The distribution of Aspergillus species in soil has been widely studied all over the world. The aim of this study was the phenotypic and genotypic characterization of species Aspergillus belonging to section Fumigati present in soils from two Argentinian semi-desert areas having different geological conditions. Altogether, 23 isolates belonging to Aspergillus section Fumigati were recovered and identified using a polyphasic approach including phenotypic and molecular identifications. Aspergillus fumigatus sensu stricto and Aspergillus fumigatiaffinis had the highest frequency, of occurrence while isolates closely related to Aspergillus udagawae and Aspergillus felis were rarely observed. A. fumigatiaffinis and isolates closer to A. udagawae were isolated for the first time from Argentinian soils and this is the first report on the occurrence of species belonging to the A. felis clade in South America. Recent scientific interests in biodiversity, as well as the increasing importance of aspergilli as causative agents of human and animal diseases increase the need to understand the diversity and occurrence of these fungi in nature.
La distribución de especies de Aspergillus en el suelo se ha estudiado ampliamente en todo el mundo. El objetivo de este trabajo fue caracterizar fenotípica y genotípicamente las especies pertenecientes a la sección Fumigati presentes en los suelos de dos zonas semidesérticas de Argentina con diferentes geologías. En total, 23 Aspergillus de la sección Fumigati fueron aislados e identificados utilizando un enfoque polifásico incluyendo identificaciones fenotípicas y moleculares. Aspergillus fumigatus sensu stricto y Aspergillus fumigatiaffinis aparecieron con mayor frecuencia, mientras que los aislamientos relacionados a Aspergillus udagawae y a Aspergillus felis se observaron raramente. Este es el primer informe de A. fumigatiaffinis y de aislamientos estrechamente relacionados a A. udagawae en suelos argentinos; también el primero sobre la ocurrencia de especies pertenecientes al clado A. felis en Sudamérica. El emergente interés científico en la biodiversidad, así como la creciente importancia de Aspergillus como agentes causales de enfermedades humanas y animales, aumentan la necesidad de conocer la diversidad y la ocurrencia de estos hongos en la naturaleza.
In the 21st century, Aspergillus and its teleomorphs have been investigated with polyphasic methods to examine variability among species. Currently, according to the polyphasic taxonomy, Houbraken et al.24 and Hubka et al.25 proposed that the genus Aspergillus is classified into four subgenera (Aspergillus, Circumdati, Fumigati and Nidulantes) and 20 sections and each includes a number of related species.
Section Fumigati is one of the most species-rich sections in the genus Aspergillus and includes species with overall significance for medicine, pharmacology, biotechnology, food and soil mycology. At present, the section consists of 51 taxa: 21 strictly anamorphic Aspergillus species and 30 Neosartorya species43.
The distribution of Aspergillus species in soil has been widely studied all over the world30. Information on the diversity of Aspergillus species was reported in more than 270 studies of microfungi from soil between 0 and 46 degrees North or South (N/S) and concluded that the relative percentage of Aspergillus species is greatest in 25–35 degrees N/S. Many rare species and most new species of Aspergillus have been reported only from tropical and subtropical soils19,22,29.
The distribution of Aspergillus species in Argentinian soils has been treated in several studies, but there are only limited data available regarding section Fumigati14,31,32. The aim of this study was the phenotypic and genotypic characterization of species belonging to section Fumigati present in soils from two Argentinian semi-desert areas having different geological conditions.
Materials and methodsAreas of studyIn winter (July) 2011 soil samples from Talampaya National Park and Pampa de Achala were collected.
Talampaya National Park is in the south west of La Rioja province (29°46′S and 67°54′O). The park covers an area of 2150 square kilometers, at an altitude of 1300 meters above mean sea level. This National Park is in a semiarid continental zone with 150–170mm annual rainfall. The climate is hot in summer, with temperatures exceeding 50°C, and going down to −7 to −9°C on winter nights. On the characteristic sandy and stony soils of the place, the vegetation is represented by xerophilous bushes and cactuses2,3.
