaceae were used4,9. In some isolates antibiotic resistances were proved by automated Vitek®2Compact system (bioMérieux, Marcy l’Etoile, France) following manufacturer's instructions.
EthicsStudies involving clinical Bcc isolates obtained as part of the hospital routine were approved by the Hospital Ethics Committee and the patients’ anonymity was preserved.
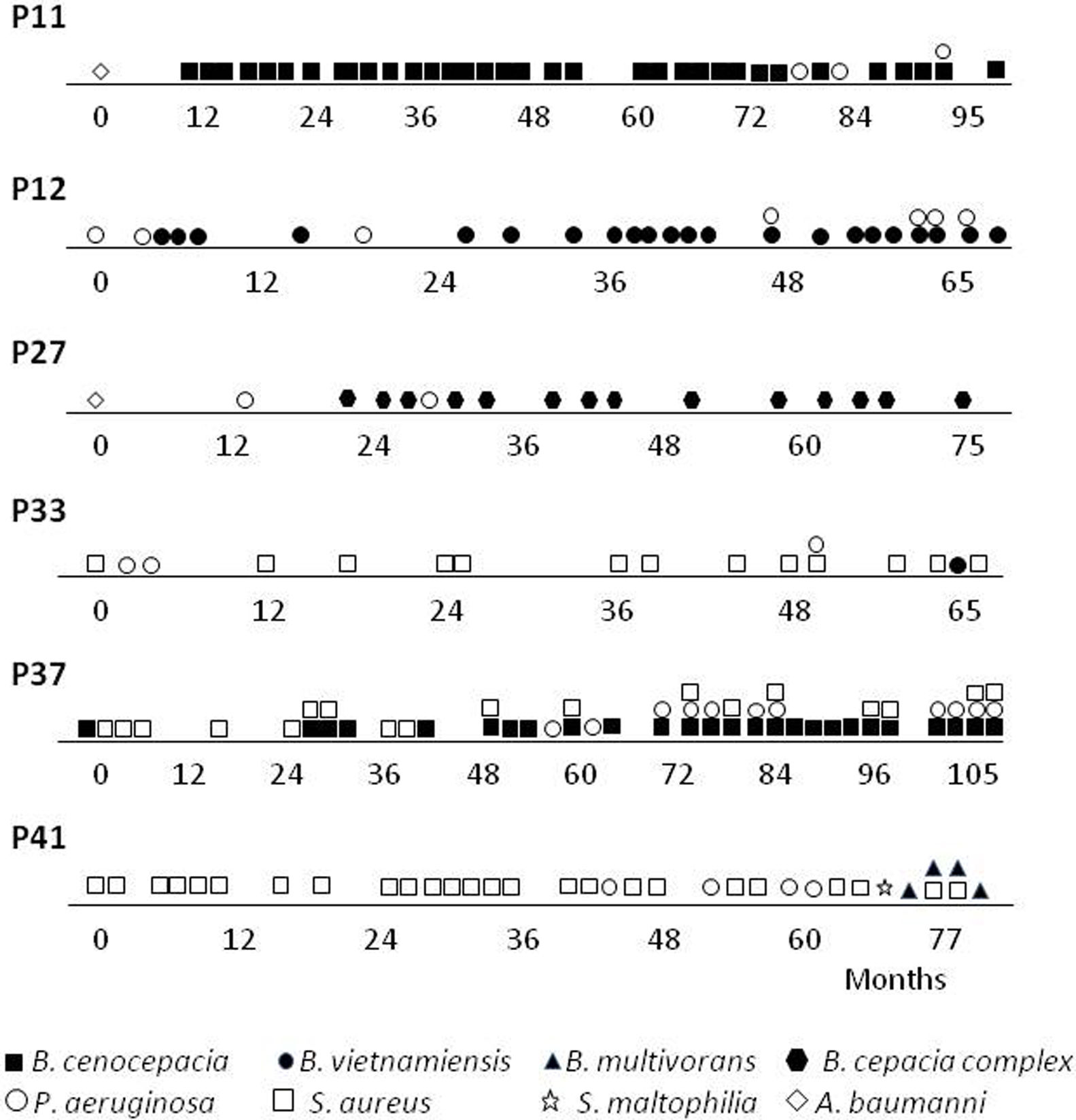
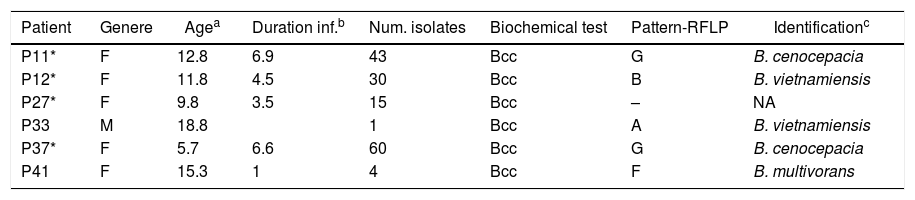
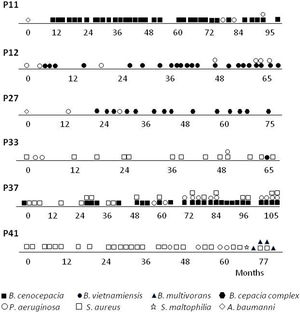
ResultsOverview of Bcc acquisitionsSince the implementation of selective media (BCSA) in 2008, Burkholderia cepacia complex is increasingly being isolated from sputum of CF patients. The initial biochemical screening performed over an 11-year period at our institution, determined a frequency of 23.7% (155/653) Bcc isolates. An additional molecular characterization made on 64 patients, based on the recA gene, confirmed 153 isolates to Bcc-level, which provides an infection prevalence of 9.4% (6 of 64 patients). The PCR-RFLP, recA sequencing data and MALDI-TOF data, for the majority of the isolates, were in agreement and corroborated with Bcc identification data. Examination of the PCR-RFLP patterns of the recA gene was performed first. Four distinct HaeIII-derived RFLP patterns described previously12 (pattern G, B. cenocepacia; pattern F, B. multivorans; patterns A and B, B. vietnamiensis), were found among isolates belonging to 5 of the studied patients. Isolated recovered from a patient (P27) were not available for molecular identification at the species level (Table 1).
Patient data and identification of isolates Bcc recovered from sputum.
| Patient | Genere | Agea | Duration inf.b | Num. isolates | Biochemical test | Pattern-RFLP | Identificationc |
|---|---|---|---|---|---|---|---|
| P11* | F | 12.8 | 6.9 | 43 | Bcc | G | B. cenocepacia |
| P12* | F | 11.8 | 4.5 | 30 | Bcc | B | B. vietnamiensis |
| P27* | F | 9.8 | 3.5 | 15 | Bcc | – | NA |
| P33 | M | 18.8 | 1 | Bcc | A | B. vietnamiensis | |
