The antibacterial activity of chitosan coatings prepared with acetic or lactic acid, as well as of composite chitosan–gelatin films prepared with essential oils, was evaluated in fresh shredded black radish samples inoculated with Listeria monocytogenes ATCC 19115 and L. monocytogenes ATCC 19112 during seven days of storage at 4°C. The chitosan coating prepared with acetic acid showed the most effective antibacterial activity. All tested formulations of chitosan films exhibited strong antimicrobial activity on the growth of L. monocytogenes on black radish, although a higher inhibition of pathogens was achieved at higher concentrations of chitosan. The antimicrobial effect of chitosan films was even more pronounced with the addition of essential oils. Chitosan–gelatin films with thyme essential oils showed the most effective antimicrobial activity. A reduction of 2.4log10CFU/g for L. monocytogenes ATCC 19115 and 2.1log10CFU/g for L. monocytogenes ATCC 19112 was achieved in the presence of 1% chitosan film containing 0.2% of thyme essential oil after 24h of storage.
Se evaluó la actividad antimicrobiana de coberturas del quitosano y de películas compuestas de quitosano-gelatina en muestras frescas de rábano negro cortado inoculadas con las cepas de Listeria monocytogenes ATCC 19115 y ATCC 19112, almacenadas durante 7 días a 4°C. Las primeras fueron preparadas con ácido acético o ácido láctico, las segundas con aceites esenciales. Las coberturas de quitosano preparadas con ácido acético mostraron la actividad antimicrobiana más eficaz. Todas las formulaciones de películas de quitosano exploradas mostraron una fuerte actividad antimicrobiana sobre el crecimiento de L. monocytogenes, aunque la mayor inhibición de estos patógenos se logró con las mayores concentraciones de quitosano. La actividad antimicrobiana de las películas de quitosano fue mayor con la adición de aceite esencial. Las películas de quitosano-gelatina con aceite esencial del tomillo fueron las que mostraron la actividad antimicrobiana más eficiente. A las 24h de almacenamiento, la película con 1% de quitosano y 0,2% de aceite esencial de tomillo produjo una reducción de 2,4log10UFC/g en L. monocytogenes ATCC 19115, y de 2,1log10UFC/g en L. monocytogenes ATCC 19112.
Nowadays, an increasing number of consumers realize the importance of fresh vegetables in their daily diet. Vegetables represent a significant source of vitamins, minerals and dietary fibers24. Moreover, it may be useful in the prevention of various diseases, especially when consumed fresh14,34. For these reasons, the increased consumption of vegetables has led to the development of the special sector that deals with the treatment of minimally processed vegetables, which have been trimmed, peeled, cut, and packaged for distribution and consumption. Since minimally processed vegetables normally do not contain any preservatives and have not been subjected to any heat or chemical treatments, they must be kept at low temperature or refrigerated storage. They can be found in the market in the form of sliced cabbage, carrots, lettuce or mixtures thereof.
During the growing process, when the outer surfaces of vegetables come in contact with soil, irrigation water, environment in the packing plants, surfaces of transportation trailers or hands of packing workers, they may be contaminated with pathogenic microorganisms1,6,11. Bacteria may be transferred from the external vegetable surfaces to the edible portions during cutting and dividing, thus contaminating fresh-cut products28,32. In addition, minimally processed products may be re-contaminated through cross-contamination and improper handling during their distribution31. Listeria monocytogenes is one of the microorganisms that represent a risk to public health. This bacterium possesses the capacity to develop at the low temperatures13 mainly used for storing minimally processed products and may multiply in vegetables in large quantities causing listeriosis, a severe disease in humans.
Listeriosis is a serious infection caused by eating food contaminated with the L. monocytogenes bacterium, particularly ready-to-eat foods, including dairy, meat and poultry, and some fruit and vegetable products. The disease primarily affects pregnant women, newborns, and adults with weakened immune systems. Although L. monocytogenes rarely causes foodborne disease outbreaks, it represents a major food safety concern due to the high mortality rate associated with listeriosis2. One of the largest listeriosis outbreaks ever occurred in several states of the USA in 2011, and was caused by the consumption of fresh cantaloupe7. This outbreak caused 146 illnesses in 28 states, including 30 deaths and 1 miscarriage.
Traditional food preservation techniques based on thermal treatments can provide microbiological stability of food; however, they are not appropriate for fresh-cut, minimally processed vegetables. To allow the microbiological stability of minimally processed vegetables in the market today, researchers are looking for non-thermal treatments that do not affect the physicochemical properties and nutritional value of these products.
A convenient agent that can provide microbiological stability of minimally processed vegetables extending shelf life and improving the quality of these products may be chitosan, a natural biopolymer which possesses unique biodegradability and bioactivity properties17. It is obtained by partial deacetylation of chitin, the second most widespread polysaccharide on Earth after cellulose. Several studies have reported the antimicrobial and antioxidant characteristics, as well as non-toxicity of chitosan3,16,18,36. The specific antimicrobial activity of chitosan in coating formulations has been previously investigated, providing evidence that it can be attributed to its molecular weight and its deacetylation degree9. The activity of chitosan can be related to a change in cell permeability; the interactions between the amino groups of chitosan and the electronegative charges on the cell surface lead to the leakage of intracellular electrolytes and protein constituents23.
The incorporation of different antimicrobial constituents, such as essential oils or organic acid, into the polymer matrices can notably change and/or improve antimicrobial and some physicochemical properties, such as mechanical properties, color or water vapor barriers, as has been described for composite films25,26 and for coatings in fruit applications4. Chitosan can be used in the form of edible coatings or films having a variety of advantages over synthetic materials, such as biodegradability, edibility, biocompatibility, also the quality of being environmentally friendly21. Several authors have studied the antimicrobial activity of chitosan in different food products8,29,33,35. However, there is no data referring to the antimicrobial activity of chitosan on black radish. Radish (Raphanussativus L.) belongs to the family Cruciferae and is grown for its edible root. In traditional kitchen, black radish is often used in winter salads. Grated fresh root, spiced with vinegar and oil, is a local specialty. This vegetable can be used as a stand-alone salad or in combination with other fruits and vegetables such as carrots, cabbage, apples, etc. Black radish mixed with honey is one of the most commonly used alternative folk medicines, suitable to treat coughs and bronchitis. Therefore, in a large number of handbooks and instructions in the field of phytotherapy, homeopathy and self-treatment, it is a very powerful hepatoprotective and well-known healing medicine in the treatment of liver disorders and bile ducts15.
The objectives of this work were (a) to study the growth of two strains of L. monocytogenes on shredded black radish at 4°C, (b) to evaluate the antimicrobial properties of chitosan coatings prepared with acetic or lactic acid on shredded black radish, c) to evaluate the antimicrobial properties of composite chitosan–gelatin films, with and without essential oils in this food category.
Materials and methodsBacterial cultureTwo pathogenic strains of L. monocytogenes, ATCC 19112 and L. monocytogenes ATCC 19115-serotype 4b were used in this investigation. These strains were purchased from ATCC (American Type Culture Collection, Rockville, MD). Before each experiment, stock cultures of bacteria were propagated through two consecutive, 24h-growth cycles, in Brain Heart Infusion agar (BHIA, HiMedia, Mumbai, India) at 37°C. Five well-isolated colonies of each strain were transferred into 10ml sterile saline solution (0.85% NaCl, w/v) to obtain a working culture containing approximately 1×108CFU/ml. One (1) ml of this suspension was added to each sample of vegetables (100g), providing an initial density in vegetables of 1×104CFU/g, approximately.
