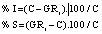The arsenic content of various water bodies in Argentina is higher than the acceptable levels for human and animal uses. Cyanobacteria are widely distributed in aquatic environments and can bioaccumulate arsenic (As). This study presents the response of indigenous cyanobacteria to As(III) and As(V), including the species Tolypothrix tenuis, Nostoc muscorum and Nostoc minutum, previously used with biotechnological purposes. As(III) resulted more toxic than As(V) in all cases, causing cell death in the range of 5–20mg/l. T. tenuis growth was sensitive to As(V) with lethal inhibition at 625mg/l, whereas the Noctoc species were stimulated. EC50 values found were 73.34mg/l for N. muscorum and 989.3mg/l for N. minutum. Batch cultures of N. minutum showed improvements in both growth parameters and photosynthetic pigment content in the presence of 1,000mg/l As(V). Increases of 66.7%, 75.5%, 40% and 20.7% in cell productivity, chlorophyll a, total carotenoids and C-phycocyanin respectively were observed, reaching a bioaccumulated arsenic value of 37.4μg/g at the stationary growth phase.
El contenido de arsénico de diversos cuerpos de agua de Argentina es superior a los niveles aceptados para consumo humano y animal. Las cianobacterias están ampliamente distribuidas en los ambientes acuáticos y pueden bioacumular As. Este estudio presenta la respuesta de cianobacterias autóctonas a As(III) y As(V), incluyendo las especies Tolypothrix tenuis, Nostoc muscorum y Nostoc minutum utilizadas previamente con fines biotecnológicos. As(III) resultó más tóxico que As(V) en todos los casos, causando muerte celular en el rango de 5–20mg/l. El crecimiento de T. tenuis fue sensible a As(V) con inhibición letal a 625mg/l. Sin embargo, las especies de Noctoc resultaron estimuladas. Los valores de EC50 encontrados fueron de 73,34mg/l para N. muscorum y 989,3mg/l para N. minutum. Los cultivos batch de N. minutum mostraron mejoras en los parámetros de crecimiento y en el contenido de pigmentos fotosintéticos en presencia de 1000mg/l As(V). Los incrementos observados en productividad celular, contenido de clorofila a, carotenoides totales y C-ficocianina fueron de 66,7 %; 75,5 %; 40 % y 20,7 % respectivamente, alcanzando un valor de arsénico bioacumulado de 37,4μg/g en la fase estacionaria.
Arsenic is a well known toxic element naturally present in the Earth crust, coming from volcanic rocks or from anthropogenic sources such as industries, agriculture, acid mine drainage and domestic wastes16,25. Its toxicity depends on the total concentration of the chemical species, with inorganic compounds being more toxic5.
Over the last few decades, numerous studies have described different responses in microorganisms exposed to this metalloid, including transport system induction27, expression levels of resistance genes8,11, phytochelatin production18 and variation of several biochemical parameters2,15.
The arsenic function as a constituent element of living organisms has recently been discussed by Wolfe-Simon et al.28, who described that arsenic can substitute phosphorus in macromolecules of eubacteria of the family Halomonadaceae.
Cyanobacteria can uptake and concentrate arsenic from the surrounding environment and arsenic-resistant genera such as Synechocystis, Oscillatoria, Anabaena and Phormidium have been reported as dominant taxa in contaminated areas of different world regions1,2,14,21,31.
Groundwater in many regions of Argentina contains As concentrations higher than the accepted levels for human and animal uses7. Regional strains of cyanobacteria of the genera Nostoc and Tolypothrix have been used mainly for biotechnological purposes as a source of phycobiliproteins, Cd biosorbents and rice-biofertilizers6,22,23. A non-viable biomass of Nostoc minutum was able to eliminate Cd by biosorption in alkali-pretreated cells and the immobilized biomass in alginate beds exhibited a higher level of Cd removal in comparison with that of free cells26.
The mass cultivation of T. tenuis, N. muscorum and N. minutum associated with their positive biosorption of As may contribute to increase the control and management of arsenic-contaminated waters. The aim of this work was to study the arsenic sensitivity of these strains under laboratory conditions, including tolerance screening, dose-response curves, growth measurement, variations in biochemical parameters and prospective As accumulation.
Materials and methodsMicroorganisms and culture conditionsThe cyanobacterial strains used were T. tenuis and N. muscorum, kindly provided by Rizobacter Argentina S.A. and N. minutum, a local strain isolated from brackish waters of the Province of San Luis, Argentina, identified by morphological and cultural characteristics6.