| P37* | F | 5.7 | 6.6 | 60 | Bcc | G | B. cenocepacia |
| P41 | F | 15.3 | 1 | 4 | Bcc | F | B. multivorans |
We examined the survival of the 64 CF patients. In 83% (5/6) of patients with Bcc, chronic colonization was established (Table 1). In the 5 patients chronically colonized, there was infection or co-infection with a single Burkholderia species. We did observe differences between the CF groups with or without Burkholderia concerning to (I) death: four of the eight deceased patients with a greater proportion attributable to B. cenocepacia; (II) age at death: with infections caused by Burkholderia species being associated with death in much older individuals (15.5 years±SD 2.65), while those who died with “Non-Burkholderia” bacteria (Staphylococcus aureus and P. aeruginosa) were younger (2.75 months±SD 1.5).
A more detailed analysis of the chronology of infection/colonization of patients with Bcc species is presented in Figure 1. The four pediatric patients (P11, P12, P27 and P37) were considered chronically infected with 50% or more of positive Bcc cultures. All patients died following long-term infection (3.5–6.5 years) and clinical deterioration. Patients P11, P12 and P27 were co-infected with P. aeruginosa and the patient P37, was also co-infected with S. aureus. Patients P33 and P41 were infected mainly with S. aureus almost 5 and 6 years; however, in the last year were co-infected with Bcc bacteria. Patient P33 was co-infected with B. vietnamiensis, which was spontaneously eradicated after 3 months since the first isolation. Patient P41 was also co-infected with P. aeruginosa and S. maltophilia, and finally with B. multivorans, which apparently replaces S. aureus. In summary, the species Bcc were associated either with chronic infection that in most CF patients (the case of patients P11, P12, P27, P37 and P41) led to death, or with a transient infection that in this case led to spontaneous clearance (Patient P33).