Sample preparationFresh black radish was purchased from local supermarkets in Šabac (Serbia). Raw, whole black radish was washed, peeled and shredded with a shredding machine (Multi Moulinette, Moulinex). Shredded vegetables were weighed into sterile containers in an amount of 100g. The bacterial suspension was added to the vegetables in the manner described above. After inoculation, bacterial suspension and vegetables were mixed well, using sterile gloves, under sterile conditions.
Preparation of chitosan coatingsChitosan of medium molecular weight (Mw 190,000–310,000Da, 75–85% deacetylation, Sigma–Aldrich, Germany) was dissolved in 1% (v/v) acetic acid solution or 1% (v/v) lactic acid solution and was stirred for 24h to ensure total solubility. The final concentration of chitosan in the solution was 0.5 and 1% (w/v). The prepared solutions of chitosan were added into the shredded vegetables.
Preparation of chitosan–gelatin filmsChitosan of medium molecular weight (Mw 190,000–310,000Da, 75–85% deacetylation, Sigma–Aldrich, Germany) was used as a matrix, into which the antimicrobial mint and thyme essential oils were incorporated. Chitosan was dissolved in 1% (v/v) aqueous solution of glacial acetic acid (Merck, Germany) and was stirred for 24h to ensure total solubility. The final concentration of chitosan in the film-forming solution was 0.5 (w/v) and 1% (w/v). A solution of white gelatin 6% (w/v) (Centrohem, Serbia) was prepared with 1% (v/v) acetic acid solution, using the following procedure: the gelatin was hydrated for 30min at room temperature, and then dissolved at 45°C (water bath) under mechanical stirring, until complete dissolution (approximately 20min). The prepared solutions of chitosan and gelatin were mixed in a ratio of 1:1 in order to obtain a homogenous mass that would become viscous upon cooling and create a composite film chitosan–gelatin. As a reference, a gelatin film contained a 6% (w/v) of gelatin was prepared. Approximately 25ml of chitosan–gelatin solution was prepared for each Petri dish, one half of which was poured into the cover, while the other half was poured onto the bottom of the Petri-dish. After spilling of the solution, a composite film was allowed to dry at room temperature under sterile conditions. Films were stored at 4°C until use. The same procedure was applied to prepare a chitosan–gelatin solution containing mint or thyme essential oils (Herbadoo, Serbia), which were added into the solution of chitosan–gelatin and mixed for 5min in order to obtain a homogenous mass. The final concentration of the tested essential oils in the coating formulations was 0.2% (w/v). To ensure emulsification of the essential oils, dimethyl sulfoxide 0.2% (w/v) (Carlo Erba Reagents, France) was added to each composite film. Gelatin films were prepared in the same manner as for the chitosan films, but without the addition of chitosan.
Application of chitosan coatingsThe coatings were applied on shredded black radish samples always using the same procedure: 10ml of chitosan solution was added to 100g of vegetables. Treatments with lactic or acetic acid (without chitosan) were used to assess the impact of the acids used for film preparation. Appropriate acid was applied in the sample using the procedure described above. Samples were thoroughly mixed in a biological safety cabinet and then inoculated with 1ml of bacterial suspension to reach a final concentration of approximately 104CFU/g. The inoculated sample of black radish without any treatment was used as a control. All samples were stored in sterile glass jars at 4°C until analysis, which was carried out every 24h for 7 days.
Application of chitosan filmsThe films were applied on fresh shredded black radish samples (40g) using the following procedure: vegetable samples were inoculated with the bacterial suspension to reach a final concentration of approximately 104CFU/g, and packed between two layers of the chitosan composite film in Petri dishes. The samples thus prepared were sealed in plastic bags and stored at 4°C until analysis, which was carried out every 24h for 7 days. A treatment with gelatin film was applied on the shredded vegetables in the same manner as for the chitosan films.
Microbial analysis of vegetable samples during refrigerated storageThe total number of tested bacteria was evaluated periodically throughout storage. Treated and untreated shredded vegetable samples (10g) were subjected to a microbial analysis during refrigerated storage at 4°C at the initial point (day 0) and after days 1–7. Under sterile conditions, 10g of sample was homogenized in 90ml sterile saline solution (0.85% NaCl, w/v) at 150rpm for 10min using a laboratory shaker (J.P. Selecta, model 3000974). Serial dilutions of each suspension were prepared. One milliliter of the appropriate dilution was used to perform a pour plate method described by Skalina and Nikolajeva30. Palcam agar (Hi Media, Mumbai, India), containing acriflavine hydrochloride (5mg/l), polymyxin B (10mg/l) and ceftazidime (20mg/l) was used as selective medium. All plates were incubated for 24h at 37°C.
Statistical analysisAll experiments were replicated three times. Results are expressed as mean values of three repetitions. Microbiological counts were converted to log10CFU/g and statistical differences were determined using Student's t-test (Microsoft Excel, 2007) after analysis of variance. Significance was set at p<0.05. Pearson's correlation coefficient was calculated in order to determine the correlation between two tested bacterial strains.
Sensory evaluationTo evaluate the sensory characteristics of shredded vegetables using chitosan films and essential oils, the method described by Faria and Yotsuyanagi12 was conducted. Five highly trained panelists from the Department of Food Technology (High Technological School of Professional Studies, Šabac, Serbia) were selected to perform the sensory analysis of the vegetables. The samples containing black radish were stored in the refrigerator at 4°C until use. The sensory quality of the vegetables was evaluated at the initial point (day 0) and after days 1–3. Vegetable samples (40g) were presented individually to the panelists in the glass Petri dishes, which were randomly stored in the plastic bags. Fresh shredded radish samples serving as the control sample were also presented to the panelists. The control has been freshly prepared for each testing day. Sensory evaluation was conducted in controlled conditions of light, temperature and humidity.
Panelists were asked to score color, odor, taste and freshness of shredded black radish using a nine-point structured scale, in which 1 is substantially worse than the control, 5 is equal to the control, and 9 is substantially better than the control. The mean and standard deviation were calculated. Analysis of variance and Student t-test were used to test the differences between the control and the treated samples of vegetables.