Cyanobacterial stock cultures were maintained in Watanabe medium23 at 30°C in a temperature-controlled room. Continuous illumination was provided by a set of nine fluorescent lamps with a light intensity of 26W/m2.
Toxicity testToxicity tests were conducted in cyanobacterial cultures exposed to different arsenic concentrations, using sodium arsenite (NaAsO2) and sodium arsenate (Na2HAsO4.7H2O) as As(III) and As(V) solutions. Two-fold dilutions from an initial concentration of 40mg/l of As(III) and 20,000mg/l of As(V) contained in one ml were added to an equal volume of Watanabe medium (2X) followed by inoculation with 50μl of cyanobacterial suspensions. The cultures were incubated during 7 days under the above mentioned conditions and growth estimation was performed by visual comparison of the blue-green apparent color (AC) of culture suspensions with and without added As. Four different apparent color standards were prepared, culture suspension with no As (1), centrifuged standard 1 resuspended in three-quarter volume (2), in half volume (3), and three-quarter dilution of standard 1 (4). The growth assessment score was: +: AC similar to standard 1, ++: AC similar to standard 2, +++: AC similar to standard 3 ±: AC similar to standard 4 and −: yellowish apparent color (cellular death). Visual comparison methods describing the apparent color are used for analyzing the presence of algae and other suspended matter in water24. Results are representative of three independent experiments performed in duplicate.
Dose-response assaysSamples of wet biomass (62±6mg) obtained from an active culture of two weeks and harvested by filtration with a sterile Buchner filter were placed into Petri dishes containing 7.5ml of Watanabe medium (2X) plus 7.5ml of different As(V) concentrations. The range of arsenic concentration used was related to the sensitivity of each cyanobacterium: T. tenuis (0.01–1,000mg/l), N. muscorum (0.10–5,000mg/l) and N. minutum (5–10,000mg/l). The Petri dishes were incubated for 14 days at 30°C under constant illumination of 26W/m2. Cell growth was estimated by dry weight determinations using the whole content of each dish, centrifuged at 3,180g for 20min, washed twice with distilled water and dried at 96°C until constant weight.
The biological response relative to the control (growth with no As) was calculated with the following equations17:
Where,
% I: Percentage growth inhibition relative to control.
% S: Percentage growth stimulation relative to control.
C: Control
GRi : Growth response [i=0.01, 0.05,….n mg/l As(V)]
The dose-response data included: EC50 [metal concentration at which cell growth was modified by 50% with 95% confidence interval (CI) and correlation coefficient (R2)], NOEC (metal concentration at which no effect is observed) and Emax (the maximal obtainable effect). The dose-response data were analyzed using the model of sigmoidal dose-response relationship of GraphPad Prism for Windows (version 5.01, GraphPad Software, San Diego, California, USA).
Kinetic studiesBased on the quantitative dose-response assays and the growth stimulation by As(V) observed for N. minutum, further kinetic studies were performed only with this strain.
N. minutum cultures were obtained in 300ml glass columns, 37mm i.d., containing 200ml of Watanabe medium with or without the addition of 1,000mg/l As(V) and air bubbled through a diffuser to improve gas mass transfer rates. Glass columns were inoculated with 175mg of wet biomass (equivalent to 5.25mg dry weight) coming from 2 week-old cultures and incubated at 30°C with continuous illumination of 26W/m2. Cyanobacterial growth (x) was estimated by optical density at 580nm using the following correlation with dry weight determinations: ×control (g/l)= 0.351 ODcontrol + 0.0724 (R2=0.987) and × +As(V) (g/l)= 0.410 OD+As(V) + 0.0604 (R2=0.998). Morphological changes in trichomes, hormogonia, and heterocysts were evaluated by light microscopy counting 1000 cells at the end of each experiment. Cell productivity, chlorophyll a, total carotenoids12, C-phycocyanin and allophycocyanin3 were determinated after cultures reached the stationary phase. Residual and bioaccumulated arsenic in cyanobacterial biomass were detected by inductively coupled plasma atomic emission spectroscopy (ICP-AES). Prior to ICP-AES analysis, the biomass was harvested and rinsed twice with deionized water. Results are representative of at least two separate experiments in duplicate.
