St. Louis encephalitis (SLEV) and West Nile (WNV) arboviruses, which circulate in Argentina, are maintained in enzootic transmission cycles involving Culex mosquitoes (vectors) and birds belonging to orders Passeriformes and Columbiformes (amplifier hosts). The objective of this work was to determine the circulation of both viruses among wild birds in a semiarid ecosystem in the Province of La Rioja through a serologic survey. During spring 2013 and fall 2014, a total of 326 wild birds belonging to 41 species were captured in areas close to the cities of La Rioja and Chilecito, in the Province of La Rioja. While exposure to SLEV and WNV was analyzed in birds’ serum through neutralizing antibody detection, viral circulation was estimated through apparent seroprevalence of neutralizing antibodies. The exposure of the avian community to viruses was 3.02% for SLEV and 1.89% for WNV, while 1.19% corresponded to coinfections. Our study confirms for the first time the circulation of SLEV and WNV in wild birds in the Province of La Rioja. Moreover, it is the first study to register neutralizing antibodies for flavivirus in the species Leptotila verreauxi (White-tipped Dove) (WNV) and Melanerpes cactorum (White-fronted Woodpecker) (SLEV). These results suggest that in semiarid ecosystems from northwestern Argentina the requirements and conditions for amplification and enzootic maintenance of SLEV and WNV would be present.
Entre los arbovirus que circulan en la Argentina se encuentran el virus de la encefalitis de San Luis (St. Louis encephalitis virus [SLEV]) y el virus del Nilo Occidental (West Nile virus [WNV]), los cuales son mantenidos en ciclos enzoóticos de transmisión que involucran a mosquitos Culex (vectores) y a aves de los órdenes Paseriformes y Columbiformes (hospedadores amplificadores). El objetivo de este trabajo fue determinar la circulación de ambos virus en aves silvestres de un ecosistema semiárido de la provincia de La Rioja (Argentina) mediante una encuesta serológica. Durante la primavera de 2013 y el otoño de 2014 se capturaron un total de 326 aves silvestres pertenecientes a 41 especies en las proximidades de las ciudades de La Rioja y Chilecito, provincia de La Rioja. Se analizó la exposición a SLEV y WNV en los sueros recolectados de aves mediante la detección de anticuerpos neutralizantes (AcNT), mientras que la circulación viral fue estimada a través de la seroprevalencia aparente de AcNT. La exposición de la comunidad aviar a los virus fue del 3,02% para SLEV y del 1,89% para WNV, mientras que el 1,19% correspondió a coinfecciones. Nuestro estudio confirma por primera vez la circulación de SLEV y WNV en aves silvestres de la provincia de La Rioja; además, representa el primer registro de AcNT de flavivirus en las especies Leptotila verreauxi (yerutí común) (WNV) y Melanerpes cactorum (carpintero del cardón) (SLEV). Estos resultados sugieren que, en ecosistemas semiáridos del noroeste argentino los requerimientos y las condiciones para la amplificación y el mantenimiento enzoótico de SLEV y WNV estarían presentes.
Many different viruses transmitted by arthropods (arbovirus) of sanitary and economic importance circulate in Argentina, Saint Louis encephalitis virus (SLEV) and West Nile virus (WNV) (Flavividiridae: Flavivirus)5 are found among them. While these viruses are maintained in enzootic transmission cycles between Culex mosquitoes and Passeriformes and Columbiformes birds, as vectors and amplifying hosts, respectively14, both of them belong to the Japanese encephalitis serocomplex22. Díaz et al.15 propose a transmission network where multiple species of hosts and vectors participate in the amplification and maintenance of SLEV and WNV in nature. Humans and other mammals are considered terminal hosts that occasionally present symptoms such as febrile illness and encephalitis21,33.