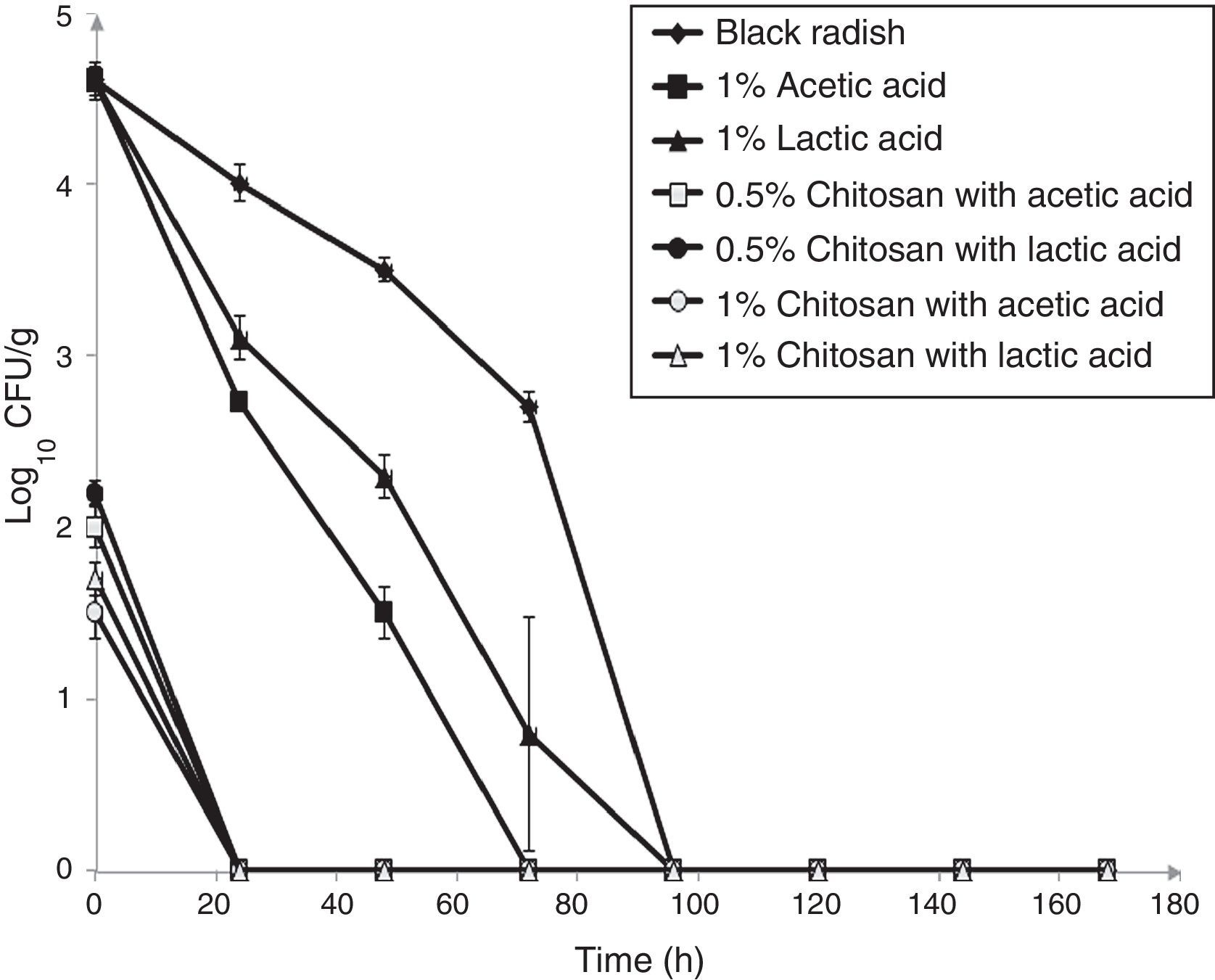
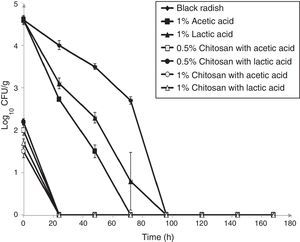
ResultsThe antimicrobial effect of two different chitosan coatings prepared with acetic or lactic acid, measured against L. monocytogenes ATCC 19115 on shredded black radish samples was evaluated during seven days of refrigerated storage at 4°C (Fig. 1). The initial number of L. monocytogenes ATCC 19115 in the control sample (black radish) decreased to an undetectable level after four days of storage. The addition of 1% acetic acid completely inhibited the population of tested bacteria on black radish after three days of storage. Based on the obtained data, acetic acid was considered to be the most effective antimicrobial agent against L. monocytogenes ATCC 19115 and was selected to be further used in the film formulations.
Effect of chitosan coatings on viable cells (CFU/g) of L. monocytogenes ATCC 19115 in shredded black radish (♦ – L. monocytogenes ATCC 19115 in shredded black radish; ▴ – 1% lactic acid; ■ – 1% acetic acid; ● – 0.5% chitosan coatings with lactic acid; □ – 0.5% chitosan coatings with acetic acid; ▵ – 1% chitosan coatings with lactic acid; ○ – 1% chitosan coatings with acetic acid). Reported populations represent the means of three values. Error bars show standard deviations.
From the experimental data it clearly emerges that black radish alone exhibited antimicrobial effect against the tested bacteria (Fig. 1). The application of the chitosan coating on black radish samples caused an immediate reduction in the L. monocytogenes population. After the addition of all tested chitosan coatings, the L. monocytogenes population on coated samples was significantly lower than in the control samples on the same day (p<0.05). In the cases of 1 and 0.5% chitosan coatings prepared with acetic acid, 3.1log10CFU/g and 2.6log10CFU/g cycle reductions were achieved immediately after chitosan addition. When 1 and 0.5% chitosan coatings were prepared with lactic acid, a decrease of 2.9log10CFU/g and 2.4log10CFU/g immediately occurred after chitosan addition, compared to control. A statistically significant difference (p<0.05) was found between the same chitosan concentrations prepared with acetic and lactic acid. After only 24h of storage there were no detectable bacteria in the coated samples, highlighting the strong bactericidal effect of the developed coating formulation.