Pampa de Achala is a hydrologic natural reserve located in the north west of Córdoba province (31°41′S and 64°50′O). It is a pampa (plain) of 146000 square kilometers with gentle slope and steep gorge located at an altitude between 1500 and 2790m above mean sea level. The climate of the region is temperate-cold; maximum temperatures are generally 30°C in the summer, falling below −20°C during the winter. Rainfall takes place from October to April and is about 800mm annually. The Pampa's vegetation is characterized by scrub and grasslands1,3.
SamplingSoil samples were collected in Talampaya National Park in a 55 km-section of national road 76. Soil samples were collected in Pampa de Achala in a 30km-section of the old provincial route 14. In both cases, sampling was carried out every 5km, moving away 200 or 300m from the main road. Five samples were collected randomly in a 20m2 radius. A pool of about 250g was made. The samples were gathered with a sterilized spoon from the superficial layer (2–3cm). In cases in which the soil was hard, a knife tip sterilized in situ using iodized alcohol was used. The soil samples were stored in sterilized paper bags at 4°C until they were analyzed.
Soil processingDilution technique: One gram of each sample was diluted in 10ml sterilized water. From this 1:10 dilution a 1:100 dilution was made. Of each dilution (1:10 and 1:100), 0.2ml were transferred to Petri dishes containing potato-dextrose-agar (PDA) with chloramphenicol (0.25g/l). Each dilution was cultured twice. Culture plates were incubated for 7 days at 37°C in order to inhibit the growth of mesophilous molds. The plates were kept for 20 days waiting for the possible growth of associated teleomorphs.
The pH of each soil sample collected (10g) was measured immediately using a pH meter (Jenco 6230), after dilution in 125ml sterile distilled water with 5min of agitation.
Isolation and morphological diagnosisPrimary culture plates were examined under a stereoscopic microscope and every Aspergillus and its teleomorphs were counted and sub-cultured on specific media. Each species was counted once in each sample, even if it appeared twice in the same plate or in the duplicate. Species frequency was calculated by its presence in total samples.
Isolates were subcultured on Czapek yeast autolysate agar (CYA), oatmeal agar (OA) and malt extract agar (MEA) and incubated for a week at 25°C. Identification was done by macroscopic and microscopic morphology criteria according to general taxonomic keys. To distinguish growing maximum temperature range, isolates were inoculated on CYA and incubated for 7 days at 10-37-42-45-48 and 50°C30,37,38,40.
Molecular identificationCultures were grown on 2ml malt peptone broth [10% (v/v) malt extract (Brix 10) and 0.1% (w/v) bacto peptone (Difco)], in 15ml tubes. The cultures were incubated at 25°C for 7 days37.
DNA was extracted from mycelia using the Masterpure™ yeast DNA purification kit (Epicenter Biotechnologies, Madison, WI, USA) according to the manufacturer's instructions.
Random amplified polymorphic DNA (RAPD)-PCRs under the conditions described by Hong18 was performed to identify Aspergillus fumigatus sensu stricto (A. fumigatus s.s.) and to detect other different band patterns. Primers PELF (5′-ATATCATCGAAGCCGC-3′) and URP1F (5′-ATCCAAGGTCCGAGACAACC-3′) were used and the A. fumigatus type strain CBS 133.61 was used as pattern.