In humans, these viruses can be neuroinvasive in a very low percentage of cases; most infections are asymptomatic or present an undifferentiated febrile condition. In Argentina, SLEV reemerged as a human pathogen in 2002 and produced the first epidemic of SLEV human encephalitis in Córdoba city in 200539. Later, a new small outbreak occurred in 2010 in the same city40, as well as sporadic human cases in several provinces in the northeast and center of Argentina. WNV strains have been isolated from sick horses in 200630, and the same year the first human cases in the country were reported. No cases of SLEV or WNV have been reported in the Province of La Rioja to date27.
In our country, SLEV is an endemic and reemergent virus39, Culex pipiens quinquefasciatus Say, Culex interfor Dyar and Culex saltanensis Dyar being the mosquito vectors associated with its maintenance and transmission2,3. Serological findings and experimental studies have allowed the identification of pigeons Columbina picui and Zenaida auriculata as important amplifier hosts of SLEV in urban environments17. In turn, neutralizing antibodies for the virus have been detected in birds belonging to families Ardeidae, Columbidae, Fringillidae, Furnariidae, Icteridae, Tyrannidae, Phytotomidae and Cotingidae13,16,20,28,29,36,38 in different regions of central-northern Argentina.
The ecology of WNV in Argentina is poorly known14. The introduction of WNV in our country dates from the end of 2004, when the exposure of resident wild birds in multiple locations in central-northern Argentina8,12,20 was first detected. Experimental trials have shown the maintenance of the WNV in C.picui14. As for the vectors, so far Culex interfor, Culex maxi Dyar and Culex spp. have been found naturally infected in the southern coast of Mar Chiquita Lagoon, in the Province of Córdoba.4 In turn, Micieli et al.26 demonstrated the intrinsic capacity of Cx. quinquefasciatus and Cx. pipiens f. molestus collected in the Province of Buenos Aires to transmit WNV strains (NY99 and WN02).
Both viruses circulate enzootically in the bird community of northern Argentina; however, epidemiological information is lacking in the Province of La Rioja. The objective of this study was to determine the circulation of SLEV and WNV in wild birds of a semi-arid ecosystem in the mentioned province, by means of a serological survey. These findings will contribute to detail the distribution of WNV and SLEV and the association with possible avian hosts involved in viral maintenance.
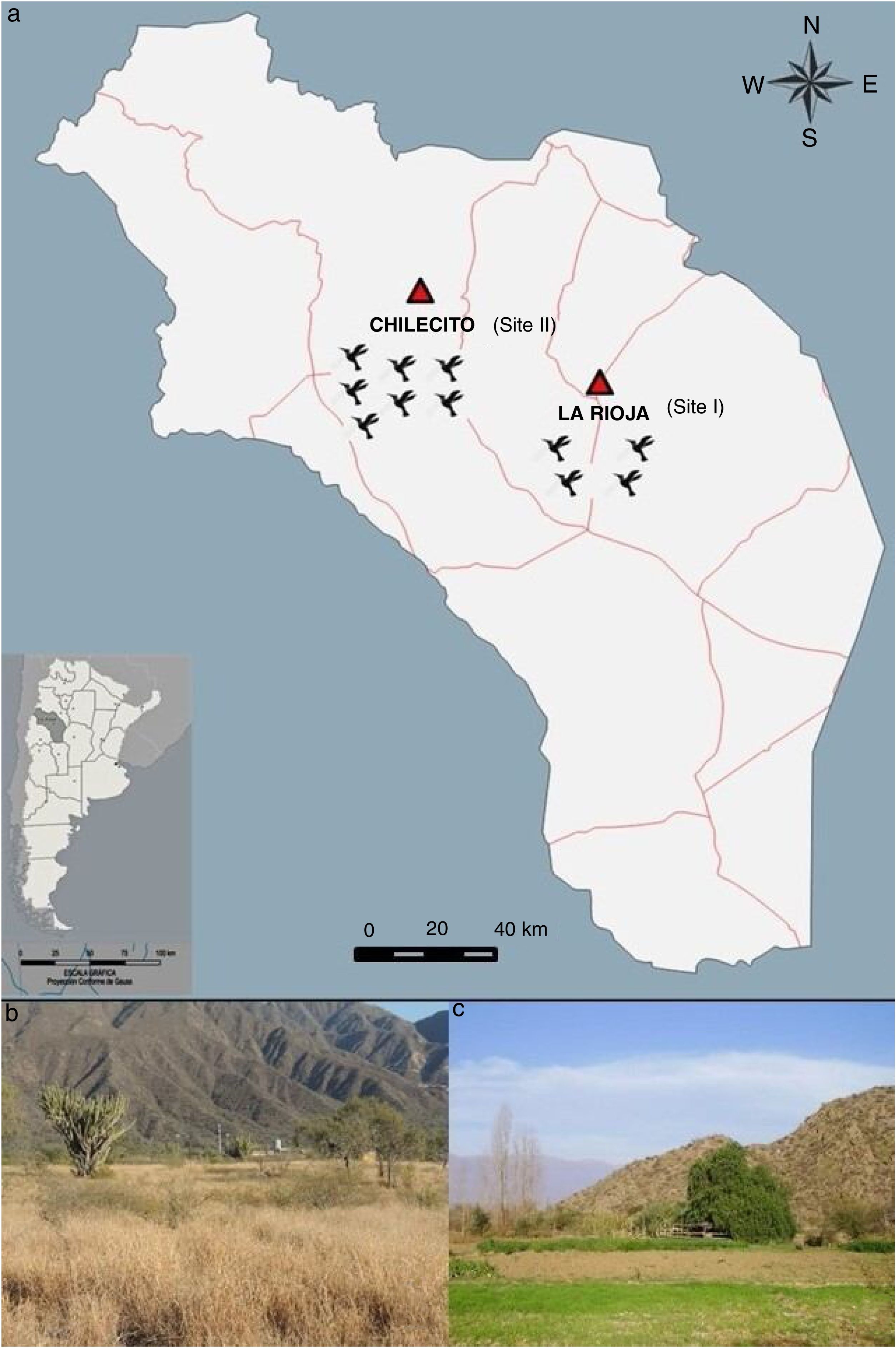
Materials and methodsStudy area and collection sitesTwo suburban sites in the Province of La Rioja, which belong to the ecoregion called Monte de Sierras yBolsones6, were selected for the bird captures (Fig. 1a). The first sampling site was located west of La Rioja city (site I: La Rioja; 29°26′22.55″S; 66°54′29.05″W; 620m.a.s.l.) (Fig. 1b). The climate is subtropical dry to semi-desertic, with annual average precipitation between 80 and 300mm concentrated mainly in the warm season (October to March), while the annual average temperature is 26°C and 12°C for the warm and cold seasons, respectively, with a daily thermal amplitude of up to 10°C7. The second sampling site was located in the outskirts of the city of Chilecito (site II: Chilecito; 29°11′15.12″S; 67°28′45.06″W; 1028m.a.s.l.) (Fig. 1c). The mean annual temperature is 24.5°C and 9.3°C in the warm and cold seasons, respectively, while rainfall ranges from 200 to 300mm annualy1,7.
Bird capture and sample collectionBirds were captured in spring (2013) and fall (2014). With the aim to obtain serum samples, eight mist nets of 12m length and 36mm weft were used34. Mist nets remained active for four days, from dawn until 10:00h and from 17:00 until sunset to reduce heat stress in the captured birds. Birds were identified at species level, sexed, weighed and aged when possible. Moreover, an alphanumeric aluminum band was placed in the tarsus to identify the individuals in the case of recapture events. Subsequently, through jugular (most species) or brachial venipuncture (columbids), 100μl of blood was withdrawn in birds with a body weight of less than 20g and 200μl in birds over 200g, preventing blood volume compromise37. Next, birds were rehydrated with a sugar solution and then released, while blood samples were placed in tubes with phosphate saline solution in order to reach 1:5 solution, and then transported refrigerated to the laboratory within 48h. The capture, manipulation, blood collection and transport of samples were approved by Fauna Silvestre and the Secretaría de Ambiente of the Province of La Rioja (Resol. S.A. 0215/13). Bird captures and handling complied with the ARRIVE guidelines and were carried out in accordance with the U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines, EU Directive 2010/63/EU for animal experiments.
