This article analyses the presence of gender bias in clinical trials of monoclonal antibodies used to treat multiple sclerosis.
Material and methodsWe performed a systematic review of controlled clinical trials of 4 monoclonal antibodies used to treat multiple sclerosis (natalizumab, rituximab, alemtuzumab, and ocrelizumab). We searched the PubMed/MEDLINE database for articles published in English before March 2020. The study was conducted in accordance with the relevant international recommendations.
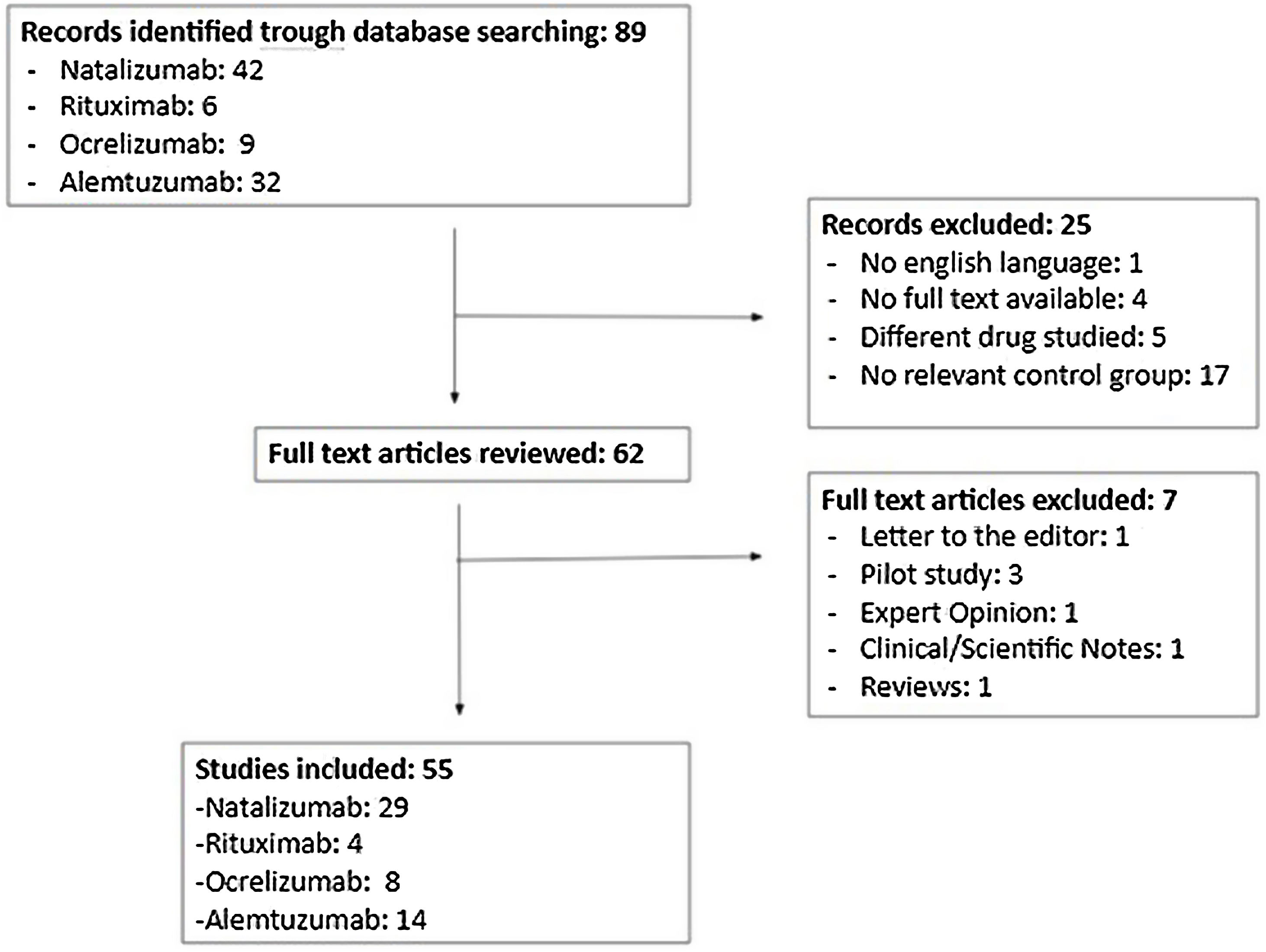
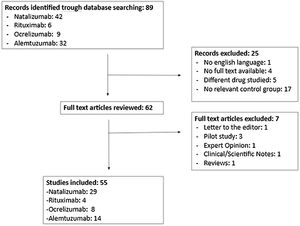
ResultsThe search identified 89 articles, 55 of which met the inclusion criteria. Of all patients included in these trials, 64.6% were women. The lead authors of 10 of the studies were women. Fifteen of the 55 studies included a sex-based analysis of the primary endpoint. Only 8 articles discussed the results separately for men and for women.
ConclusionsThe clinical trials of these 4 monoclonal antibodies present a significant gender bias. In most cases, the primary and secondary endpoints are not analyzed according to patient sex, despite the fact that international recommendations include this as a minimum requirement for ensuring scientific validity and obtaining appropriate results for extrapolation to the wider population.
Este artículo evalúa el sesgo de género presente en los ensayos clínicos sobre anticuerpos monoclonales para el tratamiento de la esclerosis múltiple.
Materiales y métodosSe realizó una revisión sistemática de ensayos clínicos controlados de 4 anticuerpos monoclonales (natalizumab, rituximab, alemtuzumab y ocrelizumab) para el tratamiento de la esclerosis múltiple a través de las bases de datos Pubmed/Medline, publicados hasta marzo de 2020 y los cuales fueron escritos en inglés. El estudio siguió las correspondientes recomendaciones internacionales.
ResultadosSe identificaron 89 artículos, de los cuales 55 cumplieron los criterios de inclusión. Se encontró que el 64,6% del total de pacientes eran mujeres. El sexo del primer autor era femenino en 10 ensayos clínicos. El análisis de la variable principal en función del sexo se realizó en 15 de los 55 artículos incluidos. Además, solo 8 ensayos clínicos discutieron los resultados separadamente de acuerdo al sexo.
ConclusionesLos ensayos clínicos de estos 4 anticuerpos monoclonales muestran un sesgo de género significativo. En su mayoría, la variable principal y secundarias no son analizadas en función del sexo. Esto se produce a pesar de las recomendaciones internacionales que lo establecen, como requisito mínimo, para dar validez científica y obtener unos resultados apropiados para extender su aplicación a la población global.
Multiple sclerosis (MS) is 2 to 3 times more frequent in women than in men.1 In the last decades, some studies have revealed that the incidence of MS in women may be increasing, being more significant in countries located in Northern latitudes.2,3 The reasons for this increase remain unknown, but some authors suggest that they could be influenced by environmental or immunological factors.4,5
On the other hand, the average age at which the first symptoms of MS appear, usually ranges between the second and the third decade of life in the general population,1 but those symptoms present even earlier in women than in men.3
Likewise, men experience a faster progression of the disease and a worse prognosis, with greater cognitive disorders and brain atrophy. However, women suffer a greater number of flares, with more inflammatory lesions that are shown on nuclear magnetic resonance imaging.3
Currently, the biological treatment with monoclonal antibodies is considered one of the most effective therapies in reducing inflammation and in the number of flares in MS patients. Furthermore, various authors advise “personalizing” this type of therapy by monitoring adverse effects and developing individual strategies to detect possible treatment failures.6 However, there is little evidence on the study of the efficacy and safety of the drugs used in MS based on gender,7 being this analysis particularly interesting to individualize patients’ treatments.
The National Institute of Health requires the inclusion of women in phase III clinical trials to ensure adequate analysis of data based on sex.8 These recommendations served as the basis for the preparation of the SAGER guidelines, a tool used to systematize data based on gender and type in scientific publications, when required.9 In 1993, “The Food and Drug Administration” (FDA) published a guide that also promotes the participation of women in clinical trials (CT)10 and that was not replicated in Europe.11 Finally, some similar initiatives have emerged from other organizations such as: “The International Committee of Medical Journal Editors”12 or “The Canadian Institute of Health”,13 which highlight the significance of promoting the perspective of gender and sex in the research and scientific articles.
Some systematic reviews have shown gender bias in different medical specialities in the design of CT. For example, a low female representation in CT has been verified with antiretroviral drugs14 or with long-acting antipsychotic drugs,15 and even in certain studies that treat different types of cancer that are not gender-specific.16 Finally, according to a review of randomized clinical trials published in high impact journals, an over-representation of men has been found in 43% of the studies without explicit exclusion of women in the selection process.17
The goal of this study was to assess if the published CTs on monoclonal antibodies authorized in the treatment of MS, followed the international recommendations to avoid gender bias.
MethodsCTs of monoclonal antibodies used in the treatment of Multiple Sclerosis (Ocrelizumab, Rituximab, Alemtuzumab and Natalizumab) were collected through the PubMed/MEDLINE database published in English until March 2020. Only monoclonal antibodies were selected in this study because they are the newest therapies in EM. Daclizumab and Opicinumab were excluded because they have not been approved by European Medicine Agency (EMA).
To obtain the full text of the articles that did not have this modality in the journal itself, they used the Virtual Library of the Public Health System of Andalusia.
The literature search strategy can be seen in Table 1.
The following filter was used: Clinical Trials.
The inclusion and exclusion criteria can be seen in Table 2.
Inclusion and exclusion criteria.
| Inclusion criteriaThe study drug had to be natalizumab, rituximab, ocrelizumab or alemtuzumab.The CT had to have a control group and use random allocation.The treated patients had to be over 18 years of age.The diagnosis of the CT participants had to be Multiple Sclerosis.Exclusion criteriaPilot studies with a small sample of patients.Reviews and meta-analyzes.Short reports, letters to the editor, expert opinions or clinical notes. |
The following study characteristics were recorded:
- •
Drug under study: ocrelizumab, alemtuzumab, rituximab o natalizumab.
- •
Publication year: divided into CT published from 2000 to 2009, and from 2010 to 2019.
- •
Location of the trial: international or other (United States, Japan, …). the post hoc studies identified the CT they were coming from.
- •
CT phase: I, II, III or IV. In those CT where phase II/III were considered phase III.
- •
Something used as a standard for comparison: placebo or active drug (in multiarm trials, whenever one of them was active drug, the comparator was considered active drug).
- •
Type of CT according to its main outcomes: efficacy and/or safety, PK/PD.
- •
Financing of the CT: pharmaceutical industry or independent (trials were considered to be promoted by the pharmaceutical industry if one of the authors was employed by a pharmaceutical company).
- •
Main diagnosis: progressive primary MS (PPMS), progressive secondary MS (PSMS), recurring remittent MS (RRMS) and progressive remittent MS (PRMS).
- •
Total number of participants.
- •
Author gender.
- •
If studies described pregnancy as an exclusion criterion.
- •
If they discussed the results analyzed based on gender.
- •
If there was an analysis of the interaction between hormone replacement therapy AND study drug, included women using hormonal contraceptives.
- •
If there was a study of the influence of the phase of the menstrual cycle on the pharmacokinetics and response to the drug.
The following main variables were analyzed: percentage of women among the total number of patients recruited and percentage of CT presenting the main results arranged by sex.
The percentage of women was estimated with the raw data (number of women among the total number of patients in each subgroup). Post hoc studies were not taken into account for the total number of patients or the number and percentage of women since they used the same population of the CT they came from. However, it was analyzed if the post hoc study included an analysis based on gender of the main variable or other variables that was not included in the analysis of the first publication of the original trial.
For the analysis of gender differences, they used the SAGER guidelines9 and the FDA guide11 mentioned above. The basic statistics of the central tendency were estimated, analyzing the following subgroups: study type, drug, place, drugs used as a standard for comparison, date of publication, CT phase. type of objective, sample size, funding and first author sex.
This systematic review was performed in accordance with the PRISMA-E 2012 guide.18
ResultsA total of 89 studies were identified in the literature search; of these, 55 complied with the inclusion and exclusion criteria (Fig. 1).
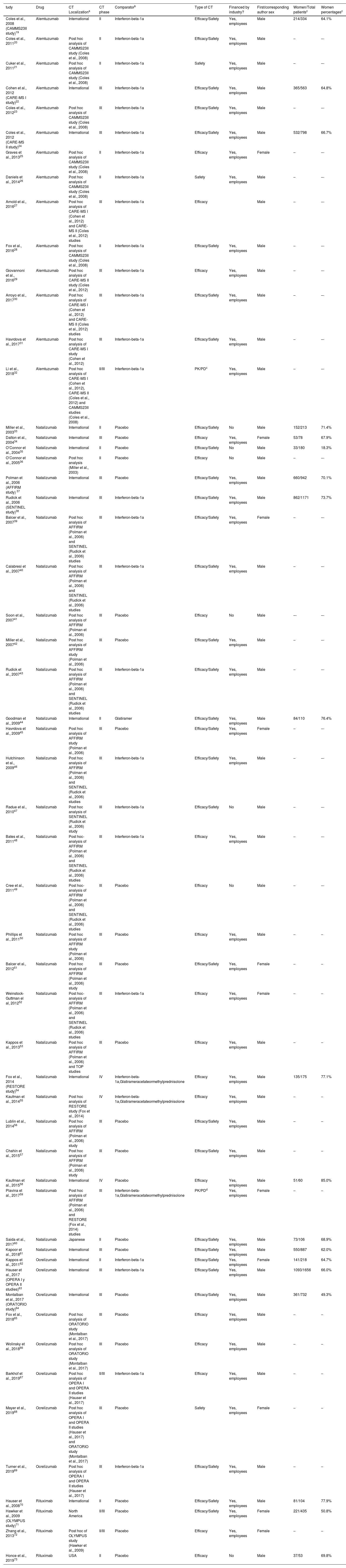
Table 3 shows the characteristics of the trials included in the study.
Features of the included clinical trials.
| tudy | Drug | CT Localizationa | CT phase | Comparatorb | Type of CT | Financed by industry? | First/corresponding author sex | Women/Total patientsc | Women percentagesc |
|---|---|---|---|---|---|---|---|---|---|
| Coles et al., 2008 (CAMMS23II study)19 | Alemtuzumab | International | II | Interferon-beta-1a | Efficacy/Safety | Yes, employees | Male | 214/334 | 64.1% |
| Coles et al., 201120 | Alemtuzumab | Post hoc analysis of CAMMS23II study (Coles et al., 2008) | II | Interferon-beta-1a | Efficacy/Safety | Yes, employees | Male | – | –- |
| Cuker et al., 201121 | Alemtuzumab | Post hoc analysis of CAMMS23II study (Coles et al., 2008) | II | Interferon-beta-1a | Safety | Yes, employees | Male | – | –- |
| Cohen et al., 2012 (CARE-MS I study)22 | Alemtuzumab | International | III | Interferon-beta-1a | Efficacy/Safety | Yes, employees | Male | 365/563 | 64.8% |
| Coles et al., 201223 | Alemtuzumab | Post hoc analysis of CAMMS23II study (Coles et al., 2008) | III | Interferon-beta-1a | Efficacy/Safety | Yes, employees | Male | – | –- |
| Coles et al., 2012 (CARE-MS II study)24 | Alemtuzumab | International | III | Interferon-beta-1a | Efficacy/Safety | Yes, employees | Male | 532/798 | 66.7% |
| Graves et al., 201325 | Alemtuzumab | Post hoc analysis of CAMMS23II study (Coles et al., 2008) | II | Interferon-beta-1a | Efficacy | Yes, employees | Female | – | –- |
| Daniels et al., 201426 | Alemtuzumab | Post hoc analysis of CAMMS23II study (Coles et al., 2008) | II | Interferon-beta-1a | Safety | Yes, employees | Male | – | –- |
| Arnold et al., 201627 | Alemtuzumab | Post hoc analysis of CARE-MS I (Cohen et al., 2012) and CARE-MS II (Coles et al., 2012) studies | III | Interferon-beta-1a | Efficacy | Male | – | –- | |
| Fox et al., 201628 | Alemtuzumab | Post hoc analysis of CAMMS23II study (Coles et al., 2008) | II | Interferon-beta-1a | Efficacy/Safety | Yes, employees | Male | – | –- |
| Giovannoni et al., 201629 | Alemtuzumab | Post hoc analysis of CARE-MS II study (Coles et al., 2012) | III | Interferon-beta-1a | Efficacy | Yes, employees | Male | – | –- |
| Arroyo et al., 201730 | Alemtuzumab | Post hoc analysis of CARE-MS I (Cohen et al., 2012) and CARE-MS II (Coles et al., 2012) studies | III | Interferon-beta-1a | Efficacy/Safety | Yes, employees | Male | – | –- |
| Havrdova et al., 201731 | Alemtuzumab | Post hoc analysis of CARE-MS I study (Cohen et al., 2012) | III | Interferon-beta-1a | Efficacy/Safety | Yes, employees | Male | – | –- |
| Li et al., 201832 | Alemtuzumab | Post hoc analysis of CARE-MS I (Cohen et al., 2012), CARE-MS II (Coles et al., 2012) and CAMMS23II studies (Coles et al., 2008) | II/III | Interferon-beta-1a | PK/PDc | Yes, employees | Male | – | –- |
| Miller et al., 200333 | Natalizumab | International | II | Placebo | Efficacy/Safety | No | Male | 152/213 | 71.4% |
| Dalton et al., 200434 | Natalizumab | International | III | Placebo | Efficacy | Yes, employees | Female | 53/78 | 67.9% |
| O’Connor et al., 200435 | Natalizumab | International | II | Placebo | Efficacy/Safety | No | Male | 33/180 | 18.3% |
| O’Connor et al., 200536 | Natalizumab | Post hoc analysis (Miller et al., 2003) | II | Placebo | Efficacy | No | Male | – | –- |
| Polman et al., 2006 (AFFIRM study) 37 | Natalizumab | International | III | Placebo | Efficacy/Safety | Yes, employees | Male | 660/942 | 70.1% |
| Rudick et al., 2006 (SENTINEL study)38 | Natalizumab | International | III | Interferon-beta-1a | Efficacy/Safety | Yes, employees | Male | 862/1171 | 73.7% |
| Balcer et al., 200739 | Natalizumab | Post hoc analysis of AFFIRM (Polman et al., 2006) and SENTINEL (Rudick et al., 2006) studies | III | Interferon-beta-1a | Efficacy/Safety | Yes, employees | Female | – | –- |
| Calabresi et al., 200740 | Natalizumab | Post hoc analysis of AFFIRM (Polman et al., 2006) and SENTINEL (Rudick et al., 2006) studies | III | Interferon-beta-1a | Efficacy/Safety | Yes, employees | Male | – | –- |
| Soon et al., 200741 | Natalizumab | Post hoc analysis of AFFIRM (Polman et al., 2006) | III | Placebo | Efficacy | No | Male | –- | –- |
| Miller et al., 200742 | Natalizumab | Post hoc analysis of AFFIRM study (Polman et al., 2006) | III | Placebo | Efficacy/Safety | Yes, employees | Male | – | –- |
| Rudick et al., 200743 | Natalizumab | Post hoc analysis of AFFIRM (Polman et al., 2006) and SENTINEL (Rudick et al., 2006) studies | III | Interferon-beta-1a | Efficacy/Safety | Yes, employees | Male | – | –- |
| Goodman et al., 200944 | Natalizumab | International | II | Glatiramer | Efficacy/Safety | Yes, employees | Male | 84/110 | 76.4% |
| Havrdova et al., 200945 | Natalizumab | Post hoc analysis of AFFIRM study (Polman et al., 2006) | III | Placebo | Efficacy/Safety | Yes, employees | Female | – | –- |
| Hutchinson et al., 200946 | Natalizumab | Post hoc analysis of AFFIRM (Polman et al., 2006) and SENTINEL (Rudick et al., 2006) studies | III | Interferon-beta-1a | Efficacy/Safety | Yes, employees | Male | – | –- |
| Radue et al., 201047 | Natalizumab | Post hoc analysis of SENTINEL (Rudick et al., 2006) study | III | Interferon-beta-1a | Efficacy/Safety | No | Male | – | –- |
| Bates et al., 201148 | Natalizumab | Post hoc-analysis of AFFIRM (Polman et al., 2006) and SENTINEL (Rudick et al., 2006) studies | III | Interferon-beta-1a | Efficacy | Yes, employees | Male | – | –- |
| Cree et al., 201149 | Natalizumab | Post hoc-analysis of AFFIRM (Polman et al., 2006) and SENTINEL (Rudick et al., 2006) studies | III | Placebo | Efficacy | No | Male | – | –- |
| Phillips et al., 201150 | Natalizumab | Post hoc analysis of AFFIRM study (Polman et al., 2006) | III | Placebo | Efficacy | Yes, employees | Male | – | – |
| Balcer et al., 201251 | Natalizumab | Post hoc analysis of AFFIRM (Polman et al., 2006) study | III | Placebo | Efficacy/Safety | Yes, employees | Female | – | – |
| Weinstock-Guttman el al, 201252 | Natalizumab | Post hoc-analysis of AFFIRM (Polman et al., 2006) and SENTINEL (Rudick et al., 2006) studies | III | Interferon-beta-1a | Efficacy | Yes, employees | Female | – | – |
| Kappos et al., 201353 | Natalizumab | Post hoc analysis of AFFIRM (Polman et al., 2006) and TOP studies | III | Placebo | Efficacy | Yes, employees | Male | – | – |
| Fox et al., 2014 (RESTORE study)54 | Natalizumab | International | IV | Interferon-beta-1a,Glatirameracetateormethylprednisolone | Efficacy | Yes, employees | Male | 135/175 | 77.1% |
| Kaufman et al., 201455 | Natalizumab | Post hoc analysis of RESTORE study (Fox et al., 2014) | IV | Interferon-beta-1a,Glatirameracetateormethylprednisolone | Efficacy | Yes, employees | Male | – | – |
| Lublin et al., 201456 | Natalizumab | Post hoc analysis of AFFIRM (Polman et al., 2006) study | III | Placebo | Efficacy/Safety | Yes, employees | Male | – | – |
| Chahin et al., 201557 | Natalizumab | Post hoc analysis of AFFIRM (Polman et al., 2006) study | III | Placebo | Efficacy/Safety | Yes, employees | Male | – | – |
| Kaufman et al., 201558 | Natalizumab | International | IV | Placebo | Efficacy | Yes, employees | Male | 51/60 | 85.0% |
| Plavina et al., 201759 | Natalizumab | Post hoc analysis of AFFIRM (Polman et al., 2006) and RESTORE (Fox et al., 2014) studies | III | Interferon-beta-1a,Glatirameracetateormethylprednisolone | PK/PDd | Yes, employees | Female | – | – |
| Saida et al., 201760 | Natalizumab | Japanese | II | Placebo | Efficacy/Safety | Yes, employees | Male | 73/106 | 68.9% |
| Kapoor et al., 201861 | Natalizumab | International | III | Placebo | Efficacy/Safety | Yes, employees | Male | 550/887 | 62.0% |
| Kappos et al., 201162 | Ocrelizumab | International | II | Interferon-beta-1a | Efficacy/Safety | Yes, employees | Female | 141/218 | 64.7% |
| Hauser et al., 2017 (OPERA I y OPERA II studies)63 | Ocrelizumab | International | III | Interferon-beta-1a | Efficacy/Safety | Yes, employees | Male | 1093/1656 | 66.0% |
| Montalban et al., 2017 (ORATORIO study)64 | Ocrelizumab | International | III | Placebo | Efficacy/Safety | Yes, employees | Male | 361/732 | 49.3% |
| Fox et al., 201865 | Ocrelizumab | Post hoc analysis of ORATORIO study (Montalban et al., 2017) | III | Placebo | Efficacy | Yes, employees | Male | – | – |
| Wolinsky et al., 201866 | Ocrelizumab | Post hoc analysis of ORATORIO study (Montalban et al., 2017) | III | Placebo | Efficacy | Yes, employees | Male | – | – |
| Barkhof et al., 201967 | Ocrelizumab | Post hoc analysis of OPERA I and OPERA II studies (Hauser et al., 2017) | II/III | Interferon-beta-1a | Efficacy | Yes, employees | Male | – | – |
| Mayer et al., 201968 | Ocrelizumab | Post hoc analysis of OPERA I and OPERA II studies (Hauser et al., 2017) and ORATORIO study (Montalban et al., 2017) | III | Placebo | Safety | Yes, employees | Female | – | – |
| Turner et al., 201969 | Ocrelizumab | Post hoc analysis of OPERA I and OPERA II studies (Hauser et al., 2017) | III | Interferon-beta-1a | Efficacy/Safety | Yes, employees | Male | – | – |
| Hauser et al., 200870 | Rituximab | International | II | Placebo | Efficacy/Safety | Yes, employees | Male | 81/104 | 77.9% |
| Hawker et al., 2009 (OLYMPUS study)71 | Rituximab | North America | II/III | Placebo | Efficacy/Safety | Yes, employees | Female | 221/435 | 50.8% |
| Zhang et al., 201372 | Rituximab | Post hoc of OLYMPUS study (Hawker et al., 2009) | II/III | Placebo | Efficacy | Yes, employees | Female | – | – |
| Honce et al., 201973 | Rituximab | USA | II | Placebo | Efficacy | No | Male | 37/53 | 69.8% |
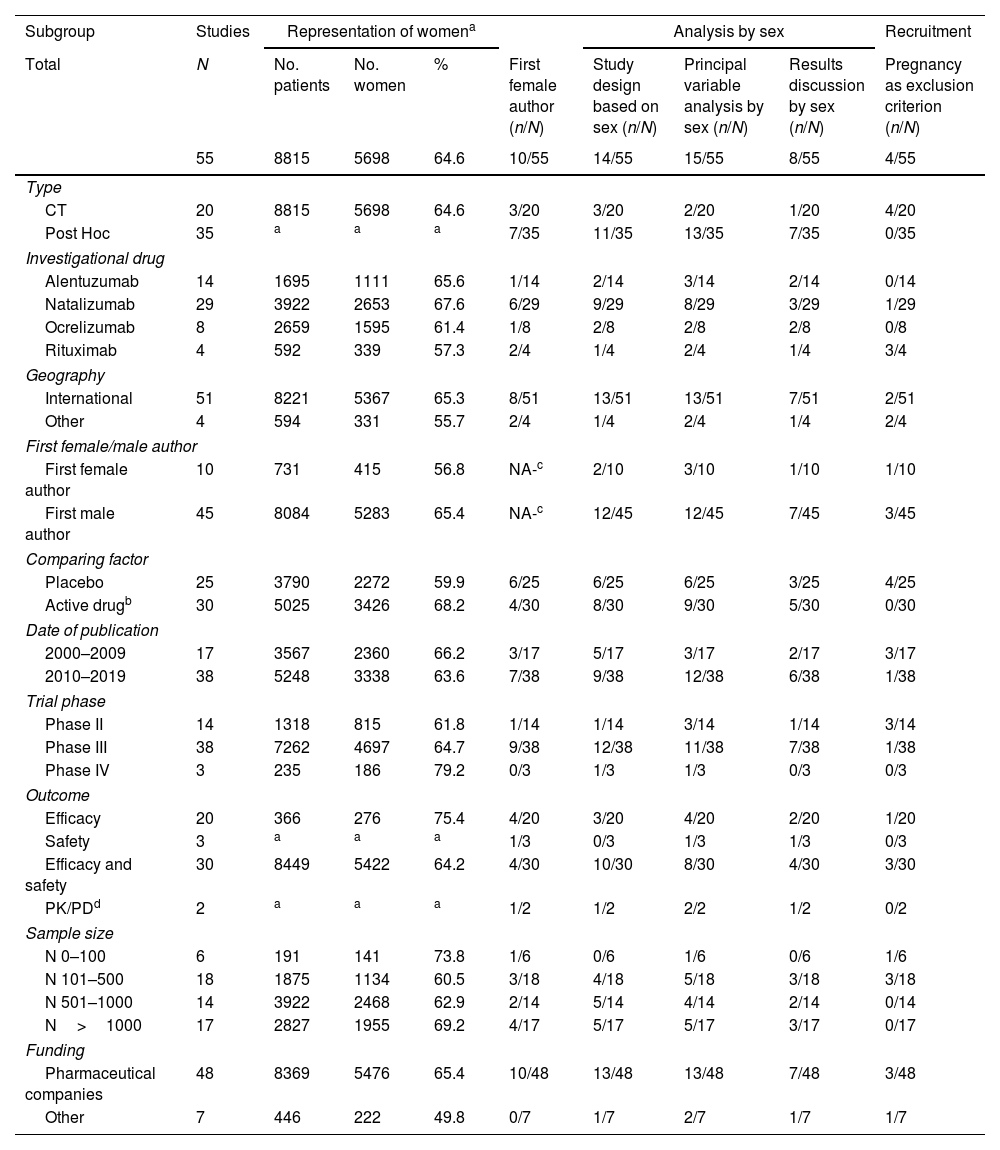
Table 4 shows the proportion of women and the evaluation of the results based on sex in the different subgroups of the CTs.
Proportion of women and other characteristics of gender allocation according to different subgroups.
| Subgroup | Studies | Representation of womena | Analysis by sex | Recruitment | |||||
|---|---|---|---|---|---|---|---|---|---|
| Total | N | No. patients | No. women | % | First female author (n/N) | Study design based on sex (n/N) | Principal variable analysis by sex (n/N) | Results discussion by sex (n/N) | Pregnancy as exclusion criterion (n/N) |
| 55 | 8815 | 5698 | 64.6 | 10/55 | 14/55 | 15/55 | 8/55 | 4/55 | |
| Type | |||||||||
| CT | 20 | 8815 | 5698 | 64.6 | 3/20 | 3/20 | 2/20 | 1/20 | 4/20 |
| Post Hoc | 35 | a | a | a | 7/35 | 11/35 | 13/35 | 7/35 | 0/35 |
| Investigational drug | |||||||||
| Alentuzumab | 14 | 1695 | 1111 | 65.6 | 1/14 | 2/14 | 3/14 | 2/14 | 0/14 |
| Natalizumab | 29 | 3922 | 2653 | 67.6 | 6/29 | 9/29 | 8/29 | 3/29 | 1/29 |
| Ocrelizumab | 8 | 2659 | 1595 | 61.4 | 1/8 | 2/8 | 2/8 | 2/8 | 0/8 |
| Rituximab | 4 | 592 | 339 | 57.3 | 2/4 | 1/4 | 2/4 | 1/4 | 3/4 |
| Geography | |||||||||
| International | 51 | 8221 | 5367 | 65.3 | 8/51 | 13/51 | 13/51 | 7/51 | 2/51 |
| Other | 4 | 594 | 331 | 55.7 | 2/4 | 1/4 | 2/4 | 1/4 | 2/4 |
| First female/male author | |||||||||
| First female author | 10 | 731 | 415 | 56.8 | NA-c | 2/10 | 3/10 | 1/10 | 1/10 |
| First male author | 45 | 8084 | 5283 | 65.4 | NA-c | 12/45 | 12/45 | 7/45 | 3/45 |
| Comparing factor | |||||||||
| Placebo | 25 | 3790 | 2272 | 59.9 | 6/25 | 6/25 | 6/25 | 3/25 | 4/25 |
| Active drugb | 30 | 5025 | 3426 | 68.2 | 4/30 | 8/30 | 9/30 | 5/30 | 0/30 |
| Date of publication | |||||||||
| 2000–2009 | 17 | 3567 | 2360 | 66.2 | 3/17 | 5/17 | 3/17 | 2/17 | 3/17 |
| 2010–2019 | 38 | 5248 | 3338 | 63.6 | 7/38 | 9/38 | 12/38 | 6/38 | 1/38 |
| Trial phase | |||||||||
| Phase II | 14 | 1318 | 815 | 61.8 | 1/14 | 1/14 | 3/14 | 1/14 | 3/14 |
| Phase III | 38 | 7262 | 4697 | 64.7 | 9/38 | 12/38 | 11/38 | 7/38 | 1/38 |
| Phase IV | 3 | 235 | 186 | 79.2 | 0/3 | 1/3 | 1/3 | 0/3 | 0/3 |
| Outcome | |||||||||
| Efficacy | 20 | 366 | 276 | 75.4 | 4/20 | 3/20 | 4/20 | 2/20 | 1/20 |
| Safety | 3 | a | a | a | 1/3 | 0/3 | 1/3 | 1/3 | 0/3 |
| Efficacy and safety | 30 | 8449 | 5422 | 64.2 | 4/30 | 10/30 | 8/30 | 4/30 | 3/30 |
| PK/PDd | 2 | a | a | a | 1/2 | 1/2 | 2/2 | 1/2 | 0/2 |
| Sample size | |||||||||
| N 0–100 | 6 | 191 | 141 | 73.8 | 1/6 | 0/6 | 1/6 | 0/6 | 1/6 |
| N 101–500 | 18 | 1875 | 1134 | 60.5 | 3/18 | 4/18 | 5/18 | 3/18 | 3/18 |
| N 501–1000 | 14 | 3922 | 2468 | 62.9 | 2/14 | 5/14 | 4/14 | 2/14 | 0/14 |
| N>1000 | 17 | 2827 | 1955 | 69.2 | 4/17 | 5/17 | 5/17 | 3/17 | 0/17 |
| Funding | |||||||||
| Pharmaceutical companies | 48 | 8369 | 5476 | 65.4 | 10/48 | 13/48 | 13/48 | 7/48 | 3/48 |
| Other | 7 | 446 | 222 | 49.8 | 0/7 | 1/7 | 2/7 | 1/7 | 1/7 |
Most of the studies were done with natalizumab, followed by alemtuzumab, ocrelizumab and rituximab. 6 trials had less than 100 patients, 18 between 101 and 500, 14 between 501 and 1000, and 17 studies had more than 1001 patients. Seventeen trials were published between 2000 and 2009 and 38 between 2010 and 2019. 92.73% of the trials were carried out worldwide. 14 trials were performed in phase II, 38 in phase III and 3 in phase IV. Something used as a standard for comparison was placebo in 25 trials and another active drug in the remaining studies. The trials measured the variables of efficacy and safety,30 efficacy,20 safety,3 PKPD parameters.2
Most of the studies (48/55) were financed by pharmaceutical companies. Moreover, in most of them (40/55) the main diagnosis was RRMS, in 3 studies it was RRMS, in 2 it was PRMS, in 1, it was PSMS and in 9 studies they included more than one type of MS. There were no clear differences between the studies that included one or more typologies related to the main variables.
Women represented 64.6% of all patients that were recruited in all CTs with a range of 18.3% to 85.0%. The first author of the publication was a woman in only 10 trials. The sex-separated analysis of the main variable was carried out in 15 out of the 55 included studies. Furthermore, only 8 CTs discussed the results based on gender. The exclusion of pregnant women is mentioned in only 4 of the 55 trials.
No trial meets any of the other 6 variables:
- -
analysis of the interaction between hormone replacement therapy and the active drug.
- -
inclusion of women using hormonal contraceptives.
- -
analysis of the interaction between hormonal contraceptives and the medication under study.
- -
analysis of the influence of the drug on the pharmacokinetics of hormonal contraceptives.
- -
analysis of the effects of the menstrual cycle phase on the pharmacodynamics of the drug.
- -
analysis of the influence of the phase of the menstrual cycle on the pharmacokinetics of the drug.
Our results show that, in the majority of the CTs of these four drugs, the percentage of women is higher than men. Contrary to what happens in similar reviews in other pathologies where women are often provided with insufficient or inadequate representation. This could be justified by the higher prevalence of the disease in women than men and because the incidence of RRMS in women is increasing.7 However, the ideal percentage of women in the trials should have been between 66% and 75% since the prevalence in women is usually 2 to 3 times higher than in men.
The data obtained is consistent, with a percentage of women of more than 50% in almost all studies. A progressive increase is observed in the phases of the CTs, with an inclusion of women of 61.83% in phase II and reaching almost 80% in phase IV. This issue could be related to the fact that previously, childbearing age women, never had to be admitted to phase I and II trials.75 However, during the 1990s and thanks to the NIH-dependent Research Office on Women's Health (ORWH) and the FDA,74 the previously mentioned prohibition was eliminated and the participation of women in all CTs was required; since the inclusion of women from the initial stages of drug development is essential for obtaining data on pharmacokinetic and pharmacodynamic differences according to gender; that lead the administration guideline in later phases.76
A lower gender bias is observed in studies funded by the pharmaceutical industry compared to independent studies. This could be explained because the development of monoclonal antibodies for MS is relatively recent and it seems that in the last few years there is a greater awareness of the pharmaceutical industry in following international recommendations that establish gender analysis as a minimum requirement of scientific validity to be able to extend the application of the results to the general patient population.15
The design of the study based on gender and analysis of the results arranged based on gender (main variable) is very rarely performed. The vast majority of the studies that analyze that design, are post hoc studies that come from original CT that do not take these variables into account. In fact, only one original CT discusses these results based on gender.71 However, the influence of factors related to gender and type should be previously determined on the basis of their hypothetical role in the efficacy of the treatment. Authors should refrain from conducting a post hoc gender analysis if the study design is insufficient to allow meaningful conclusions.9 Chilet et al. discuss the possibility that, in some trials, the analysis of the results arranged based on gender is performed, but this information is not finally published because, probably, the researchers do not find it relevant if no differences are found. However, knowing that there are no differences is also important.75
In two natalizumab CT where a sex-based analysis was performed, no significant differences were found in the efficacy of the natalizumab treatment between men and women.47,56 However, in Hutchinson et al. trial, natalizumab showed a significant reduction in the progression of MS in women compared to the interferon beta 1.46 In a clinical trial with 435 patients, it was determined that there are no differences in response to rituximab based on gender.71 With regard to ocrelizumab, two clinical trials also reveal the same clinical benefit of the ocrelizumab treatment in women and men.66,69 Finally, no significant differences were detected in the pharmacokinetics and pharmacodynamics of alemtuzumab according to gender,32 however, those differences were detected in the prevalence of thyroid disorders in patients treated with alemtuzumab (higher in women, 29.7% vs. 15.8% of men).26
We found that only four of the trials indicate that pregnancy has been a reason for exclusion. It is likely that in the rest of the trials it was also a reason for exclusion, although it was not explicitly stated in the text of the article. In any case, it would have been important to express it so as not to raise doubts. Previous studies have shown that pregnancy has a strong influence on MS activity with a reduction in the frequency of relapses. During the third trimester the relapse rate is even 70% lower than before pregnancy. Postpartum, MS often worsens, with an increase in relapses 3–6 months after giving birth.3,4 The improvement during pregnancy could be due to an increase in the proliferation of oligodendrocytes and in the number of myelinated axons in the maternal CNS, leading to greater remyelination.76
Furthermore, during pregnancy, sex hormones (estriol, progesterone, prolactin, and others) increase, producing an immunomodulatory and neuroprotective effect.76,77
Due to the lack of adequate and well-controlled studies of these four drugs in pregnant women, the FDA classifies all of them as category C, that is, the use during pregnancy should be avoided unless the potential benefit to the mother exceeds the potential risk to the fetus.78–81
As we have previously commented, no trial analyzes the interaction between hormonal contraceptives and the study drug. Because oral contraceptives contain estrogens (or estrogen receptor regulators) and progestogens, their influence on MS has been researched. Some studies suggest a positive effect of oral contraceptives, not only on symptoms but also on MRI activity and disease progression, while others were associated with a slightly increased risk of multiple sclerosis and clinical syndrome isolated.3
The low number of women as first authors of these studies are also noteworthy. Giovanni et al. concluded,82 that few women have reached the peak of academic neurology despite the availability of a considerable number of high-level academic neurologists and neuroscientists who have been successful worldwide. This could be due to the existence of gender bias in publication practices (editorial boards, scientific programs for professional meetings and recognition awards, etc.). However, it is curious that in studies where the first author is a woman, there is a lower representation of women and a greater gender bias.
The highlight of our work is that it is the first to study the gender differences of the biological drugs used in MS, considered one of the best options in certain types of MS, such as in PPMS, which continues to be developed to improve their efficacy tolerability and safety.
As the CT is the basic tool for evaluating the efficacy of drugs, the study population should be as real as possible. Therefore, women should be included in the study of drugs potentially used by them, the results should be shown sex-stratified results in the final drug approval reports and in the publication of CT, so that drug information can be obtained more personalized to promote a differential care for women and men if there is any difference in the efficacy or safety of the drugs between the two groups.
In the future, it would be desirable that the ethical committees do not allow the performance of CT which do not analyze gender in their design. Reviewers and editors of the journals also take this aspect into account as a requirement of scientific validity.
Author disclosure statementAll authors have approved the final version for publication; they are responsible and can guarantee that all the aspects that make up the manuscript, have been reviewed and discussed among the authors so that they are exposed with the utmost precision and integrity.
Funding statementThe present investigation has not received specific aid from agencies from the public sector, commercial sector or non-profit entities.
Conflict of interestsThe authors declare that they have no conflict of interest.