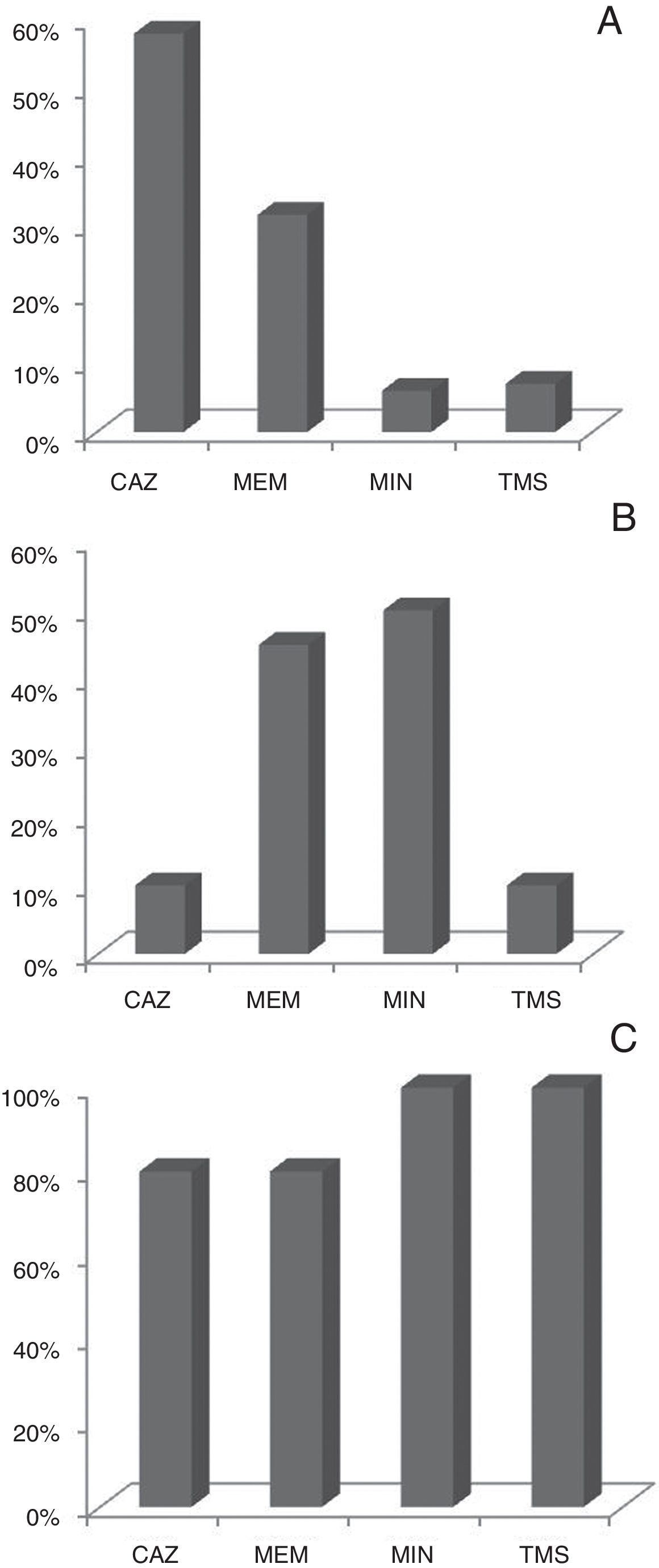
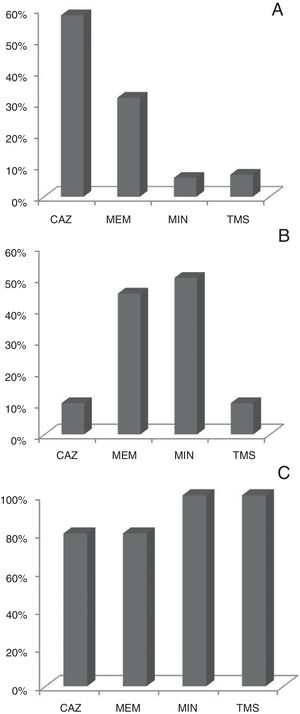
Antimicrobial susceptibilityBcc isolates were tested for 11 antimicrobial agents by disk diffusion method and Vitek®2Compact instrument. Ceftazidime and meropenem were the most effective antimicrobials tested in vitro with 53% and 46% of the isolates being susceptible to these compounds respectively. Figure 2 shows the profile of antibiotic susceptibility by species. The resistance rates of the Bcc isolates to remaining antibiotics, ranged from approximately 27% to 100%, and were markedly dissimilar among the three Bcc species examined (Table S1).