Data analysisThe statistical analysis of N. minutum growth stimulation by As(V) was based on variations in kinetic parameters and pigment levels. All the data are presented as mean ± standard deviation. Statistical analysis was carried out by the Student's t-test using GraphPad Prism for Windows to compare the differences among the treatment and control batch cultures. Significance was indicated at 0.05 levels.
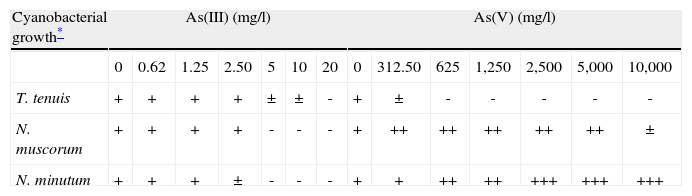
Results and discussionThe screening sensitivity of the cyanobacterial strains assayed to As showed that As(III) was more toxic than As(V) (Table 1). Although As(III) exerted the strongest toxic effect causing cell death in the range of 5–20mg/l, differences between the strains were found, the Nostoc species were markedly sensitive, whereas T. tenuis was able to withstand a concentration of 10mg/l before total inhibition. Conversely, the response to As(V) showed that T. tenuis was the most sensitive microorganism with total growth inhibition at 625mg/l, while a notorious growth stimulation was observed with the Nostoc strains. N. minutum grew in the whole range of As concentration and N. muscorum showed a similar behavior up to 5,000mg/l, with partial inhibition, cell aggregation and changes in pigmentation at 10,000mg/l. The arsenic toxicity to freshwater microalgae is dependent on the chemical species of As. As(V) was more toxic than As(III) to freshwater green algae4,10; however, the opposite was found for Chlorella vulgaris13. On the other hand, a comprehensive study made with cyanobacteria present in a soil of India showed different tolerance limits to As(V); from six genera of cyanobacterial species, only Phormidium and Nostoc were able to survive at 10,000mg/l, a concentration at which the other genera were dead21.
Cyanobacterial growth tolerance to arsenic solutions using NaAsO2 and Na2HAsO4.7H2O as As(III) and As(V) and incubated at 30°C with constant illumination of 26W/m2 during 7 days.
| Cyanobacterial growth* | As(III) (mg/l) | As(V) (mg/l) | ||||||||||||
| 0 | 0.62 | 1.25 | 2.50 | 5 | 10 | 20 | 0 | 312.50 | 625 | 1,250 | 2,500 | 5,000 | 10,000 | |
| T. tenuis | + | + | + | + | ± | ± | - | + | ± | - | - | - | - | - |
| N. muscorum | + | + | + | + | - | - | - | + | ++ | ++ | ++ | ++ | ++ | ± |
| N. minutum | + | + | + | ± | - | - | - | + | + | ++ | ++ | +++ | +++ | +++ |
Growth was estimated by visual comparison of the blue-green apparent color (AC) with the following standards: 1. culture suspension with no As; 2. centrifuged standard 1 resuspended in three-quarter volume; 3. in half volumen and 4. threequarter dilution of standard 1. The assessment score was: +: AC similar to standard 1, ++: AC similar to standard 2, +++: AC similar to standard 3, ±: AC similar to standard 4 and - : yellowish apparent color (cellular death).