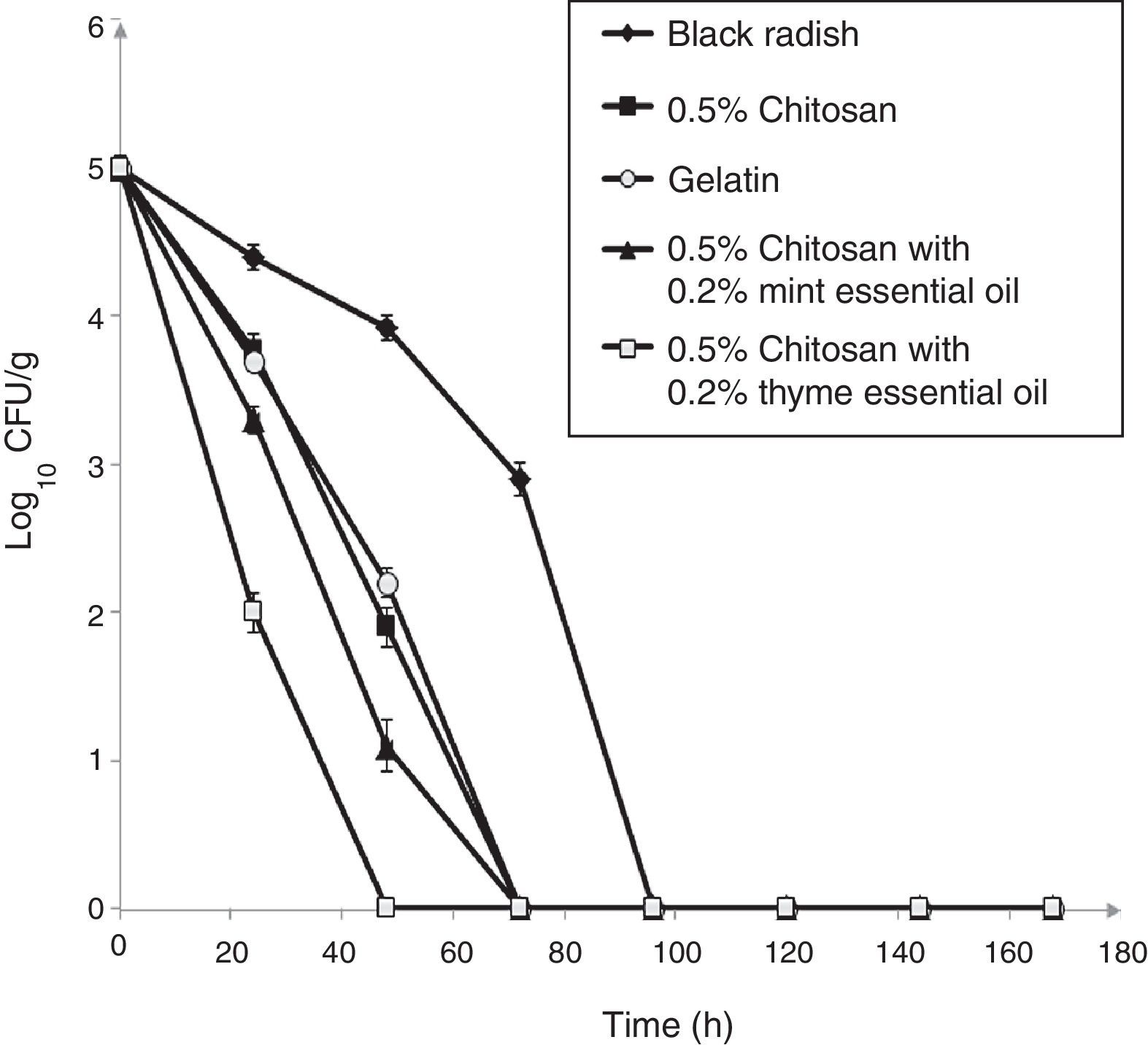
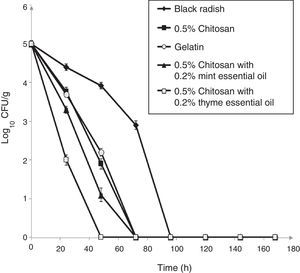
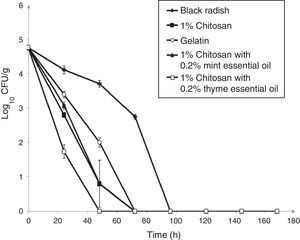
Populations of L. monocytogenes ATCC 19115 inoculated on shredded black radish in the presence of 0.5% chitosan–gelatin film decreased from 5log10CFU/g to undetectable levels after three days at 4°C (Fig. 2). Acetic acid which was present in the gelatin treatments affected the reduction of the bacterial population to a level that could not be detected after 72h of storage (Fig. 2). A significant difference (p<0.05) was observed between the samples stored in the presence of 0.5% chitosan composite films and samples stored in the presence of gelatin with acetic acid, after 48h storage. From the experimental data it clearly emerges that the 0.5% chitosan film exhibited similar activity against L. monocytogenes ATCC 19115 as the gelatin film. The modest antibacterial activity of chitosan in film form primarily depended on the presence of acetic acid (Fig. 2).
Effect of 0.5% chitosan–gelatin films on viable cells (CFU/g) of L. monocytogenes ATCC 19115 in shredded black radish. (♦ – L. monocytogenes ATCC 19115 in shredded black radish; ■ – 0.5% chitosan–gelatin film; ○ – 6% gelatin film prepared with acetic acid; ▴ – 0.5% chitosan–gelatin film enriched with 0.2% mint essential oils; □ – 0.5% chitosan–gelatin film enriched with 0.2% thyme essential oils). Reported populations represent the means of three values. Error bars show standard deviations.
The combined treatment of 0.5% chitosan films and thyme essential oils showed a significant reduction (p<0.05) of L. monocytogenes ATCC 19115 compared to the control sample (black radish). A complete reduction of the tested strain was achieved with this treatment after 48h of storage. The treatment with chitosan and mint essential oils had slightly weaker antimicrobial activity than the film prepared with thyme essential oil (Fig. 2).
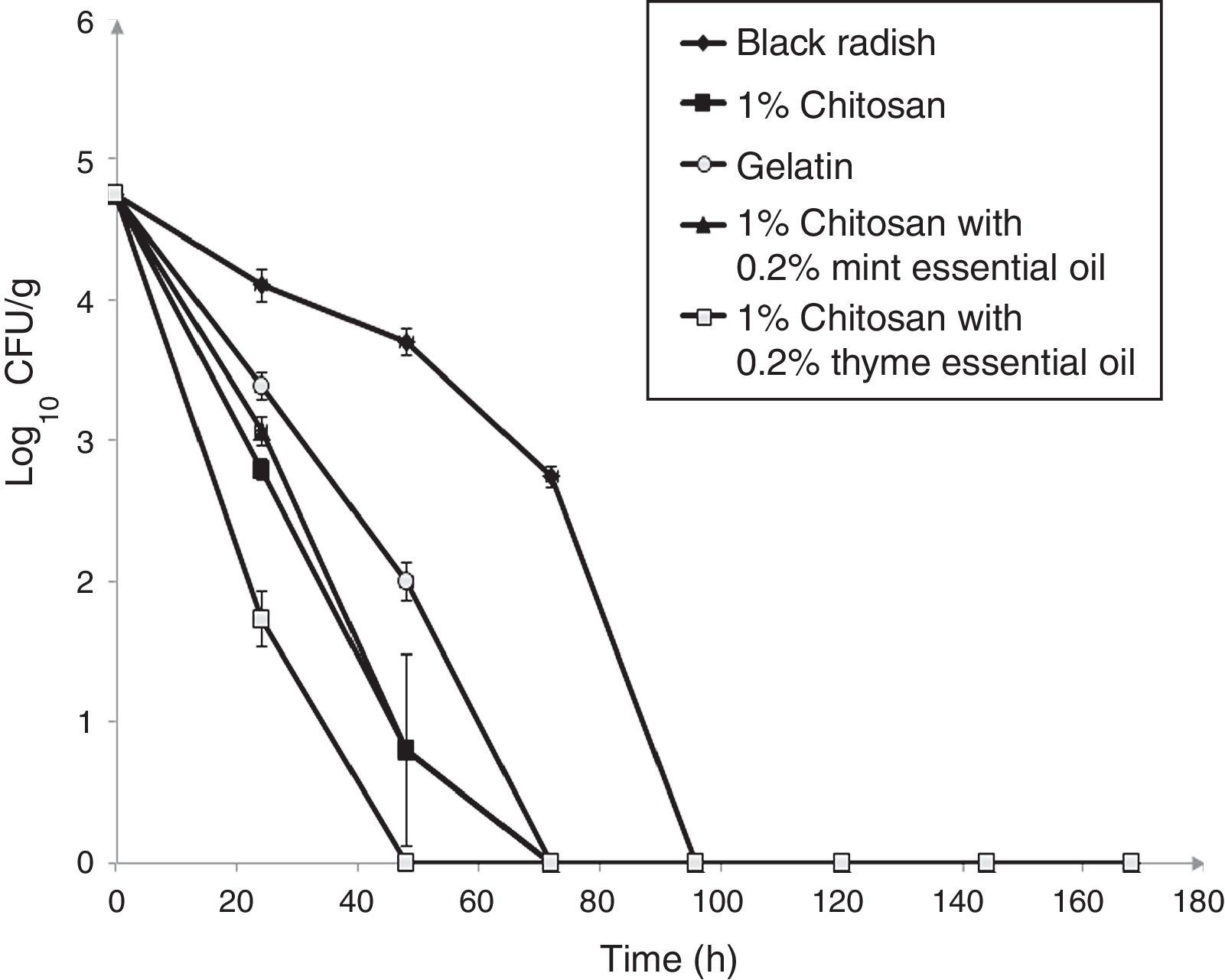
