Ochratoxin A (OTA) is a mycotoxin produced by filamentous fungi with high impact in food safety due to its toxicity. In the last decade, the presence of OTA was widely reported in different foods. In this study, the ability of Lactobacillus (L.) plantarum CRL 778 to control growth and OTA production by Aspergillus (A.) niger 13D strain, at different water activity (aw) values (0.955, 0.964, 0.971, 0.982, and 0.995) was determined in vitro. Both parameters were significantly (p<0.05) reduced by the lactobacilli and the effect depended on aw. Greatest growth rate inhibition (46.9%) was obtained at aw=0.995, which is the most suitable value for growth and production of antifungal metabolites (lactic acid, acetic acid, phenyllactic and hydroxyl-phenyllactic acids) by L. plantarum CRL 778. Besides, morphological changes and inhibition of melanin synthesis were observed in colonies of A. niger 13D in presence of L. plantarum CRL 778 at aw ranged between 0.971 and 0.995. In addition, maximum reduction (90%) of OTA production took place at aw=0.971, while inhibition of fungi growth was more evident at aw=0.995. These findings suggest that L. plantarum CRL 778 could be used for control of ochratoxigenic fungal growth and OTA contamination in different fermented foods with aw values between 0.971 and 0.995.
Ocratoxina A (OTA) es una micotoxina producida por hongos filamentosos con un alto impacto en la seguridad alimentaria debido a su toxicidad. En la última década se ha reportado ampliamente a nivel mundial, la presencia de OTA en diversos alimentos. En este estudio se evaluó in vitro, la capacidad de Lactobacillus (L.) plantarum CRL 778 de controlar el crecimiento y la producción de OTA por Aspergillus (A.) niger 13D, a diferentes valores de actividad de agua (aw): 0.955, 0.964, 0.971, 0.982 y 0.995). La cepa láctica redujo significativamente (p<0.05) ambos parámetros, siendo el efecto dependiente del valor de aw. La mayor inhibición del crecimiento (46.9%) se obtuvo a aw=0.995, valor más adecuado para el crecimiento y producción de metabolitos antifúngicos (ácido láctico, ácido acético, ácidos fenil-láctico e hidroxi-fenil láctico) por la cepa láctica. Además, se observaron cambios morfológicos en las colonias de A. niger 13D, crecidas en presencia de L. plantarum CRL 778 a valores de aw de 0.971 y 0.995. El porcentaje máximo de reducción en la producción de OTA (90%) por la cepa láctica se observó a un valor de aw=0.971, mientras la inhibición del crecimiento fúngico fue mayor cuando aw=0.995. Estos hallazgos sugieren que L. plantarum CRL 778 podría emplearse para el control de la contaminación por hongos ocratoxigénicos en alimentos con valores de aw comprendidos entre 0.971-0.995.
Ochratoxin A (OTA) is a mycotoxin produced by filamentous fungi with nephrotoxic, immunotoxic, teratogenic and carcinogenic effects27,31,33. In the last decade, its presence has been extensively reported worldwide in common foodstuff and beverages. This has aroused significant public concern and also constitutes a major economic problem2,3,26.
The OTA production by fungi of genera Aspergillus (A.) and Penicillium is influenced by environmental and nutritional factors such as pH, temperature, water activity (aw) and nitrogen and carbon sources17–19. At present, several physical and chemical methods have been reported to prevent the ochratoxigenic fungi growth and OTA detoxification in products for human and animal consumption; however none of these strategies were completely successful10,16. Currently, some molds acquired resistance to some chemical additives such as potassium sorbate, sorbic and benzoic acids, which were considered effective preservatives in the past29. Besides, current legislation restricts the use of some preservatives for food manufacture. Moreover, consumer increasingly demand reduction of chemical compounds in food or feed and production of natural and healthy products. In this context, in the last decade researchers have shown interest for biological methods, being lactic acid bacteria (LAB) the most powerful prokaryotes when it comes to antifungal potential6,11,14. Nevertheless, there is a lack of publications on antifungal LAB against black Aspergilli and ochratoxigenic fungi. Kapetanakou et al.17 reported that none of the six LAB strains tested as potential inhibitors of A. carbonarius growth and OTA production had positive results. More recently, de Melo Pereira et al.16 reported that Lactobacillus (L.) brevis LPBB03 was able to inhibit the ochratoxigenic strain A. westerdijkiae in vitro. However, this study did not evaluate the antimycotoxin effect of the LAB.