Serological assaysDetection of neutralizing antibodies was performed through the plaque-forming unit (PFU) neutralization test in the Vero Cl76 cell line (ATCC CRL-587)19. The screening test serum samples were incubated with 100 PFUs of each virus: CbaAr-4005 of SLEV11 and the E/7229/06 of WNV30, the cultured sample achieving a 1:10 dilution. Sera that neutralized at least 80% of the 100 PFUs included in the test were considered positive. The positive control corresponded to a known serum with neutralizing antibodies for each virus, and the negative control corresponded to a serum without neutralizing antibodies. To determine the neutralizing antibody titer of the sera positive to screening, serial dilutions factor 2 from 1:10 were performed. Titers were assigned as reciprocals of the maximum dilution in which >80% of the PFUs were neutralized. Heterotypic responses with titers higher than 20 were considered positive for both viruses16.
Statistical analysisExposure to SLEV and WNV in wild birds was studied by detecting neutralizing antibodies, and viral circulation was estimated through the apparent seroprevalence of neutralizing antibodies. The seroprevalences and 95% confidence interval were calculated with the binom library18 using the Pearson–Kloper method in R32. Data were grouped by avian species, sites and seasons (spring and fall). Statistical differences (α=0.05) and magnitude (Odds Ratio, OR) in the frequency of exposure to viruses due to the joint and additive effect on the site-season were analyzed by means of generalized linear models (GLM) with binomial distribution.
Results and discussionA total of 326 birds belonging to 41 species were captured in the study sites during the sampling period. At site I-La Rioja the species most frequently caught were: C. picui (Picui Ground-Dove), Saltatricula multicolor (Many-colored Chaco Finch) and Zonotrichia capensis (Rufous-collared Sparrow), while in site II-Chilecito, the best represented species were C. picui (Picui Ground-Dove), Leptotila verreauxi (White-tipped Dove), Poospiza melanoleuca (Black-capped Warbling Finch), Pipraeidea bonariensis (Blue-and-yellow Tanager) and Z. capensis (Rufous-collared Sparrow).
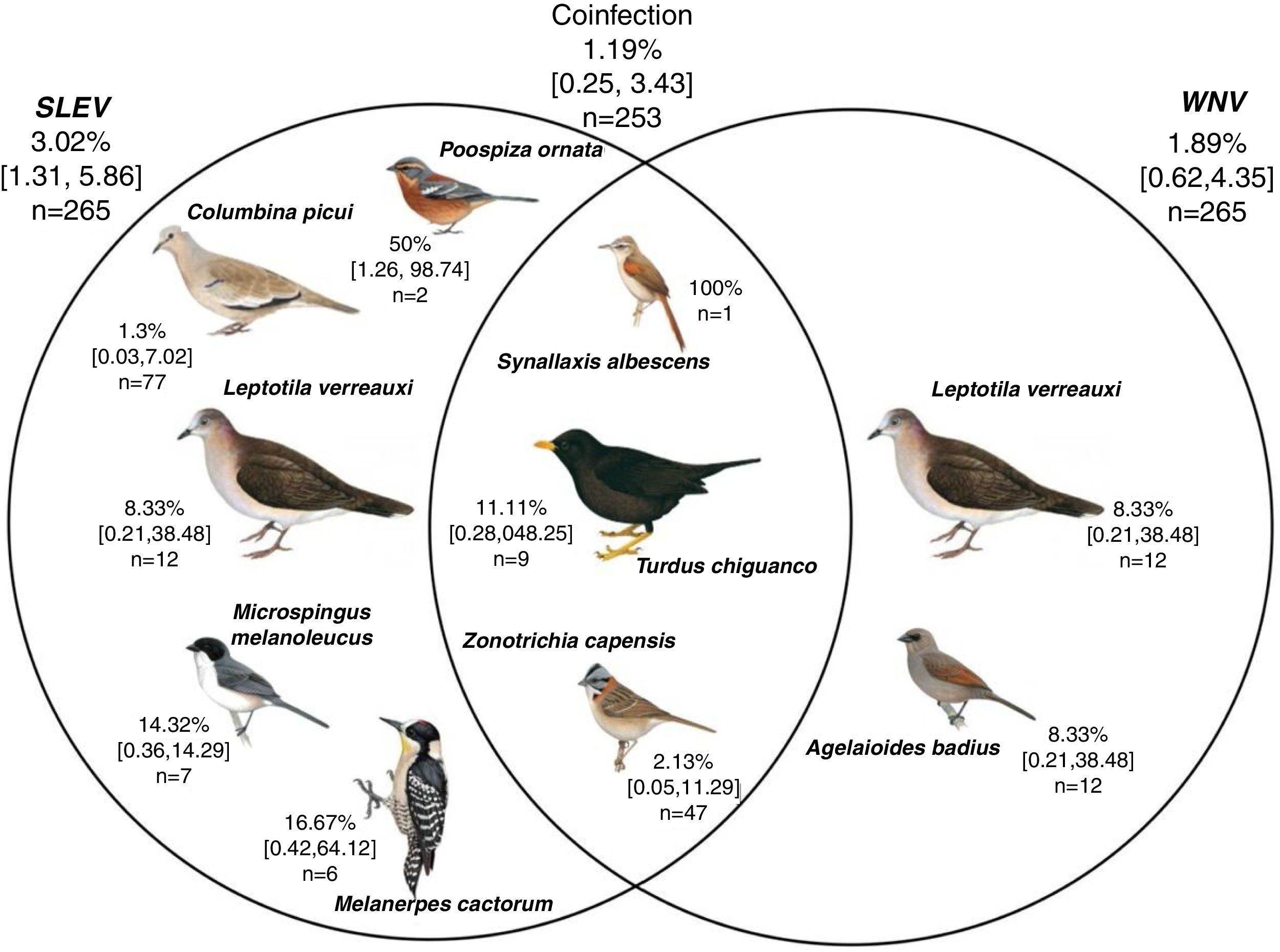
Of the total number of birds captured in this study, only 265 (81.28%; 39 species) could be analyzed to detect neutralizing antibodies. The avian community exposition to viruses was 3.02% (8/265; IC95% [1.31–5.86]) for the SLEV, 1.89% (5/265; IC95%[0.62–4.35]) for the WNV, and 1.19% belonged to coinfections (3/253; IC95% [0.25–3.43]) (Fig. 2). This study observed a lower prevalence of exposed birds compared to other regions in Argentina4,20,31 (Table 1).
Exposure of the avian community to the viruses St. Louis encephalitis and West Nile in the Province of La Rioja, Argentina. Numbers represent infection prevalence (%), confidence intervals [], and number of sampled individuals (n). Analyzed birds (n: La Rioja – n: Chilecito): Leptotila verreauxi (1–11), Columbina picui (59–20), Colaptes melanochloros (0–1), Melanerpes cactarum (1–5), Rhinocrypta lanceolata (0–1), Pseudoseisura lophotes (2–3), Furnarius rufus (0–3), Leptasthenura fuliginiceps (0–1), L. platensis (0–1), Coryphistera alaudina (1–0), Cranioleuca pyrrhophia (0,2), Synallaxis albescens (2–0), S. frontalis (2–0), Tarphonomus certhioides (0–3), Asthenes baeri (1–0), A. dorbignyi (0–1), Phytotoma rutila (0–5), Troglodytes aedon (1–3), Elaenia sp. (1–0), Knipolegus aterrimus (1–0), Stigmatura budytoides (2–2), Serpophaga subscristata (0–1), Geothlypis aequinoctialis (1–0), Turdus amaurochalinus (0–5), T. chiguanco (2–7), Saltator multicolor (11–0), S. aurantiirostris (0–4), Coryphospingus cucullatus (1–0), Catamenias analis (1–1), Lophospingus pusillus (5,0), Piranga flava (0,3), Pipraeidea bonariensis (1–16), Poospiza ornata (2–0), Microspingus melanoleuca (1–7), M. pectoralis (1–0), Sporophila caerulescens (2–0), Zonotrichia capensis (21–30), Icterus pyrrhopterus (0,1), Agelaioides badius (9–3). *The birds’ scientific name follows Remsen et al.35 recommendations, and the images belong to del Hoyo et al.10
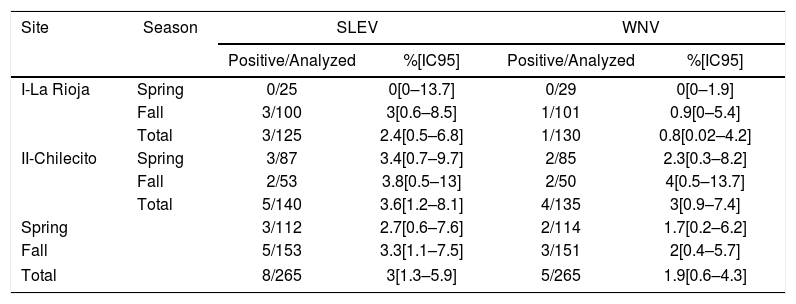
Seroprevalence for the virus St. Louis encephalitis (SLEV) and West Nile (WNV) in wild birds captured during the period 2013–2014 in the Province of La Rioja, Argentina.
| Site | Season | SLEV | WNV | ||
|---|---|---|---|---|---|
| Positive/Analyzed | %[IC95] | Positive/Analyzed | %[IC95] | ||
| I-La Rioja | Spring | 0/25 | 0[0–13.7] | 0/29 | 0[0–1.9] |
| Fall | 3/100 | 3[0.6–8.5] | 1/101 | 0.9[0–5.4] | |
| Total | 3/125 | 2.4[0.5–6.8] | 1/130 | 0.8[0.02–4.2] | |
| II-Chilecito | Spring | 3/87 | 3.4[0.7–9.7] | 2/85 | 2.3[0.3–8.2] |
| Fall | 2/53 | 3.8[0.5–13] | 2/50 | 4[0.5–13.7] | |
| Total | 5/140 | 3.6[1.2–8.1] | 4/135 | 3[0.9–7.4] | |
| Spring | 3/112 | 2.7[0.6–7.6] | 2/114 | 1.7[0.2–6.2] | |
| Fall | 5/153 | 3.3[1.1–7.5] | 3/151 | 2[0.4–5.7] | |
| Total | 8/265 | 3[1.3–5.9] | 5/265 | 1.9[0.6–4.3] | |
SLEV: Site*Season (LRT X2=1.05, p=0.30); Site (LRT X2=1.54 p=0.21); Season (LRT X2=0.01 p=0.92).
WNV: Site*Season (LRT X2=0.29, p=0.59); Site (LRT X2=0.01, p=0.91); Season (LRT X2=0.5, p=0.48).
Avian species C. picui, Poospiza ornata, L. verreauxi, Microspingus melanoleucus and Melanerpes cactorum were found to be seropositive for SLEV (Fig. 2). Similar results were obtained in other studies carried out in different regions of Argentina, where neutralizing antibodies for SLEV were detected in C.picui4,16,20,31. Furthermore, two avian species L. verreauxi and Agelaiodes badius were registered as seropositive for WNV in our study. The latter has been detected in nature infected with WNV in other studies4,13,20. Finally, three species: Synallaxis albescens, Turdus chiguanco and Z. capensis, were registered as coinfected with both viruses. The Rufous-collared Sparrow had been detected as coinfected in previous studies carried out in the country4,20.
This study is the first to register neutralizing antibodies for flavivirus in L. verreauxi (White-tipped Dove) (WNV) and Melanerpes cactorum (White-fronted Woodpecker) (SLEV) species. In spite of the results obtained by Diaz et al.13, Berrón4 and Flores20, no neutralizing antibodies for WNV were detected in C. picui. The results obtained regarding A. badius are important since it is considered that this species could maintain WNV in nature. In experimental inoculations it developed a high and long-lasting viremia13. Further studies would be necessary to determine the role of this species in the maintenance of WNV in the Province of La Rioja.
Spatial and seasonal stability was noted in the viral exposure. No additive effect was observed in the chances of exposure to SLEV (LRT X2=1.05, p=0.30); WNV (LRT X2=0.29, p=0.59), nor in coinfections (LRT X2=1.80, p=0.18) with regard to the sites and seasons analyzed. Neither was there a variation in the seasonal tendency for each site observed (Table 1). However, differences among sites and seasons could occur even when the magnitude of viral circulation is low, the amount of sampled birds being a limitation to evidence differences in the exposure.
Certain seasonality regarding maximum viral circulation and wild bird exposure in fall could be expected. Yet, the accumulative effect of birds exposed in previous seasons could hide the seasonality of the exposure, since birds exposed in fall could be detected in spring. The low neutralizing antibody titers in SLEV and WNV (1:20–1:160, data not shown) could suggest that the exposures occurred in the same season. It is worth mentioning that the highest proportion of seropositive birds were adults of resident species (7/8). Some of them demonstrated to be competent in laboratory, suggesting that the virus is not recently established in the region13,17, therefore, this evidence would confirm the enzootic maintenance of SLEV and WNV in the Province of La Rioja. The role of migratory birds in WNV and SLEV maintenance would be less relevant4,31. Even though Poospiza ornata moves east in winter9, the introduction of these viruses would not be expected since mosquito activity ceases during winter and transmission stops23.
Recent entomological studies indicate the occurrence of mosquitoes associated with arbovirus transmission in the region, Culex quinquefasciatus and Aedes aegypti being the most abundant species41. The low prevalence of viral exposure that we found in this work could be related to the limiting effect of temperature and rainfall in arbovirus transmission. Thus, variations in temperature and rainfall determine changes in the abundance, feeding behavior, longevity, length of the gonotrophic cycle and the extrinsic incubation period of the virus in the mosquito, and finally affecting vectorial capacity23,24. Besides, the composition of the mosquito community is affected by low precipitations in semiarid ecosystems. For example, the most abundant mosquito species in the study area breeds in artificial containers that people use mainly to store water41. For that it is important to consider the environmental modifications caused by human activities which can alter the dynamics and composition of the associated biological communities, and thus allowing species to develop in areas where previous conditions were not favorable for them. The effect of urban ecosystems over mosquito communities is of epidemiological interest since those environments offer a wide variety of breeding sites for mosquitoes, provide refuge and adequate microclimate conditions to survive the unfavorable season, and could favor or limit host availability as a blood source for adult females25, which could be of particular interest in arid and semiarid regions. However, there is a lack of information regarding the intrinsic potential of mosquito populations for the SLEV and WNV transmission in the Province of La Rioja. All of the above evidences a scenario of arboviral transmission which would suggest the need to extend and intensify entomological surveillance, and reinforce arbovirus control programs in the region.
Our study confirms, for the very first time, the circulation of SLEV and WNV in wild birds of the Province of La Rioja, suggesting that requirements and conditions for amplification and enzootic maintenance of SLEV and WNV would be present in semiarid ecosystems from northwestern Argentina. In addition to mosquito detection41, the above information encourages the study of the viral circulation dynamics in host and vector communities, including phylogeographic analysis of circulating strains, in order to understand the mechanisms of SLEV and WNV maintenance in semiarid ecosystems.
Funding sourcesThis work was carried out with the financial assistance from CICyT – Universidad Nacional de La Rioja (Resol. CICyT No. 094/2011).