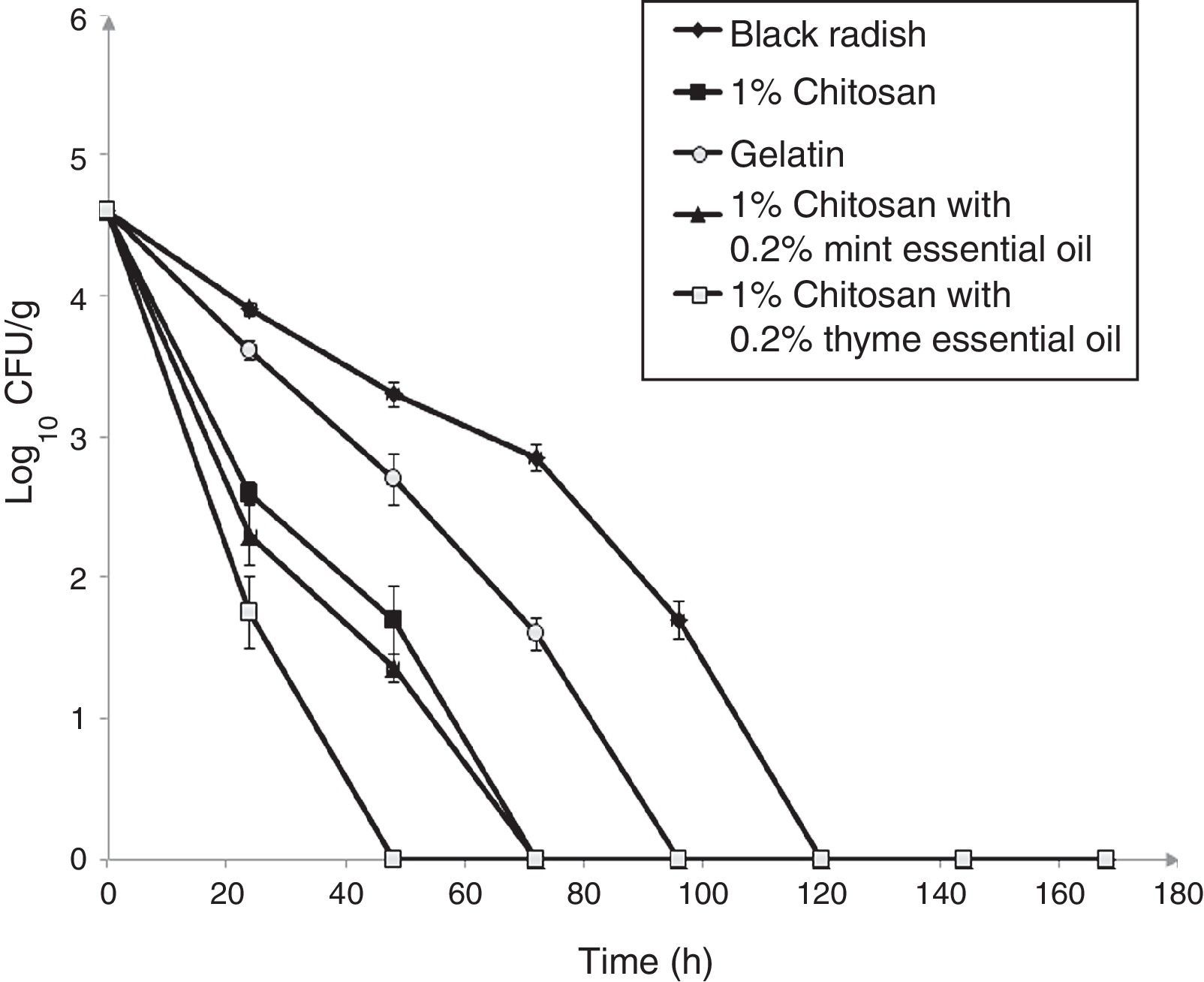
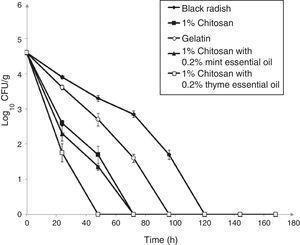
The survival of L. monocytogenes ATCC 19115 on fresh shredded black radish in the presence of 1% chitosan–gelatin film is shown in Fig. 3. the population of L. monocytogenes ATCC 19115 decreased from 4.74log10CFU/g to an undetectable level after three days, in the presence of 1% chitosan–gelatin film (Fig. 3). A significant difference (p<0.05) was observed between samples stored in the presence of 1% chitosan–gelatin composite films and samples stored in the presence of gelatin with acetic acid, after 24h of storage. Our results show that the 1% chitosan film evidenced a strong antimicrobial activity in the inactivation of L. monocytogenes ATCC 19115. Black radish samples treated with 1% chitosan film showed a microbial reduction of 1.3log10CFU/g, already after 24h of storage, compared to the control (Fig. 3). The combined treatment of 1% chitosan film and thyme essential oil showed a significant reduction (p<0.05) of 2.4log10CFU/g in the case of L. monocytogenes ATCC 19115 after 24h of storage at 4°C. A complete reduction of L. monocytogenes ATCC 19115 with this treatment was achieved after 48h of storage. The treatment with chitosan and mint essential oil showed a significant reduction of L. monocytogenes ATCC 19115 populations already after 24h of storage (p<0.05), compared to control. In the samples treated with 1% chitosan film and mint essential oil there were no detectable bacteria after 72h of storage.
Effect of 1% chitosan–gelatin films on viable cells (CFU/g) of L. monocytogenes ATCC 19115 in shredded black radish. (♦ – L. monocytogenes ATCC 19115 in shredded black radish; ■ – 1% chitosan–gelatin film; ○ – 6% gelatin film prepared with acetic acid; ▴ – 1% chitosan–gelatin film enriched with 0.2% mint essential oils; □ – 1% chitosan–gelatin film enriched with 0.2% thyme essential oils). Reported populations represent the means of three values. Error bars show standard deviations.