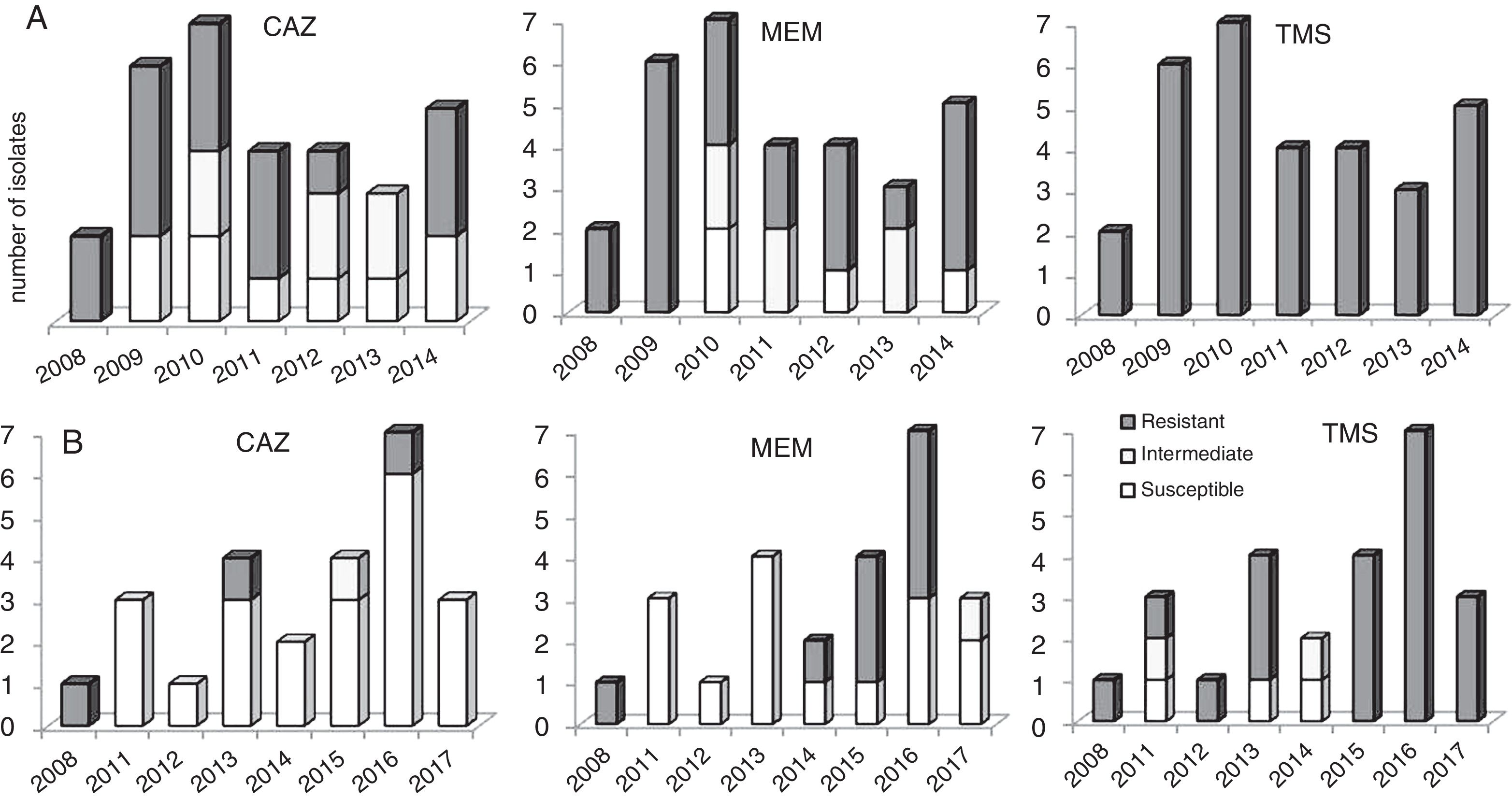
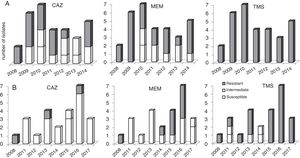
Variation of the antimicrobial susceptibility profiles in parallels genotypes of B. cenocepacia isolates obtained during chronic infectionAn appreciable variation of the antimicrobial susceptibility pattern of sequential isolates obtained from chronic infected patients was registered. This was dependent on the antibiotics tested, the co-infecting species, and the colonized CF patient clinical situation. Basically, this variation can be considered as a fluctuation of the susceptibility level of isogenic variants during the colonization period, from resistant (R) to sensitive (S), or vice versa, eventually passing through an intermediate susceptibility phenotype (I). We highlight the cases of two patients (P11 and P37) infected with same bacteria (B. cenocepacia) where the first isolate obtained from a specific patient was resistant to a certain antimicrobial and during persistent infection, either susceptible or resistant variants were isolated. For example, the isolates obtained from patient P11 in the first and last isolation dates were resistant to ceftazidime, while most of the intermediary isolates obtained throughout a period of six years were susceptible; the opposite case, as observed for patient P37 (Fig. 3).