Conflict of interestThe authors declare that they have no conflicts of interest
The authors thanks to SECyT (UNLaR) for financial support. Thanks to Hernán Brizuela for permission and support to carry on sampling activities in the Camping Legislativo, in the city of La Rioja. Thanks to Cynthia Tompkins, PhD, Arizona State University, USA, for manuscript revision in English.




![Exposure of the avian community to the viruses St. Louis encephalitis and West Nile in the Province of La Rioja, Argentina. Numbers represent infection prevalence (%), confidence intervals [], and number of sampled individuals (n). Analyzed birds (n: La Rioja – n: Chilecito): Leptotila verreauxi (1–11), Columbina picui (59–20), Colaptes melanochloros (0–1), Melanerpes cactarum (1–5), Rhinocrypta lanceolata (0–1), Pseudoseisura lophotes (2–3), Furnarius rufus (0–3), Leptasthenura fuliginiceps (0–1), L. platensis (0–1), Coryphistera alaudina (1–0), Cranioleuca pyrrhophia (0,2), Synallaxis albescens (2–0), S. frontalis (2–0), Tarphonomus certhioides (0–3), Asthenes baeri (1–0), A. dorbignyi (0–1), Phytotoma rutila (0–5), Troglodytes aedon (1–3), Elaenia sp. (1–0), Knipolegus aterrimus (1–0), Stigmatura budytoides (2–2), Serpophaga subscristata (0–1), Geothlypis aequinoctialis (1–0), Turdus amaurochalinus (0–5), T. chiguanco (2–7), Saltator multicolor (11–0), S. aurantiirostris (0–4), Coryphospingus cucullatus (1–0), Catamenias analis (1–1), Lophospingus pusillus (5,0), Piranga flava (0,3), Pipraeidea bonariensis (1–16), Poospiza ornata (2–0), Microspingus melanoleuca (1–7), M. pectoralis (1–0), Sporophila caerulescens (2–0), Zonotrichia capensis (21–30), Icterus pyrrhopterus (0,1), Agelaioides badius (9–3). *The birds’ scientific name follows Remsen et al.35 recommendations, and the images belong to del Hoyo et al.10 Exposure of the avian community to the viruses St. Louis encephalitis and West Nile in the Province of La Rioja, Argentina. Numbers represent infection prevalence (%), confidence intervals [], and number of sampled individuals (n). Analyzed birds (n: La Rioja – n: Chilecito): Leptotila verreauxi (1–11), Columbina picui (59–20), Colaptes melanochloros (0–1), Melanerpes cactarum (1–5), Rhinocrypta lanceolata (0–1), Pseudoseisura lophotes (2–3), Furnarius rufus (0–3), Leptasthenura fuliginiceps (0–1), L. platensis (0–1), Coryphistera alaudina (1–0), Cranioleuca pyrrhophia (0,2), Synallaxis albescens (2–0), S. frontalis (2–0), Tarphonomus certhioides (0–3), Asthenes baeri (1–0), A. dorbignyi (0–1), Phytotoma rutila (0–5), Troglodytes aedon (1–3), Elaenia sp. (1–0), Knipolegus aterrimus (1–0), Stigmatura budytoides (2–2), Serpophaga subscristata (0–1), Geothlypis aequinoctialis (1–0), Turdus amaurochalinus (0–5), T. chiguanco (2–7), Saltator multicolor (11–0), S. aurantiirostris (0–4), Coryphospingus cucullatus (1–0), Catamenias analis (1–1), Lophospingus pusillus (5,0), Piranga flava (0,3), Pipraeidea bonariensis (1–16), Poospiza ornata (2–0), Microspingus melanoleuca (1–7), M. pectoralis (1–0), Sporophila caerulescens (2–0), Zonotrichia capensis (21–30), Icterus pyrrhopterus (0,1), Agelaioides badius (9–3). *The birds’ scientific name follows Remsen et al.35 recommendations, and the images belong to del Hoyo et al.10](https://static.elsevier.es/multimedia/03257541/0000005300000002/v1_202106160540/S0325754120300857/v1_202106160540/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)