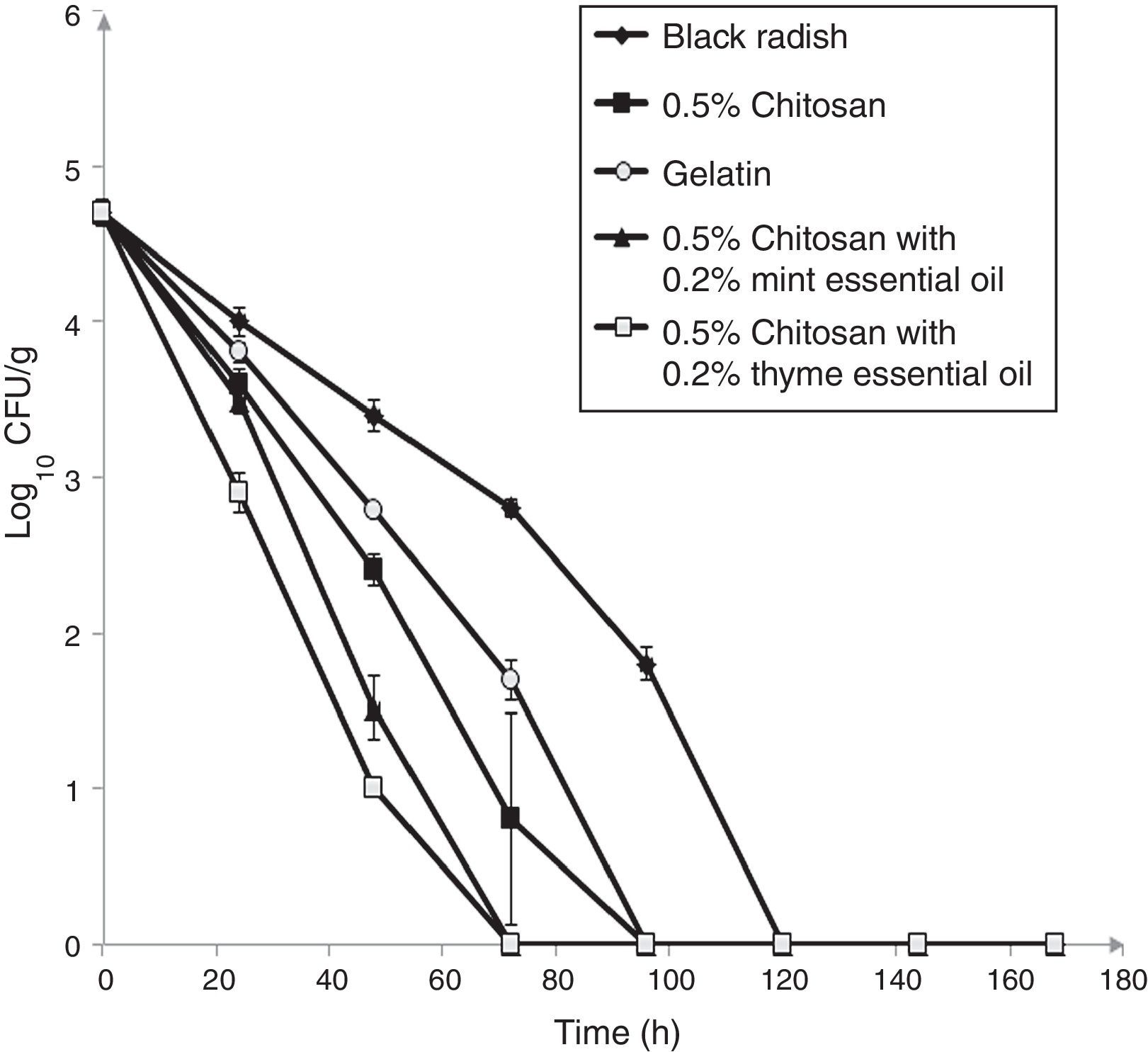
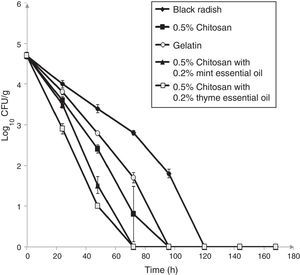
The populations of L. monocytogenes ATCC 19112 inoculated on shredded black radish in the presence of 0.5% chitosan–gelatin film decreased from 4.7log10CFU/g to an undetectable level after four days at 4°C (Fig. 4). Acetic acid, which was present in the gelatin film affected the reduction of the bacterial population to a level that could not be detected after 96h of storage (Fig. 4). A significant difference (p<0.05) was observed between samples stored in the presence of the 0.5% chitosan composite film and those stored in the presence of gelatin with acetic acid after 24h of storage. It clearly emerges from the experimental data that the 0.5% chitosan film evidenced stronger antimicrobial activity in the inactivation of L. monocytogenes ATCC 19115 (Fig. 2) when compared to strain L. monocytogenes ATCC 19112 (Fig. 4).
Effect of 0.5% chitosan–gelatin films on viable cells (CFU/g) of L. monocytogenes ATCC 19112 in shredded black radish.
(♦ – L. monocytogenes ATCC 19112 in shredded black radish; ■ – 0.5% chitosan–gelatin film; ○ – 6% gelatin film prepared with acetic acid; ▴ – 0.5% chitosan–gelatin film enriched with 0.2% mint essential oils; □ – 0.5% chitosan–gelatin film enriched with 0.2% thyme essential oils). Reported populations represent the means of three values. Error bars show standard deviations.
Our research showed that the 0.5% chitosan–gelatin film possesses very good antimicrobial potential against L. monocytogenes ATCC 19112. A significant reduction in the number of bacteria (p<0.05) was observed in the black radish samples treated with the 0.5% chitosan–gelatin film. A strong antimicrobial effect, i.e. a microbial reduction of 1log10CFU/g already occurred after 48h of storage, in the presence of 0.5% chitosan films compared to the control sample. After 96h of refrigerated storage, there were no detectable bacteria on the samples treated with the 0.5% chitosan film.
The combined treatment of the 0.5% chitosan–gelatin films and thyme essential oils showed a significant reduction (p<0.05) of 1.1log10CFU/g of L. monocytogenes ATCC 19112 after 24h compared to control. The treatment with chitosan and mint essential oil showed a significant reduction (p<0.05) of 1.9log10CFU/g after 48h of storage at 4°C.
The populations of L. monocytogenes ATCC 19112 inoculated on shredded black radish in the presence of 1% chitosan–gelatin film decreased from 4.6log10CFU/g to an undetectable level after 72h at 4°C (Fig. 5). During the first three days of testing, a significant difference (p<0.05) was observed between the samples stored in the presence of the 1% chitosan–gelatin composite film and the samples stored in the presence of gelatin with acetic acid. It clearly emerges from the experimental data that the 1% chitosan–gelatin film evidenced stronger antimicrobial activity in the tested bacteria than the 0.5% chitosan–gelatin film.
Effect of 1% chitosan–gelatin films on viable cells (CFU/g) of L. monocytogenes ATCC 19112 in shredded black radish. (♦ – L. monocytogenes ATCC 19112 in shredded black radish; ■ – 1% chitosan–gelatin film; ○ – 6% gelatin film prepared with acetic acid; ▴ – 1% chitosan–gelatin film enriched with 0.2% mint essential oils; □ – 1% chitosan–gelatin film enriched with 0.2% thyme essential oils). Reported populations represent the means of three values. Error bars show standard deviations.
It can be seen that the 1% chitosan–gelatin film exhibits strong antimicrobial properties against this strain (Fig. 5). Black radish samples treated with the 1% chitosan–gelatin film showed a significant reduction in the L. monocytogenes population (p<0.05), compared to control. In the presence of 1% chitosan–gelatin film, a reduction of 1.3log10CFu/g already occurred after 24h of storage, while there were no detectable bacteria after 72h of refrigerated storage.