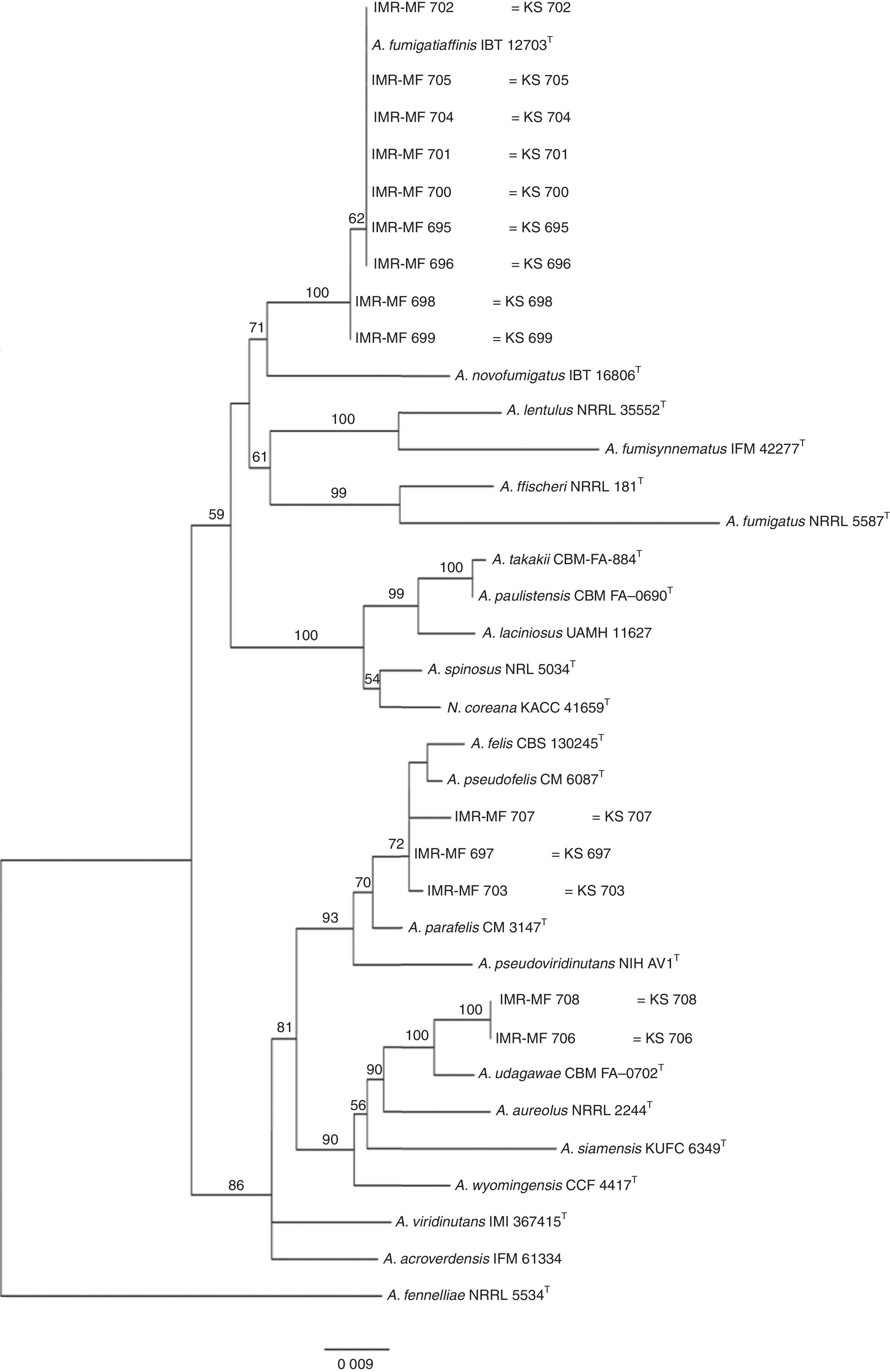
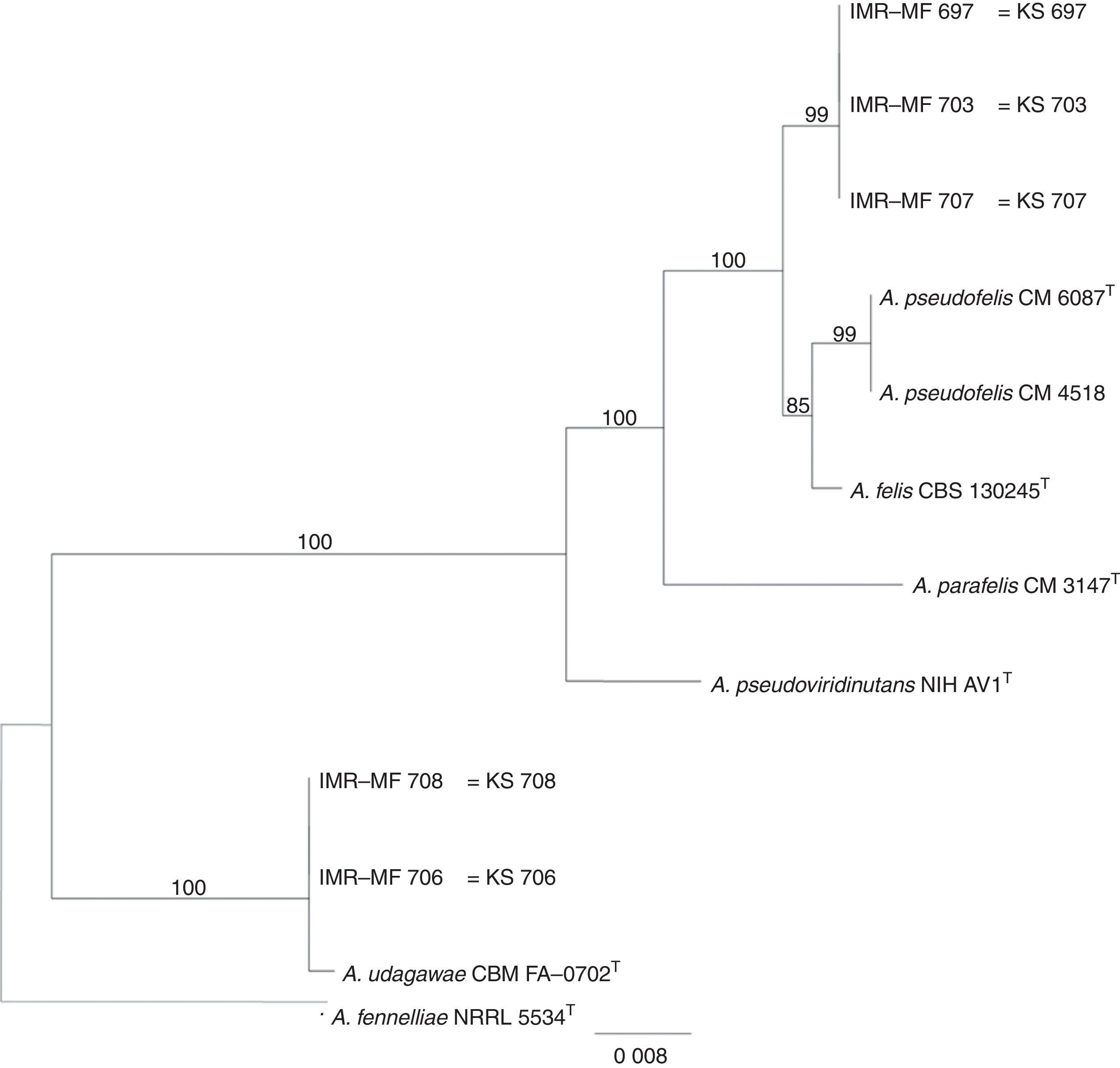
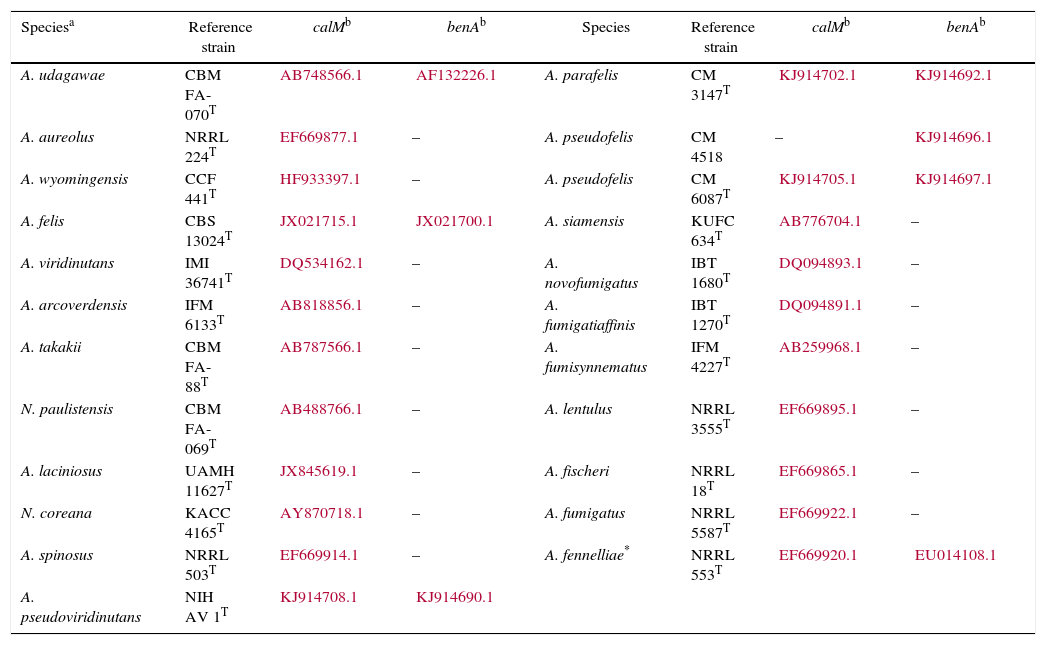
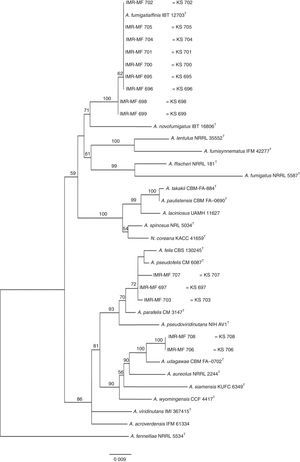
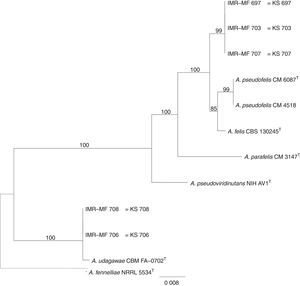
Isolates genetically different from type strain A. fumigatus were sent to the Department of Microbiology, Faculty of Science and Informatics, University of Szeged, Hungary for molecular identification. DNA was extracted as mentioned above. Amplification of the partial calmodulin gene (calM) and β-tubulin (benA) was carried out using primers cmd5 (5′-CCGAGTACAAGGAGGCCTTC-3′), cmd6 (5′-CCGATAGAGGTCATAACGTGG-3′)18, and Bt2a (5′-GGTAACCAAATCGGTGCTGCTTTC-3′), Bt2b (5′-ACCCTCAGTGTAGTGACCCTTGGC-3′)15, respectively. The amplified DNA fragments were purified by the QIAquick PCR purification kit (Qiagene, Hilden, Germany). Sequence analyses were performed with the BigDye Terminator 3.1 Cycle Sequencing Ready Reaction Kit (ABI 0401041, Foster City, California) for both strands. Sequences were analyzed on the ABI PRISM 310 Genetic Analyzer (Applied Biosystems, Carlsbad, CA, USA). Alignment of partial calM and benA sequences was done using MAFFT v7.149b with the L-INS-i option28. Phylogenetic reconstruction was conducted using Maximum Likelihood analyses in raxmlGUI v1.3.1 under the GTR+Γ model41. The analysis was run in 1000 bootstrap replicates. The calM and benA sequences of type isolates of section Fumigati species were obtained from the database of the National Center for Biotechnology Information (NCBI) (Table 1).