In previous works, L. plantarum CRL 778 and other lactobacilli proved to be effective in inhibiting the growth of spoilage molds, isolated from contaminated bread and commercial citrus fruits13,14. The antifungal effect was related to the production of lactic, acetic, and phenyllactic acids. From these results, a ready-to-use biopreserver (SL778: fermented mixture of wheat flour, sucrose, skimmed milk and water) for packed bread was developed and its technologies properties were evaluated12. It was observed lower growth and OTA production by A. niger in breads made with SL778 and, remarkably, the effect was dependent on storage water activity11. The aim of the present study was to determine in vitro, the ability of L. plantarum CRL 778 to control OTA production and the growth of A. niger 13D at different values of water activity, in order to propose it inclusion in different fermented food.
Materials and methodsMicroorganismsL. plantarum CRL 778, isolated from homemade wheat sourdough, was obtained from the culture collection of Centro de Referencia para Lactobacilos (CERELA-CONICET, Tucumán, Argentina). For the assays, 16-h old cultures in MRS broth (Oxoid, Ltd., Basingstoke, England) at 37°C were centrifuged (8936×g, 10min, 4°C) and cells obtained were washed twice with sterile potassium phosphate buffer 0.1M (pH 6.5) and suspended in the same buffer. The ochratoxigenic A. niger 13 D was obtained from the culture collection of National University of Río Cuarto (UNRC), Córdoba, Argentina and stored at −20°C in 40% glycerol. The mold strain was grown on malt extract agar (MEA; Difco Laboratories, Detroit, Michigan, USA) plates at 25°C for 7 days. Conidia were collected in sterile soft agar (Tween 80, 0.5g/l and agar, 1.0g/l) and counted microscopically in a hemocytometer chamber to adjust its concentration to 104 conidia per milliliter in sterile water.
In vitro inhibition assaysThe inhibitory effect of L. plantarum CRL 778 on A. niger 13 D was evaluated on Petri plates (90mm diameter×15mm) containing yeast extract agar medium (CYA)28. L. plantarum cell suspension (3×109CFU/ml) was inoculated (2%, v/v) in plates containing molten and cooled (45°C) CYA with aw adjusted to 0.955, 0.964, 0.971, 0.982, and 0.995. Different amounts of glycerol:water were used to modified aw values; they were checked with an AquaLab Series 3 (Decagon Devices, Inc., WA, USA). These aw values correspond to those found in different foods as cheddar cheese, vegetable, milk and fresh meat, among other. Inoculated Petri dishes (CYA-L. plantarum CRL 778) were sealed in polyethylene bags and incubated 24h at 30°C. After this, Petri dishes were inoculated with spore suspension of A. niger 13 D by central puncture. Plates containing CYA agar medium were adjusted to different aw values, inoculated with A. niger 13 D and used as control. All dishes were sealed in closed plastic containers, each one containing a glycerol: water solution at a determined aw30. Quadruplicate sets of each assay were incubated 7 days at 30°C and the lag phase and the mycelial extension were used as growth fungal parameters22. Temporal mycelial extension rates were determined daily in two directions at right angles to each other until the medium was fully colonized. Radial extension rates were plotted against time and the growth rates were calculated using linear regression (mm/day) at each aw. The extraction of OTA was performed after 7 days of incubation of Petri dishes at 30°C. On each sampling, three agar plugs were removed from different points of the mycelium growth and extracted with 1ml of methanol. The mixture was centrifuged at 14,000×g during 10min, evaporated to dryness and kept at 5°C until use. The OTA concentration was determined by reversed phase HPLC according to Gerez et al.11
Effect of aw on the growth and production of organic acid by L. plantarum CRL 778Growth and organic acids production by L. plantarum CRL 778 were determined in CYA broth (200ml) adjusted to different aw values as described above. After incubation (30°C for 24h), samples were withdrawn from cell cultures and bacterial growth was ascertained by optical density at 580nm (Spectrophotometer 1000 series, Cecil, Cambridge, England). The pH values (pHmeter Altronix-TPX1 Ph, mV-Meter, Sartorius, Goettingen, Germany) and organic acids [lactate, acetate, phenyllactic acid (PLA) and hydroxyphenyllactic acid (OH-PLA)] concentrations were also determined. The organic acids were determined by High Performance Liquid Chromatography14 (HPLC) using an ion-exclusion Aminex HPX-87H column (300mm×7.8mm, Bio Rad, USA) under the following conditions: mobile phase H2SO4 (5mmol/l) at a flow rate of 0.6ml/min and column temperature of 45°C. A refractive index detector was used to identify lactate and acetate while an UV detector set at 210nm was used to identify PLA and OH-PLA. Both detectors were connected to the software Peak Simple II (Knauer Company, Berlin, Germany) for data analysis. Organic acids were quantified on the basis of detector response compared with the respective standards (Sigma–Aldrich).