The combined treatment of 1% chitosan–gelatin film with thyme essential oil showed a significant reduction (p<0.05) of 2.1log10CFU/g, in L. monocytogenes ATCC 19112 after 24h of storage, compared to control. The treatment with chitosan–gelatin film and thyme essential oil achieved a complete reduction of the tested bacteria after 48h, while the 1% chitosan–gelatin film and mint essential oil showed a complete reduction in the L. monocytogenes ATCC 19112 population after 72h of storage (p<0.05).
The value of Pearson's correlation coefficient (r=0.98) shows a significant correlation between the tested strains (p<0.05), in the presence of the 0.5% and 1% chitosan–gelatin films. These results clearly indicate that there is a possible biological significance between the tested strains in the studied systems.
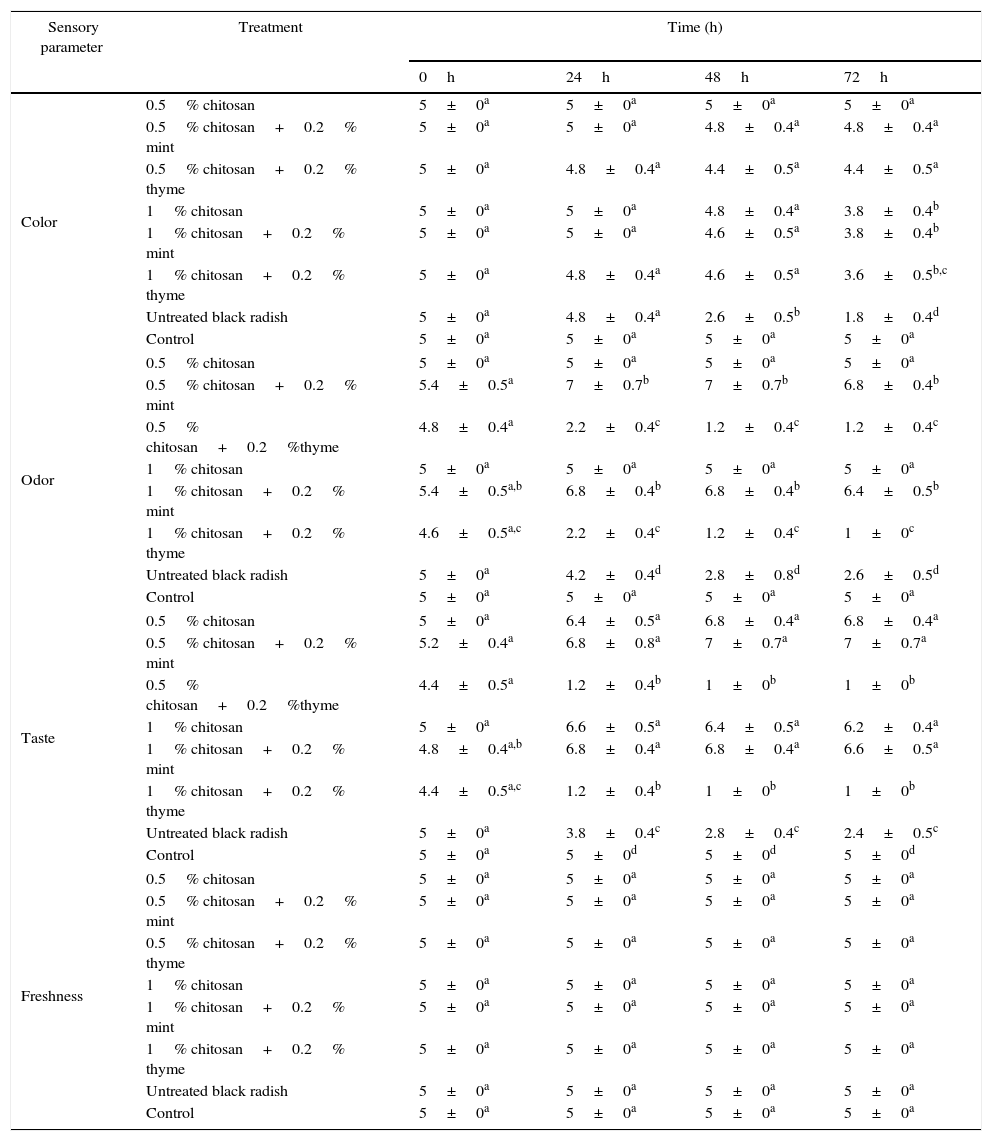
The results of the sensory evaluation are presented in Table 1.
Score of a sensory evaluation of shredded black radish in the presence of chitosan films
| Sensory parameter | Treatment | Time (h) | |||
|---|---|---|---|---|---|
| 0h | 24h | 48h | 72h | ||
| Color | 0.5% chitosan | 5±0a | 5±0a | 5±0a | 5±0a |
| 0.5% chitosan+0.2% mint | 5±0a | 5±0a | 4.8±0.4a | 4.8±0.4a | |
| 0.5% chitosan+0.2% thyme | 5±0a | 4.8±0.4a | 4.4±0.5a | 4.4±0.5a | |
| 1% chitosan | 5±0a | 5±0a | 4.8±0.4a | 3.8±0.4b | |
| 1% chitosan+0.2% mint | 5±0a | 5±0a | 4.6±0.5a | 3.8±0.4b | |
| 1% chitosan+0.2% thyme | 5±0a | 4.8±0.4a | 4.6±0.5a | 3.6±0.5b,c | |
| Untreated black radish | 5±0a | 4.8±0.4a | 2.6±0.5b | 1.8±0.4d | |
| Control | 5±0a | 5±0a | 5±0a | 5±0a | |
| Odor | 0.5% chitosan | 5±0a | 5±0a | 5±0a | 5±0a |
| 0.5% chitosan+0.2% mint | 5.4±0.5a | 7±0.7b | 7±0.7b | 6.8±0.4b | |
| 0.5% chitosan+0.2%thyme | 4.8±0.4a | 2.2±0.4c | 1.2±0.4c | 1.2±0.4c | |
| 1% chitosan | 5±0a | 5±0a | 5±0a | 5±0a | |
| 1% chitosan+0.2% mint | 5.4±0.5a,b | 6.8±0.4b | 6.8±0.4b | 6.4±0.5b | |
| 1% chitosan+0.2% thyme | 4.6±0.5a,c | 2.2±0.4c | 1.2±0.4c | 1±0c | |
| Untreated black radish | 5±0a | 4.2±0.4d | 2.8±0.8d | 2.6±0.5d | |
| Control | 5±0a | 5±0a | 5±0a | 5±0a | |
| Taste | 0.5% chitosan | 5±0a | 6.4±0.5a | 6.8±0.4a | 6.8±0.4a |
| 0.5% chitosan+0.2% mint | 5.2±0.4a | 6.8±0.8a | 7±0.7a | 7±0.7a | |
| 0.5% chitosan+0.2%thyme | 4.4±0.5a | 1.2±0.4b | 1±0b | 1±0b | |
| 1% chitosan | 5±0a | 6.6±0.5a | 6.4±0.5a | 6.2±0.4a | |
| 1% chitosan+0.2% mint | 4.8±0.4a,b | 6.8±0.4a | 6.8±0.4a | 6.6±0.5a | |
| 1% chitosan+0.2% thyme | 4.4±0.5a,c | 1.2±0.4b | 1±0b | 1±0b | |
| Untreated black radish | 5±0a | 3.8±0.4c | 2.8±0.4c | 2.4±0.5c | |
| Control | 5±0a | 5±0d | 5±0d | 5±0d | |
| Freshness | 0.5% chitosan | 5±0a | 5±0a | 5±0a | 5±0a |
| 0.5% chitosan+0.2% mint | 5±0a | 5±0a | 5±0a | 5±0a | |
| 0.5% chitosan+0.2% thyme | 5±0a | 5±0a | 5±0a | 5±0a | |
| 1% chitosan | 5±0a | 5±0a | 5±0a | 5±0a | |
| 1% chitosan+0.2% mint | 5±0a | 5±0a | 5±0a | 5±0a | |
| 1% chitosan+0.2% thyme | 5±0a | 5±0a | 5±0a | 5±0a | |
| Untreated black radish | 5±0a | 5±0a | 5±0a | 5±0a | |
| Control | 5±0a | 5±0a | 5±0a | 5±0a | |
Score was given as mean±SD. Means with different lowercase letters within the same column are significantly different (p<0.05).