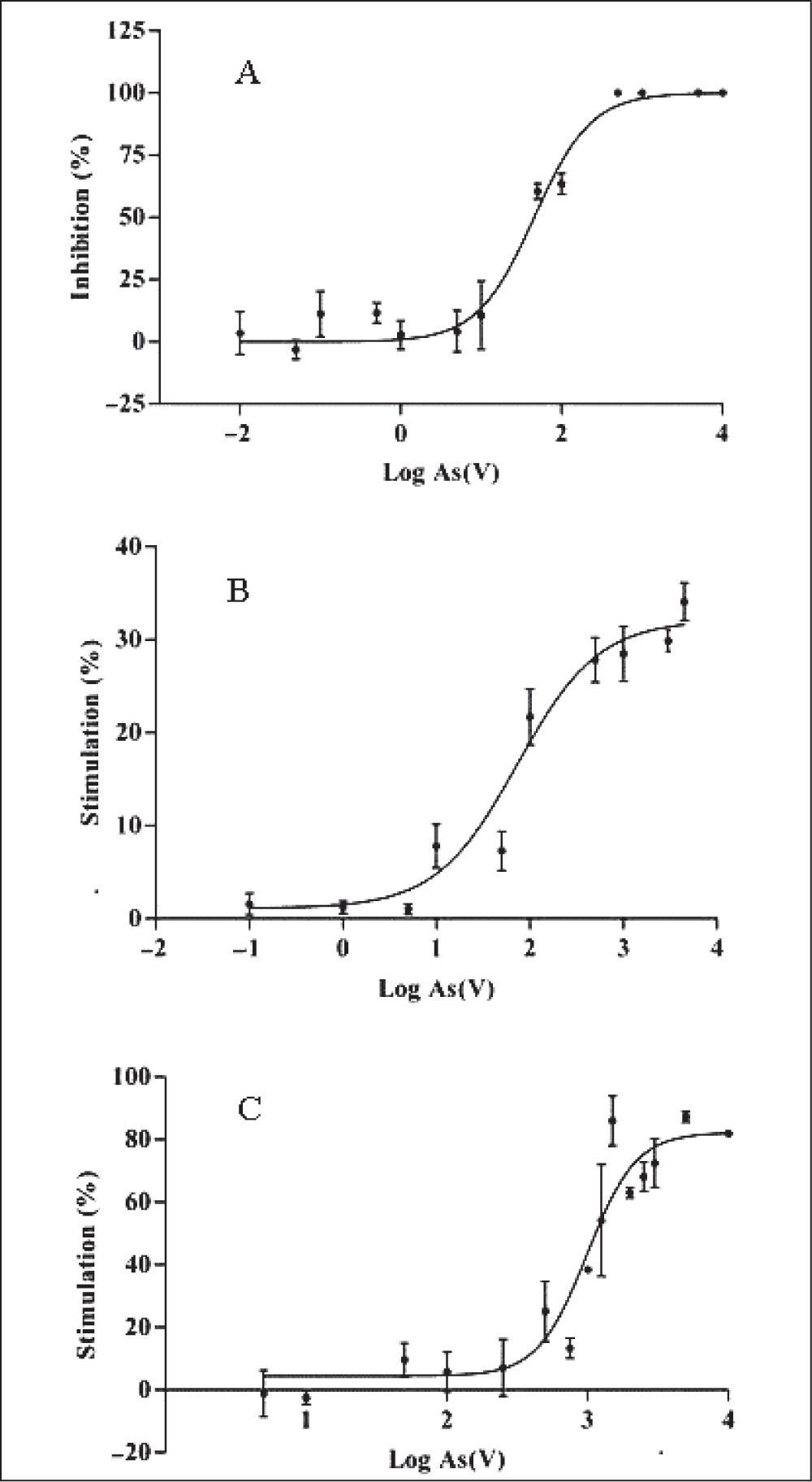
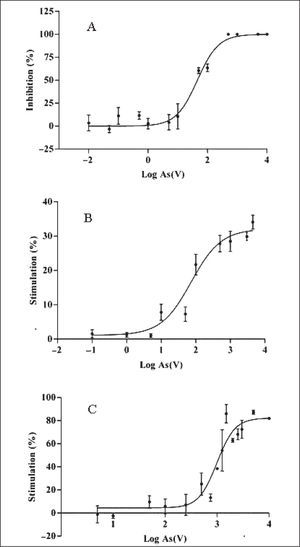
The different responses to As(V) displayed by the strains in the initial screening were further analyzed by quantitative dose-response assays in terms of EC50, NOEC and Emax. Although studies on uptake, transformation and volatilization of arsenic by cyanobacteria have been performed9,19,30, scarce reports describe the arsenic toxicity on Nostoc species on quantitative grounds14,21, and to the authors’ knowledge no reports exist for T. tenuis. T. tenuis was the only sensitive species to As(V) that caused growth inhibition [NOEC: 0.19mg/l, EC50: 51.31mg/l (95% CI: 35.86 to 73.43mg/l, R2: 0.96)] with cellular death at 500mg/l (Fig. 1A).
N. muscorum and N. minutum showed a sigmoidal relationship of growth stimulation by As(V) with the following EC50 values: 73.07mg/l (95% CI: 40.39–132.20mg/l, R2: 0.91) and 989.30mg/l (95% CI: 739.80–1323mg/l, R2: 0.85), respectively. The growth of N. minutum stimulated in the presence of As(V) presented a higher EC50 value than that reported for tolerant microorganisms28. The N. minutum NOEC (70.59mg/l) was an order of magnitude greater than that of N. muscorum (3.13mg/l). The stimulatory Emax was 31.95% at 1,600mg/l for N. muscorum and 82.28% at 3,100mg/l for N. minutum, (Figs. 1B and 1C).