calM and benA sequences of type isolates of section Fumigati used for phylogenetic analysis
| Speciesa | Reference strain | calMb | benAb | Species | Reference strain | calMb | benAb |
|---|---|---|---|---|---|---|---|
| A. udagawae | CBM FA-070T | AB748566.1 | AF132226.1 | A. parafelis | CM 3147T | KJ914702.1 | KJ914692.1 |
| A. aureolus | NRRL 224T | EF669877.1 | – | A. pseudofelis | CM 4518 | – | KJ914696.1 |
| A. wyomingensis | CCF 441T | HF933397.1 | – | A. pseudofelis | CM 6087T | KJ914705.1 | KJ914697.1 |
| A. felis | CBS 13024T | JX021715.1 | JX021700.1 | A. siamensis | KUFC 634T | AB776704.1 | – |
| A. viridinutans | IMI 36741T | DQ534162.1 | – | A. novofumigatus | IBT 1680T | DQ094893.1 | – |
| A. arcoverdensis | IFM 6133T | AB818856.1 | – | A. fumigatiaffinis | IBT 1270T | DQ094891.1 | – |
| A. takakii | CBM FA-88T | AB787566.1 | – | A. fumisynnematus | IFM 4227T | AB259968.1 | – |
| N. paulistensis | CBM FA-069T | AB488766.1 | – | A. lentulus | NRRL 3555T | EF669895.1 | – |
| A. laciniosus | UAMH 11627T | JX845619.1 | – | A. fischeri | NRRL 18T | EF669865.1 | – |
| N. coreana | KACC 4165T | AY870718.1 | – | A. fumigatus | NRRL 5587T | EF669922.1 | – |
| A. spinosus | NRRL 503T | EF669914.1 | – | A. fennelliae* | NRRL 553T | EF669920.1 | EU014108.1 |
| A. pseudoviridinutans | NIH AV 1T | KJ914708.1 | KJ914690.1 |
Sequences of calM of the 14 Aspergillus section Fumigati isolates were submitted to the European Nucleotide Archive (ENA) and assigned accession numbers KP824727–KP824740. Sequences of benA of the isolates related to A. udagawae and to A. felis were submitted to the European Nucleotide Archive (ENA) and assigned accession numbers LT674551–LT674557.
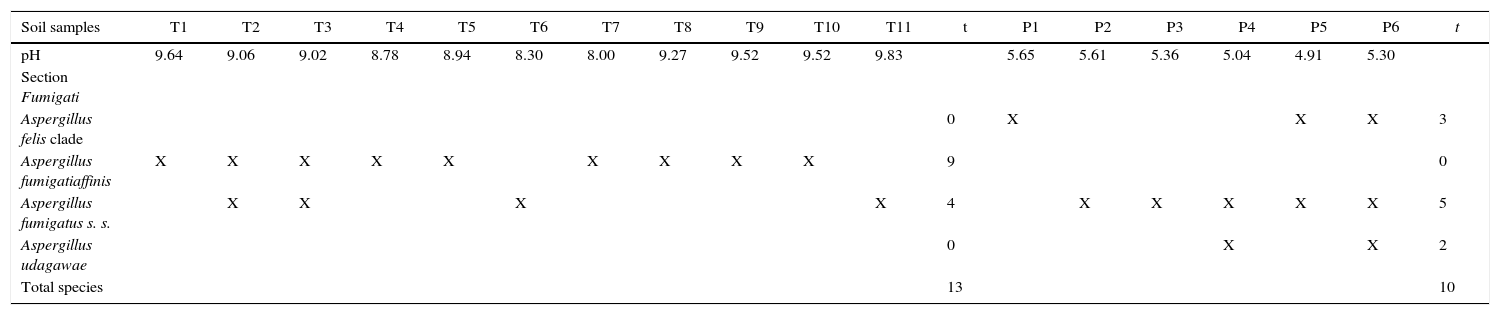
ResultsA total of 17 soil samples was collected, 11 in Talampaya National Park and 6 in Pampa de Achala. The pH values of Talampaya National Park soil samples were alkaline, ranging from 8.00 to 9.83. In contrast, the pH values of Pampa de Achala soil samples were acid, ranging from 4.91 to 5.65 (Table 2).
Frequency of Aspergillus section Fumigati isolated and pH of soil samples
| Soil samples | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | T11 | t | P1 | P2 | P3 | P4 | P5 | P6 | t |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 9.64 | 9.06 | 9.02 | 8.78 | 8.94 | 8.30 | 8.00 | 9.27 | 9.52 | 9.52 | 9.83 | 5.65 | 5.61 | 5.36 | 5.04 | 4.91 | 5.30 | ||
| Section Fumigati | |||||||||||||||||||
| Aspergillus felis clade | 0 | X | X | X | 3 | ||||||||||||||
| Aspergillus fumigatiaffinis | X | X | X | X | X | X | X | X | X | 9 | 0 | ||||||||
| Aspergillus fumigatus s. s. | X | X | X | X | 4 | X | X | X | X | X | 5 | ||||||||
| Aspergillus udagawae | 0 | X | X | 2 | |||||||||||||||
| Total species | 13 | 10 |
T: Talampaya National Park; P: Pampa de Achala; t: total.
From these 17 samples, 39 Aspergillus isolates were obtained. According to their phenotypic features, 23/39 belong to subgenus Fumigati, 8/39 to subgenus Nidulantes and 8/39 to subgenus Circumdati.
Within subgenus Fumigati only isolates belonging to section Fumigati were found, which were present in all the 17 samples analyzed.
The RAPD-PCR for 23 isolates of Aspergillus section Fumigati was conducted. Nine (9/17, 52.94%) isolates were genetically similar to type strain A. fumigatus s.s. (CBS 133.61) (data not shown) and the 14 genetically different isolates were identified by sequencing parts of the calM gene (Fig. 1) and the benA gene (Fig. 2). Nine (9/17, 52.94%) isolates were clearly identified as A. fumigatiaffinis using the calM gene. Two (2/17, 11.76%) isolates were closely related to A. udagawae and 3/17 (17.65%) isolates belong to the A. felis clade sequencing the calM and benA genes. Table 2 shows the Aspergillus section Fumigati identified and the areas where they were found.
A. fumigatus s.s. were identified by morphological and growth characteristics: dark blue-green color and velutinous colony, fast and abundant sporulation on MEA and CYA. Most isolates had subclavate vesicles (13-26μm) and the conidiophore stipe diameter ranged from 5 to 9μm. All A. fumigatus s.s. did not grow at 10°C and grew at 50°C on CYA, as was expected according to the literature18,20,38,40.
A. fumigatiaffinis, and the isolates closely related to A. felis and A. udagawae showed less sporulation on MEA and CYA than A. fumigatus s.s. and the colonies were white, with a dull green center. Most isolates of A. fumigatiaffinis showed (sub)globose vesicles (15–23μm), while vesicles of isolates belonging to the A. felis clade were subclavate (15–17μm) and isolates closely related to A. udagawae exhibited hemispherical to flask-shaped vesicles (11–16μm). The width of conidiophore stipes in A. fumigatiaffinis ranged from 6 to 8μm, while in isolates belonging to the A. felis clade from 5 to 9μm and in the isolates closely related to A. udagawae from 4 to 6μm. All these isolates were able to grow at 10°C and unable to grow at 50°C.
All isolates were deposited at Szeged Microbiological Collection of the Department of Microbiology, Faculty of Science and Informatics, University of Szeged, Hungary under the assigned numbers KS695–KS708.
DiscussionIn a vast compilation of studies on Aspergillus species in soil it was found that Aspergillus species most frequently occur in subtropical zones, between 25 and 35 degrees N/S22,23,29. Considering that the area studied is included in those latitudes, the Aspergillus spp. frequencies found probably ratify that assertion.
Section Fumigati was represented in both eco-regions studied, although with different species distributions. A. fumigatus s.s. and A. fumigatiaffinis were the most frequent species, followed by isolates closely related to A. felis and A. udagawae. However, unlike the other species, A. fumigatus s.s. occurred in both areas, confirming its capability of adaptation and ubiquity. A. fumigatiaffinis and isolates closely related to A. udagawae and belonging to the A. felis clade were isolated for the first time from Argentinian soils.
A. fumigatiaffinis was only isolated in Talampaya Park. The climatic characteristics and height above mean sea level of Talampaya Park are similar to those of Socorro city (USA), where A. fumigatiaffinis was reported for the first time18.
The presence of two isolates closely related to A. udagawae only in Pampa de Achala provides more data about the plasticity of this species because, even though it was described in Brazil in a humid subtropical area, it has also been identified as infecting humans and domestic animals in areas with extreme environments23,26,27,42.
A. felis is an important species in Aspergillus section Fumigati described recently10. A. felis (neosartorya-morph) is phenotypically similar to A. viridinutans; however, it differs by its ability to grow at 45°C and is phylogenetically related to A. aureolus and A. udagawae27. In our study, the isolates belonging to the A. felis clade were only isolated in Pampa de Achala. Although A. felis was isolated in soils of Wyoming, USA, this is the first report on the ambient occurrence of a species of this clade in the South American continent34.
Aspergillus is a large genus of ubiquitous and cosmopolitan fungi. Their high adaptability allows them to survive at different temperatures, low water activity and variations of pH and O2 concentration in soil29,39,45. This could be an explanation of the isolation of these strains in the extreme environments studied.
Fungi can tolerate a wide pH range39. The available evidence for the fungal growth–pH relationship thus indicates a significantly weaker direct connection with pH than in the case of bacteria, although pure culture studies have shown preference for certain pH values for different taxa of soil fungi12,39,45. In our study, A. fumigatiaffinis was isolated only from alkaline soils of Talampaya Park. In contrast, the isolates closely related to A. felis and A. udagawae were isolated only from Pampa de Achala where the soil pH was slightly acid. Further investigations are essential for correlating the preference for certain pH values for these taxa in order to allow definitive conclusions.
Even though A. fumigatus is the most prevalent agent of aspergillosis, several other species of section Fumigati have also been reported from clinical samples as causative agents of disease: A. lentulus7, A. udagawae16,26,42, A. felis9,10,44 and A. fumigatiaffinis4. In Argentina, a recent work with clinical isolates shows the circulating Aspergillus section Fumigati species. A. fumigatus s.s. was the most frequent followed by A. udagawae17.
Furthermore, these species show high in vitro MICs of azole drugs and amphotericin B, therefore they are frequently refractory to standard antifungal therapy4,8.
In this work, morphological and physiological characteristics were useful for differentiating isolates belonging to section Fumigati from those belonging to other sections. However, sequence-based methods are needed to assign isolates of section Fumigati at the species level. In this study, the species identification of isolates related to A. udagawae (IMR-MF 706 and IMR-MF 708) and to A. felis (IMR-MF 697, IMR-MF 703, IMR-MF 707) was not clear using only the partial calM sequences. The results of the analysis of partial benA sequences were necessary to suggest that IMR-MF 706 and 708 isolates were closer to A. udagawae and to the others isolate belonging to A. felis clade.
Identification of environmental and clinical isolates by molecular techniques is common practice in European countries and in the USA4,6,18,20–22,34,40,42,43,46 but there is scant information in South America5,11,13,17,33,35,36.
In medical mycology, as molecular methods become more available, they will allow the accurate identification of fungal infectious agents. The correct identification of species within section Fumigati could help to predict the severity of the disease and guide antifungal therapy.
The present work was carried out as a contribution to the knowledge of the ecology of section Fumigati, to understand where this section occurs in nature due to the increasing importance of these opportunistic pathogens.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors have no conflict of interest to declare.
We would like to thank to the chemistry technician Claudio Szarfsztejn for their assistance in the sampling and determination of the pH of the samples obtained