Statistical analysisResults of two independent assays are presented as mean values±standard deviation (SD). Data were analyzed by ANOVA and Tukey's test. The statistical analysis was carried out with the Statistica 5.5 program (Statsoft, Tulsa, OK, USA). Results were considered significantly different at p<0.05.
ResultsInhibitory effect of L. plantarum CRL 778A. niger 13D was able to grow in CYA medium at all aw assayed (Table 1). In general, in the absence of L. plantarum CRL 778, the specific growth rate increased at higher aw values, while the lag phase showed the opposite behavior. On the whole, the inhibitory effect of L. plantarum CRL 778 depended on the aw value of the medium. In fact, in the presence of CRL 778 strain (13D-CRL 778), a decrease in the A. niger 13D growth rate and a lengthening of the lag phase were observed, specially at aw=0.995 and 0.982. In addition, colonies of A. niger 13D growing in the presence of L. plantarum CRL 778 exhibited morphological variations compared to the control (13D), such as visible loss of conidia melanization in their outline (0.971–0.995 aw) (Fig. 1).
Lag phase and growth rate (μmax) of A. niger 13D in CYA agar with and without L. plantarum CRL 778 at different aw.
| Culture | Water activity (aw) | |||||
|---|---|---|---|---|---|---|
| 0.955 | 0.964 | 0.971 | 0.982 | 0.995 | ||
| Lag phase(h) | 13D | 22.35±0.961,a | 18.29±1.611,b | 16.65±2.111,c | 14.36±0.841,c,d | 12.16±0.881,d |
| 13D-CRL778 | 23.61±1.751,a | 21.23±1.732,a | 22.54±5.282,a | 25.05±2.592,a | 22.34±2.942,a | |
| Fungal growth rate (cm/d) | 13D | 1.11±0.041,a | 1.18±0.071,a | 1.35±0.111,b | 1.47±0.021,b | 1.81±0.031,c |
| 13D-CRL778 | 1.25±0.051,a | 1.38±0.021,a,b | 1.02±0.252,a,c | 1.09±0.062,a,c | 0.96±0.082,c | |
13D=A. niger 13D; 13 D-CRL778=A. niger 13D with L. plantarum CRL 778. Fungal growth rate (μmax): cm of colony/days. Means with the same superscript letter in the same row and the same number in the same column show no significant differences between them (p<0.05) by the Tukey test.
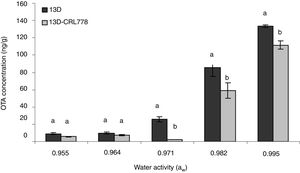
Production of OTA (at 7 days) by A. niger 13D alone and mixed with L. plantarum CRL 775 (13D-CRL778) in CYA agar at different aw is shown in Figure 2. In general, production of OTA by A. niger (13D) was dependent on aw, i.e. at higher water activity, an increment in OTA concentration was observed, being 0.995 aw the optimal condition for OTA production (133±1.5ng/g). Mycotoxin concentration was inhibited (16–90%) by L. plantarum CRL778 (13D-CRL778) in most of aw value tested. The maximum reduction (90%) was observed at 0.971 aw, in which toxin was almost negligible. On the contrary, no significant differences in the concentration at both 0.955 and 0.964 aw were observed.
Effect of aw on growth and antifungal acids production by L. plantarum CRL 778Taking into account that antifungal and antimycotoxigenic activity of CRL 778 varied significantly with aw levels, growth and antifungal metabolite production of the LAB strain were studied in the same conditions. As shown in Figure 3, L. plantarum CRL 778 was able to grow at all aw tested, with a maximum (A580nm=1.39) at aw=0.995 and minimum (A580nm=0.40) at aw=0.955. Production of lactic, acetic, phenyllactic (PLA) and hydroxy-phenyllactic (OH-PLA) acids (antifungal metabolites) increased as increased aw levels and were also greater (5.3, 4.6, 1.9 and 2.2 times, respectively) at aw=0.995.