Statistical differences were indicated with letters.
For the color evaluation, there was no significant difference (p<0.05) between the control and all the treated samples for the first 24h of storage. The greatest color changes were observed after 72h of storage, in an untreated black radish sample and all the samples containing 1% chitosan. The 0.5% chitosan treatment received the highest average score (6.8) compared to the control (5), indicating that this treatment can help improve the taste of the product after 72h storage. Furthermore, the treatment with mint essential oil had the highest score in the evaluation of odor and taste (Table 1). However, there was no significant difference with regard to freshness (p<0.05) between the samples which were evaluated for three days. There were no significant differences in the panelists’ scoring of taste and odor for each of the individual samples with the thyme essential oil evaluated for three days (Table 1). According to the panelists’ evaluation, the most acceptable vegetable samples were those with chitosan with or without the addition of mint essential oil.
DiscussionThe chitosan coating treatment reduced the initial population of L. monocytogenes in black radish. Populations decreased significantly in all radish samples analyzed within seven days of storage at 4°C. The most prominent inhibitory activity was observed in the coatings prepared with acetic acid and 1% chitosan. It is notable that all applied concentrations of chitosan coatings exhibited a pronounced bactericidal activity on shredded black radish. Experimental data indicated that the tested bacterial strains could not be detected in the black radish samples starting from the first day of storage until the end of the test. These results can be explained by the presence of antimicrobial substances in radish which can act synergistically with chitosan.
The observed antimicrobial activity of chitosan coatings on L. monocytogenes is in accordance with the results reported by other authors. Chitosan activity can be explained by changes in cell permeability, the interaction between the amino group of chitosan and the electronegative charge on the cell surface which leads to leakage of the intracellular protein and electrolyte19,23. Moreover, according to Brodelius et al.5, a high concentration of chitosan may cause cell death due to membrane permeabilization.
Durango et al.10 examined the edible coating of chitosan and starch in order to determine their antimicrobial ability on the rings of carrots which were dipped into a film of chitosan and starch, and stored at 15°C. The total number of mesophilic aerobic bacteria, coliform bacteria, Escherichia coli, Staphylococcus aureus, yeasts, fungi and lactic acid bacteria was determined in thus prepared samples. According to these authors, coatings using 0.5% chitosan had the ability to control the growth of aerobic mesophilic species, yeasts and fungi only during the first five days of storage. Coatings using 1.5% chitosan exhibited reduced mesophilic aerobic species, yeasts and fungi, for 1.34 and 2.5 log in comparison with the control, which was not treated with the edible coating. Furthermore, the total number of lactic acid bacteria was inhibited by the addition of 1.5% chitosan. These authors recommended an edible coating of chitosan and starch as an alternative to control microbiological activity in minimally processed vegetables. According to Sánchez-González et al.27, the edible coatings derived from chitosan, with or without essential oil of bergamot, offer a good alternative for storing grape, preventing weight loss and preservation of fruit firmness during storage.
Our results showed that all the investigated formulations of chitosan–gelatin films possess a potential for L. monocytogenes inhibition in black radish. Antimicrobial potential increased with the increase in chitosan concentrations. Moreover, essential oils have contributed to an increased antimicrobial activity of all chitosan films. The most prominent activity was shown in the films containing 0.2% thyme essential oil. The observed antimicrobial activity of chitosan - gelatin films with the addition of essential oils into L. monocytogenes are in accordance with the results reported by other authors.
According to Petrou et al.22 chitosan applied alone or in combination with oregano essential oil can extend the shelf life of chicken filets packed in a modified atmosphere for 6 or 14 days. Perdones et al.20 reported that chitosan enriched with essential oils can prevent the occurrence of gray mold on strawberries. The research conducted by Vu et al.35 also showed that edible coatings of chitosan with the addition of thyme, lemon and peppermint essential oils can be very effective in preventing spoilage of strawberries in a period of 14 days at 4°C.
In addition, Chi's8 results showed that the application of chitosan film can reduce the number of pathogens in salami from 1 to 3log. This author reported that the 4log reduction in L. monocytogenes and E. coli O157:H7 on sausage pieces that were kept at 10°C for 5 days has been enabled by the application of a chitosan film enriched with the addition of 1% and 2% oregano essential oil. Vasilatos and Savvaidis33 examined the antimicrobial activity of chitosan with the addition of 0.25% (v/w) rosemary essential oil on the growth of various microorganisms on turkey filets. The strongest antimicrobial effect on the growth of Lactobacillus spp., Brochothrix thermosphacta, Pseudomonas spp., Enterobacteriaceae, yeasts and molds was shown by a combination of chitosan and rosemary essential oils at a concentration of 1.5% (w/v).
The results of the sensory analysis revealed that the acceptance of products was related to the type of essential oil. Consequently, the addition of mint essential oil to the chitosan films could be a promising method for enhancing the odor and taste of fresh shredded vegetables. However, the samples containing thyme essential oil were completely unacceptable after three days of evaluation.
The edible coatings made from chitosan and acetic acid could be a good alternative for the inactivation of two different strains of L. monocytogenes on shredded black radish. Furthermore, chitosan–gelatin composite films, with or without essential oils, are also suitable for the preservation of fresh shredded black radish. Chitosan–gelatin films with thyme essential oil proved to be the most effective against pathogen bacteria. In addition to antimicrobial activity, chitosan films enriched with selected essential oils could contribute to the appropriate sensory characteristics of packaged products, e.g. to develop a pleasant taste and aroma, color change, etc. Although the chitosan film enhanced with thyme essential oil showed the highest antimicrobial activity, it also had the greatest impact on the flavor and aroma of fresh shredded vegetables, which is unacceptable for consumers. Since the chitosan films with mint essential oil also showed good antimicrobial properties and had acceptable impact on the flavor and aroma of fresh shredded vegetables, they are the most recommended for the preservation of black radish.
Conflict of interestThe authors claim that they have no conflicts of interest to declare.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.