DiscussionThe present study reports regarding the epidemiology of B. cepacia complex bacteria infecting CF patients in Posadas city (Misiones, Argentina). The overall prevalence (9.4%) of Bcc in 11-year of surveillance at our institution is similar to results reported from previous studies of CF patients in Argentina12. Recent worldwide epidemiological studies concerning the distribution of Bcc species in CF patients have cited B. multivorans and B. cenocepacia as the most frequently recovered species (85% of all Bcc infections)6,7,10,12. In this study, of 153 isolates collected from 6 patients attending our CF, the majority (67.3%) belonged to B. cenocepacia; followed by B. vietnamiensis and B. multivorans with a prevalence of 20.3% and 2.6% respectively. Approximately 10% of the isolates could not be identified (frozen isolates were not recovered). Interestingly, this distribution of species observed is opposed to results from previous studies of Bcc bacteria infecting Argentinean CF patients3,12.
Reports indicate the female sex as a risk factor for the result in Bcc infections22. In this study there are not substantial data for support this fact/hypothesis (Table 1). Reports from Canada and other countries show that females generally experience a survival disadvantage in CF22. Therefore, female sex per se could be confounding this observation; nonetheless, the mortality rate seen in our female patients infected with B. cenocepacia and B. vietnamiensis are striking.
Transmission of Bcc bacteria is considered an important factor in the colonization of CF patients. Bcc bacteria in Argentina are mainly characterized by the acquisition and spread of B. contaminans strains3,12. Fortunately, throughout the study period there was no evidence of substantial clonal spread between our patients (Table 1, pattern RFLP and sequence recA gene). Possibly, the only exception was a B. cenocepacia isolate that was common between two patients. Although the origin could not be detected, probably that is the result of inadvertent contact between the two patients in a hospital common room or social contact. B. cenocepacia (pattern G, MLST – recA gene: allele 14) was first detected in a patient (P11) in January 2008 and it was detected by second time eight months later in a different patient (P37). In both isolates, the sequencing and comparison of the recA gene revealed the same deletion (A, in position 32). The factors determining the relative transmissibility of a given isolate are not understood, infection control remains important for all Bcc bacterial infections in CF22.
One of the major concerns about Bcc pathogens is their intrinsic resistance to many commonly used antibiotics. The Bcc species are resistant to a number of antibiotic classes including aminoglycosides, polymyxins, quinolones, trimethoprim, chloramphenicol and to the host antimicrobial peptides with significant differences among species9,13,16,19. In our study, the isolates of B. cenocepacia showed high resistance to minocyclin (74%) and meropenem (54%) and high sensitivity to ceftazidime. Whereas, B. vietnamiensis showed resistance to ceftazidime (90%) and intermediate to high sensitivity to meropenem and minocyclin. B. cenocepacia and B. vietnamiensis were resistant to trimethoprim/sulfamethoxazole unlike B. multivorans. Unfortunately, there is no breakpoint for the antibiotic cefepime in Bcc. However, the sensitivity values observed by Vitek®2Compact would indicate this drug as a good therapeutic agent to be considered (Supplementary material).
There is evidence B. cenocepacia clonal expansion during chronic lung colonization, presumably as the result of mutations and selective pressures occurring in the CF lung environment7,11. A clinically important consequence of this phenotypic diversity is related to antimicrobial susceptibility testing in diagnostic laboratories, which are typically carried out on either single isolates or more colonies acting as representatives of morphotypes. Nevertheless, there is almost always considerable diversity in the antimicrobial susceptibilities within the population isolated from an individual sputum sample9 and not considering this fact could lead to a therapeutic failure.
In conclusion, this study represents the first systematic study of Burkholderia infections in our CF population since the beginning of monitoring and treatment, and highlights the importance of continued longitudinal studies on the health outcomes of patients infected and co-infected with these bacteria.
FundingThis work was supported by the Agencia Nacional de Promoción Científica y Tecnológica (grant BID PICT 2017-2941) and a grant from Foundation A. J. Roemmers.
Author's contributionJF and MVS coordinated the design and data acquisition. PFM, JF, MM and MVS analyzed and interpreted the results. SR, PFM, MM, MVS and LL were responsible for the microbiology analysis. PFM and MM drafted the manuscript. All authors read and approved the manuscript.
Conflict of interestThe authors declare that they have no competing interests.
To the doctors Guillermo Frada and Carolina Barrías for their contributions.