DiscussionFungal growth and OTA production can be influenced by intrinsic factors of food matrixes, such as aw. Our results demonstrated that A. niger 13D was able to grow and also produce OTA over a wide range of aw (0.955–0.995) in CYA medium. These observations could explain why A. niger is considered the most common Aspergillus species responsible for food contamination and is also among the most frequently fungi isolated from dried products1. It is important to note that both, fungus growth and mycotoxin production were affected by aw; being the highest aw assayed (0.995), the optimal condition for both parameters. Other authors also showed a positive influence of this abiotic factor on growth and OTA accumulation of the A. section Nigri strains and others ochratoxigen fungi4,15,21. In fact, higher water activities seem to favor both parameters. On the contrary, Esteban et al.9 reported that optimal conditions for growth were different from those for OTA production in some A. niger strains tested in CYA medium.
Some biostrategies have been proposed as alternatives to chemicals in order to reduce growth of ochratoxigenic mold and OTA contamination of foods. However, relevant use of antifungal LAB requires thorough knowledge of the parameters involved in modulating antifungal properties. In this study, growth of A. niger 13 D was significantly reduced by L. plantarum CRL 778 in CYA medium, being the inhibition related to aw. Thus, the highest inhibitory effect (46.9% reduction in growth rate) was observed at aw=0.995, optimum condition for growing and producing antifungal metabolites (lactic and acetic acids, PLA and OH-PLA) by L. plantarum CRL 778. In addition, morphological and melanization effects were observed in colonies of A. niger 13D growing in presence of L. plantarum CRL 778 at aw=0.971–0.995 (Fig. 1). Also, inhibition of sporulation and slower radial growth were observed in these colonies. Synthesis of melanin would play a fundamental role in the resistance of certain fungi to antifungal compounds32. In fact, it was reported that melanin could provide structural support to resist osmotic changes as well as protection from oxidative damage and antifungal metabolites in many fungal genera24. Hence, inhibition of melanin synthesis by CRL 778 strain would be related to its antifungal effect.
In general, a decrease in the mycelium formation leads to less production of toxins. However, several researches have shown that there is not always correlation between restriction of growth and potential inhibition of mycotoxin production. Our results showed that L. plantarum CRL778 (13D-CRL778) was also able to inhibit the OTA production, observing the maximum percentage reduction (90%) at aw=0.971. Differences observed between optimum growth conditions and OTA production inhibition may be due to other antagonistic mechanisms generated by the LAB, besides acids production. In fact, it was reported that during cell growth and lysis, bacteria may release potentially inhibitory compounds against fungal growth and mycotoxin production5. Moreover, some authors reported that certain Lactobacillus and Lactococcus, were able to bind certain mutagenic compounds such as mycotoxins to their cell wall7.
Although there are several reports regarding antifungal potential of LAB, they are mostly focused on growth inhibition but not on the mycotoxin production8,20,25. In this sense, Kapetanakou et al.17 reported that none of six LAB strains tested as potential inhibitors of A. carbonarius were able to inhibit both, growth and OTA production, in culture media and beverages. On the contrary, four Lactobacillus strains showed. in vitro, interesting antifungal activity on ochratoxin-producing Aspergillus species (A. carbonarius, A. niger, A. ochraceus, A. westerdijkiae)6,23, however, the later study did not evaluate antimycotoxin effect. In the present study, L. plantarum CRL 778 proved to be efficient in inhibiting the OTA-producing A. niger 13 D strain in CYA medium at different aw values (0.971–0.995), besides its antifungal activity. In addition, the maximum reduction (90%) of OTA production took place at aw=0.971, while fungi growth inhibition was more evident at aw=0.995. These results agree with those reported by Esteban et al.9, who found that optimal growth condition was different from those of OTA production in some A. niger strains tested in CYA medium. On the other hand, these results confirm previous study, in which a biopreservative with L. plantarum CRL 778 was effective to control OTA production and growth of A. niger 13D in bread11. Although additional studies are needed, our results strongly suggest that L. plantarum CRL 778 could be used to control ochratoxigenic fungi growth and OTA contamination in others food with aw values 0.971–0995, such as cheddar cheese, vegetable and fresh meat, among other.
Conflicts of interestNo potential conflict of interest was reported by the authors.
This work was funded by Technical Research Council (CONICET-PIP 512) and Universidad de San Pablo-T Argentina (IC-502).