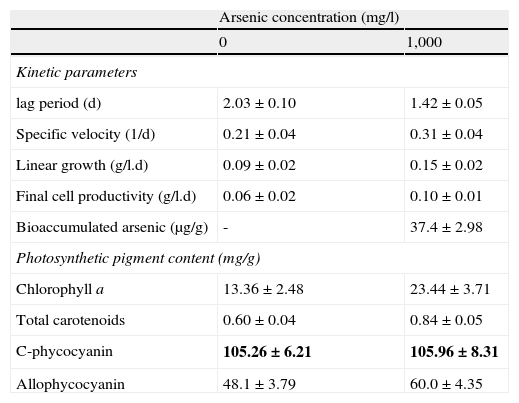
A more comprehensive analysis of the stimulatory effect of As(V) on N. minutum was based on the evaluation of growth kinetic and photosynthetic pigment content of batch cultures supplemented with 1,000mg/l of As(V) (Table 2). The growth under nitrogen and carbon fixation conditions in the presence of As(V) showed improvements in kinetic parameters and cell productivity (Table 2). The lag period was shortened, with higher specific growth rate and linear growth with respect to cultures developed with no As(V). The increase in cell productivity was 1.7 times higher with no significant variations in the morphology of trichomes, hormogonia, and heterocysts (p>0.05), changes that have been reported for Anabaena azollae cultures exposed to arsenate20. With the exception of C-phycocyanin, the content of photosynthetic pigments, including chlorophyll a, total carotenoids and allophycocyanin increased in the presence of As(V) with values that were 75.5%, 40% and 20.7% higher, respectively. Additionally the culture of N. minutum added with As(V) produced a more compact pellet with easy separation after centrifugation. The As(V) concentration in the supernatant decreased by 17% at the end of the culture, retaining 0.62% of As in the biomass, with a bioaccumulation of 37.4μg/g. The overall improvement in growth kinetic parameters and photosynthetic pigments of As-supplemented cultures, at concentrations similar to the EC50, denote a possible unknown physiological role of As(V) on the nutritional state of the cultures.
Growth kinetic and photosynthetic pigments of N. minutum in the presence of arsenic. N. minutum cultures were obtained in Watanabe medium with or without the addition of 1,000mg/l As(V) using glass columns and incubated at 30°C with continuous illumination of 26W/m2.
| Arsenic concentration (mg/l) | ||
| 0 | 1,000 | |
| Kinetic parameters | ||
| lag period (d) | 2.03±0.10 | 1.42±0.05 |
| Specific velocity (1/d) | 0.21±0.04 | 0.31±0.04 |
| Linear growth (g/l.d) | 0.09±0.02 | 0.15±0.02 |
| Final cell productivity (g/l.d) | 0.06±0.02 | 0.10±0.01 |
| Bioaccumulated arsenic (μg/g) | - | 37.4±2.98 |
| Photosynthetic pigment content (mg/g) | ||
| Chlorophyll a | 13.36±2.48 | 23.44±3.71 |
| Total carotenoids | 0.60±0.04 | 0.84±0.05 |
| C-phycocyanin | 105.26±6.21 | 105.96±8.31 |
| Allophycocyanin | 48.1±3.79 | 60.0±4.35 |
All data were significantly different from respective controls with the Student's t-test (p<0.05), except for numbers in bold (p>0.05).
The normal growth rate of cyanobacteria in the presence of arsenate 1M has been reported, which was attributed to a probable failure in the incorporation of arsenate in phosphate sufficient cells29. That was not the case with Nostoc minutum as it bioaccumulated a slight amount of As(V) in the biomass. Higher levels of As bioaccumulation were reported for species of the genera Nostoc and Synechocystis30,31; however, these higher values can be the result of As bioconcentration in long-term naturally exposed cyanobacterial biomass2.
The cyanobacterial biomass utilization with agronomical or phycoremediation purposes can be considered a green and sustainable technology. The capacity of certain strains of thriving in waters with high As concentrations widens its possible application in areas with chronic contamination to increase the control of polluted waters. However, the bioaccumulation of As in the biomass would impair its use in waste water treatment, with the risk of As entering the food chain.
Conflicts of interestThe authors declare that they have no conflicts of interest.
This work was supported by the Secretaría de Ciencia y Técnica, UNSL, and the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina.