In this article, we present our experience on optic neuritis (ON) and provide a diagnostic/therapeutic protocol, intended to rule out other aetiologies (particularly infection), and a fact sheet for parents.
MethodsWe conducted a descriptive, retrospective study of patients with ON over a 27-year period (1990–2017). A review of the available scientific evidence was performed in order to draft the protocol and fact sheet.
ResultsOur neuropaediatrics department has assessed 20 744 patients in the last 27 years, of whom 14 were diagnosed with ON: 8 had isolated ON, 1 had multiple sclerosis (MS), 1 had clinically isolated syndrome (CIS), 3 had acute disseminated encephalomyelitis (ADEM), and 1 had isolated ON and a history of ADEM one year previously. Patients’ age range was 4–13 years; 50% were boys. Eight patients were aged over 10: 7 had isolated ON and 1 had MS. Nine patients had bilateral ON, and 3 had retrobulbar ON. MRI results were normal in 7 patients and showed involvement of the optic nerve only in 2 patients and optic nerve involvement+central nervous system demyelination in 5. Thirteen patients received corticosteroids. One patient had been vaccinated against Meningococcus-C the previous month. Progression was favourable, except in the patient with MS. A management protocol and fact sheet are provided.
ConclusionsON usually has a favourable clinical course. In children aged older than 10 years with risk factors for MS or optic neuromyelitis (hyperintensity on brain MRI, oligoclonal bands, anti-NMO antibody positivity, ON recurrence), the initiation of immunomodulatory treatment should be agreed with the neurology department. The protocol is useful for diagnostic decision-making, follow-up, and treatment of this rare disease with potentially major repercussions. The use of protocols and fact sheets is important.
Se presenta nuestra experiencia en neuritis óptica (ON) y se elabora un protocolo diagnóstico-terapéutico, que contempla descartar otras causas, principalmente infecciosas y se elabora una hoja informativa para padres.
Material y métodoEstudio descriptivo retrospectivo de los pacientes con ON en 27 años (1990–2017). Revisión de evidencia científica para elaboración del protocolo y hoja informativa.
ResultadosEn nuestra sección de neuropediatría se valoraron 20.744 niños en 27 años, 14 con ON: 8 ON aisladas, 1 esclerosis múltiple (EM), 1 episodio clínicamente aislado (CIS), 3 encefalomielitis agudas diseminadas (EMAD) y 1 paciente con ON aislada que el año anterior había sufrido una EMAD. Edades entre 4-13 años, 50% varones. Mayores de 10 años, 8 pacientes: 7 ON aisladas y 1EM. Bilaterales 9, retrobulbares 3. Resonancia magnética (RM) cerebral normal en 7, sólo afectación del nervio óptico en 2 y con desmielinización del SNC en 5 casos. Recibieron corticoterapia 13/14. Un caso vacunado de Meningococo-C el mes anterior. Todos evolucionaron favorablemente, salvo la EM. Se presentan el protocolo y la hoja de información.
ConclusionesHabitual curso favorable. En niños a partir de 10 años, con factores de riesgo de desarrollar EM o neuromielitis óptica (presencia de hiperseñales en RM cerebral, bandas oligoclonales, anti-NMO, recurrencia de ON), se consensúa con Neurología el inicio de tratamiento inmunomodulador. Utilidad del protocolo para la toma de decisiones diagnósticas, de seguimiento y tratamiento, de una patología poco frecuente pero con posibles repercusiones importantes. Importancia de la protocolización y hojas informativas.
Optic neuritis (ON) is an inflammatory demyelinating disease that causes acute vision loss (usually monocular), pain upon eye movement, and colour vision loss.
ON may occur in isolation, as recurrent episodes of vision loss (recurrent ON), or as one of several symptoms of demyelination associated with acute disseminated encephalomyelitis (ADEM); it may also appear in association with transverse myelitis in patients with neuromyelitis optica (NMO) or as the initial clinical manifestation of multiple sclerosis (MS).1
The condition affects mainly women (male-to-female ratio of 2:3) aged 20–40 years.2,3 The incidence of ON in paediatric patients is unknown, although it is thought to be considerably lower than in adults.
We analyse 27 years of experience at a paediatric neurology unit and present a management protocol and an information sheet for parents.
Material and methodsWe conducted a retrospective, descriptive study of patients diagnosed with ON and included in the database of the paediatric neurology unit at Hospital Infantil Universitario Miguel Servet in Zaragoza, Spain, from May 1990 (when the unit was created) to August 2017.4,5 Hospital Infantil Universitario Miguel Servet is a tertiary care hospital with 170 beds for paediatric patients; the paediatric neurology unit serves as the reference unit for the healthcare district of Aragon, La Rioja, and Soria. The database includes all patients assessed by the paediatric neurology unit, either on an outpatient basis or during hospitalisation. Diagnosis of ON was based on clinical symptoms and physical and ophthalmological examination results.
We reviewed these patients’ medical histories to gather data on the year of diagnosis, age at diagnosis, sex, symptoms, ophthalmological examination results, complementary tests, differential diagnoses, possible trigger factors, hospitalisation time, treatment, and outcome. In August 2017 we telephoned the patients who were no longer attended at our unit to ascertain whether they had experienced any further neurological or ophthalmological symptoms.
We designed and implemented a diagnostic and treatment protocol based on current scientific evidence; the protocol was designed according to the consensus of all medical professionals involved in the management of these patients (paediatric neurologists, paediatric ophthalmologists, neuroradiologists, and neurologists).
We also drafted an information sheet for parents, providing information about the disease, the tests involved, treatment options, and outcomes.
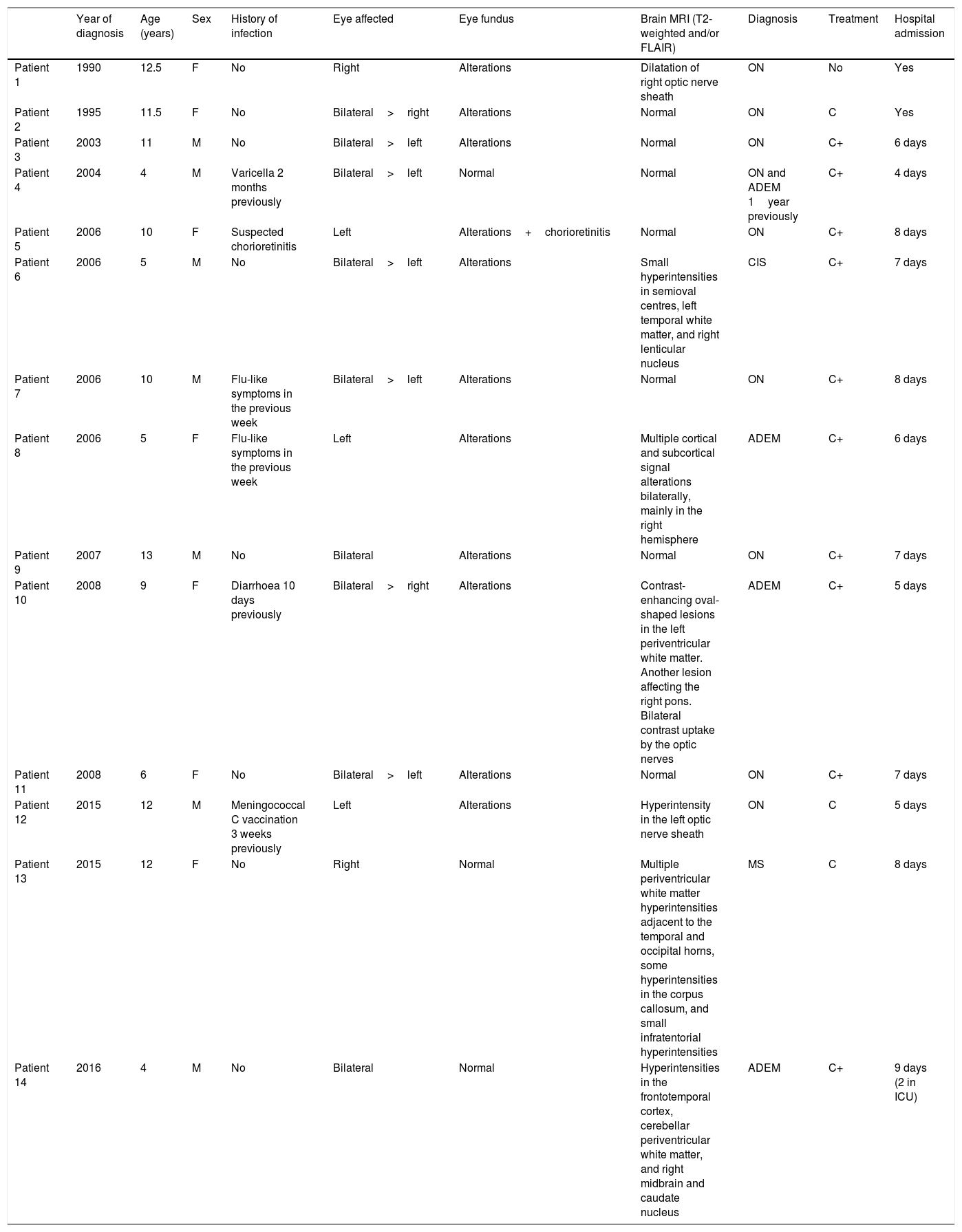
ResultsThe unit has evaluated 20 744 children over the past 27 years. Fourteen of these patients were diagnosed with ON: 8 with isolated ON, one with MS, one with clinically isolated syndrome, 3 with ADEM, and one with isolated ON who had presented ADEM the previous year. Age at diagnosis ranged from 4 to 13 years; half of the patients were male. Eight patients were older than 10 years: 7 had isolated ON and one had MS. Optic nerve involvement was bilateral in 9 patients and retrobulbar in 3. Brain MRI findings were normal in 7 patients, compatible with optic nerve involvement in 2 patients, and indicative of CNS demyelination in 5. Thirteen patients received corticosteroids, with different treatment schedules. Hospitalisation time ranged from 4 to 8 days. All patients progressed favourably, presenting no recurrences or sequelae, except for the patient with MS (patient 13). Patients were contacted by telephone in August 2017; none had presented any further recurrences or complications. Mean follow-up time (from diagnosis until August 2017) was 9.7 years (SD: 4.8 years; range, 17 months to 16.9 years). Table 1 shows the demographic and clinical characteristics of our sample.
Characteristics of our sample.
| Year of diagnosis | Age (years) | Sex | History of infection | Eye affected | Eye fundus | Brain MRI (T2-weighted and/or FLAIR) | Diagnosis | Treatment | Hospital admission | |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 1990 | 12.5 | F | No | Right | Alterations | Dilatation of right optic nerve sheath | ON | No | Yes |
| Patient 2 | 1995 | 11.5 | F | No | Bilateral>right | Alterations | Normal | ON | C | Yes |
| Patient 3 | 2003 | 11 | M | No | Bilateral>left | Alterations | Normal | ON | C+ | 6 days |
| Patient 4 | 2004 | 4 | M | Varicella 2 months previously | Bilateral>left | Normal | Normal | ON and ADEM 1year previously | C+ | 4 days |
| Patient 5 | 2006 | 10 | F | Suspected chorioretinitis | Left | Alterations+chorioretinitis | Normal | ON | C+ | 8 days |
| Patient 6 | 2006 | 5 | M | No | Bilateral>left | Alterations | Small hyperintensities in semioval centres, left temporal white matter, and right lenticular nucleus | CIS | C+ | 7 days |
| Patient 7 | 2006 | 10 | M | Flu-like symptoms in the previous week | Bilateral>left | Alterations | Normal | ON | C+ | 8 days |
| Patient 8 | 2006 | 5 | F | Flu-like symptoms in the previous week | Left | Alterations | Multiple cortical and subcortical signal alterations bilaterally, mainly in the right hemisphere | ADEM | C+ | 6 days |
| Patient 9 | 2007 | 13 | M | No | Bilateral | Alterations | Normal | ON | C+ | 7 days |
| Patient 10 | 2008 | 9 | F | Diarrhoea 10 days previously | Bilateral>right | Alterations | Contrast-enhancing oval-shaped lesions in the left periventricular white matter. Another lesion affecting the right pons. Bilateral contrast uptake by the optic nerves | ADEM | C+ | 5 days |
| Patient 11 | 2008 | 6 | F | No | Bilateral>left | Alterations | Normal | ON | C+ | 7 days |
| Patient 12 | 2015 | 12 | M | Meningococcal C vaccination 3 weeks previously | Left | Alterations | Hyperintensity in the left optic nerve sheath | ON | C | 5 days |
| Patient 13 | 2015 | 12 | F | No | Right | Normal | Multiple periventricular white matter hyperintensities adjacent to the temporal and occipital horns, some hyperintensities in the corpus callosum, and small infratentorial hyperintensities | MS | C | 8 days |
| Patient 14 | 2016 | 4 | M | No | Bilateral | Normal | Hyperintensities in the frontotemporal cortex, cerebellar periventricular white matter, and right midbrain and caudate nucleus | ADEM | C+ | 9 days (2 in ICU) |
ADEM: acute disseminated encephalomyelitis; C: intravenous methylprednisolone; C+: intravenous methylprednisolone followed by oral prednisone; CIS: clinically isolated syndrome; F: female; ICU: intensive care unit; M: male; MS: multiple sclerosis; ON: isolated optic neuritis.
All patients underwent brain MRI scans, an ophthalmological examination, CSF cytochemical and microbiological studies, and blood serology testing.
All patients showed decreased visual acuity at the time of diagnosis. All but 2 patients fully recovered visual function within weeks. All patients showed reduced retinal nerve fibre layer thickness throughout follow-up, regardless of whether optical coherence tomography (OCT) had detected oedema in the retinal nerve fibre layer at the time of diagnosis. At diagnosis, visual field campimetry revealed a diffuse reduction in sensitivity in one patient, centrocecal scotoma in 2, concentric visual field defect in one, and an enlarged blind spot in another patient. Visual evoked potentials were recorded at diagnosis for 8 patients (patients 4, 6, 8, 9, 10, 11, 12, and 13); alterations were observed in all cases.
The 2 most recent patients (patients 13 and 14) underwent a CSF autoimmunity study and oligoclonal band testing. Patient 14 was a 4-year-old boy with ADEM presenting anti-MOG antibodies in the CSF; he tested negative for other antibodies, including anti-NMO antibodies (anti-AQP4 antibodies).
Patient 13 was a 12-year-old girl who reported blurred vision, which was bilateral at onset and subsequently became more marked in the right eye, in addition to headache and dizziness. She developed strabismus in the right eye. Eye fundus examination revealed no alterations; campimetry detected vision loss in the right eye. MRI showed numerous white matter hyperintensities, which were predominantly periventricular and mainly located adjacent to the temporal and occipital horns, with some corpus callosum and infratentorial hyperintensities. The patient tested positive for CSF oligoclonal bands and negative for blood anti-NMO antibodies. Despite treatment with intramuscular interferon beta–1a dosed at 30μg per week, the patient presented 2 relapses (at one and 5 months), which were managed with intravenous methylprednisolone for 5 days. During the relapses, the patient presented vertigo and sixth nerve palsy, but no ON recurrences. Interferon beta–1a was replaced with oral fingolimod dosed at 0.5mg daily. The patient remains stable; an MRI scan conducted 4 months ago showed a decrease in the size of the lesions, with no additional hyperintensities.
Patient 4 was a 4-year-old boy with retrobulbar ON and normal MRI results who had presented varicella 2 months previously. One year earlier, the patient had been admitted to our hospital due to ADEM, which had resolved with no sequelae; a T2-weighted MRI scan revealed multiple disseminated hyperintensities involving the cerebral peduncle, pons, periaqueductal region, right lenticular nucleus, internal capsule, left globus pallidus, and bilateral paraventricular subcortical white matter.
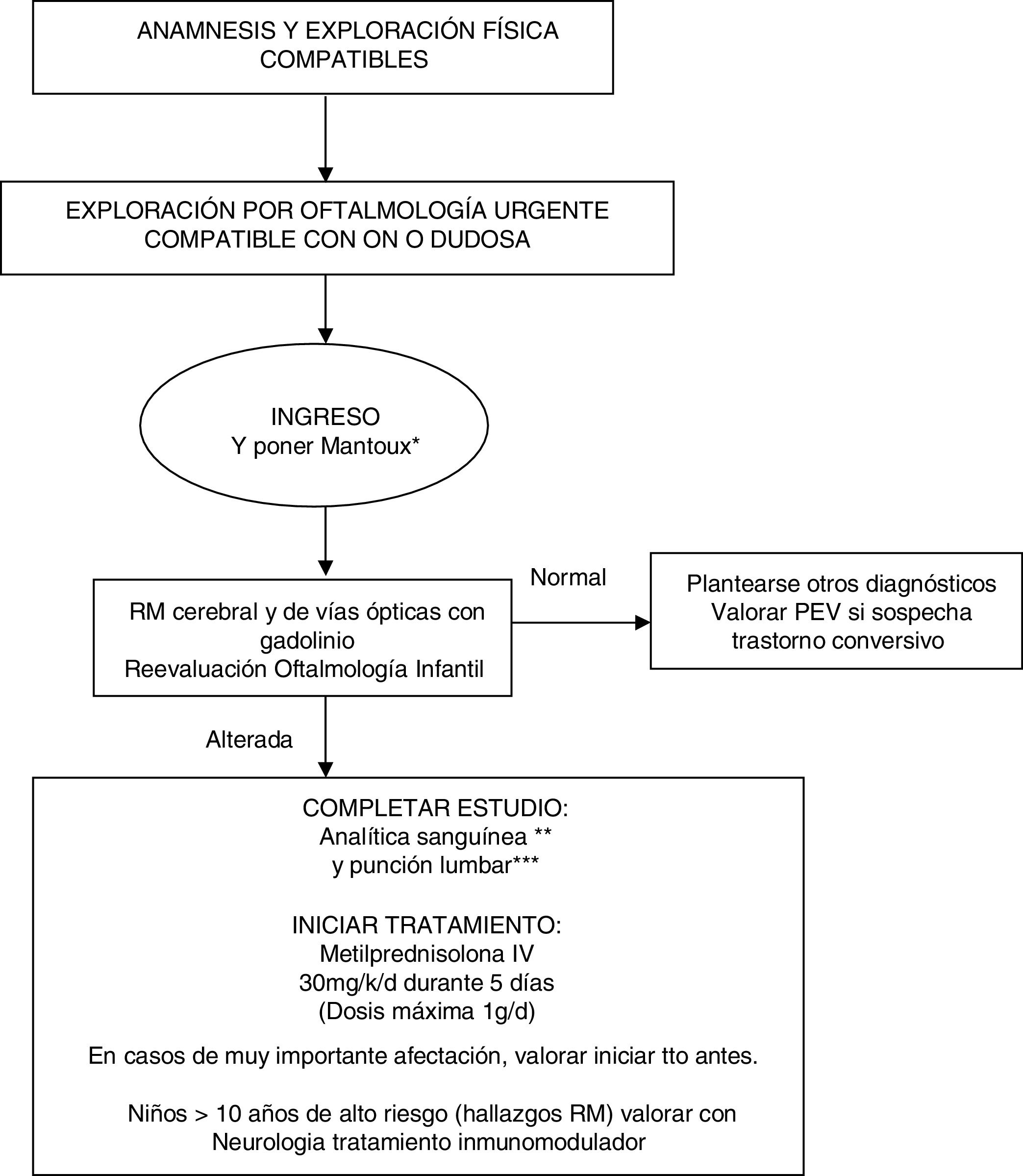
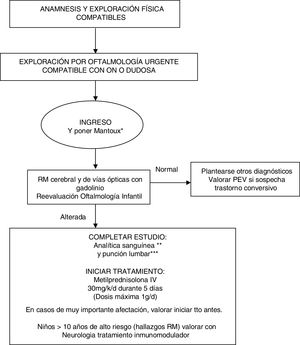
Our evidence-based management algorithm and the information sheet for parents are shown in Fig. 1 and Fig. 2.
Management algorithm for treating children with suspected optic neuritis.
* Routine Mantoux test in view of the possible need for high-dose corticosteroid therapy.
** Blood analysis: complete blood count, C-reactive protein, erythrocyte sedimentation rate, biochemical analysis, ions, creatinine, liver profile, albumin, protein test, immunoglobulins, thyroid hormones, carcinoembryonic antigen test, vitamin B12, folic acid, vitamin D, autoimmunity study (ANA, etc), oligoclonal bands, anti-NMO antibodies. Blood serology: neurotropic viruses (CMV, EBV, herpes simplex, enterovirus), Mycoplasma, Borrelia, Brucella, Chlamydia, syphilis, and HIV.
*** CSF analysis: cell count, biochemical analysis, adenosine deaminase, protein test, immunoglobulins, and oligoclonal bands. Gram stain, viral and bacterial cultures.
IV: intravenous; MRI: magnetic resonance imaging; MS: multiple sclerosis; ON: optic neuritis; VEP: visual evoked potentials.
The clinical characteristics of ON in adults were systematically described in the Optic Neuritis Treatment Trial,3 which included 457 patients with unilateral acute ON aged 18–46 years. The condition is characterised by vision loss developing within 2 weeks, accompanied with pain exacerbated by eye movement. Eye movement may also trigger photopsia. Colour vision loss disproportionate to the loss of visual acuity is specific to optic nerve involvement; the most common visual field defect is central scotoma.
ON is rare in children and has different clinical characteristics to ON in adults.6 In 39% to 60% of cases, childhood ON is associated with viral infection, although the pathogen is rarely identified. Some cases of ON have been described within 30 days of a vaccination, as in patient 12 from our sample, who had been vaccinated against meningococcal C disease; however, this occurs in less than 5% children with ON.7 Unlike in adults, involvement is usually bilateral8 and the condition is frequently associated with papillitis: in our series, 9 patients showed bilateral involvement and 11 presented papillitis.9,10
Diagnosis is based on medical history and examination results. Ophthalmological examination is essential: the results of an eye fundus examination, combined with clinical signs, may help distinguish typical and atypical cases. To evaluate visual involvement, we recommend using both structural (OCT) and functional (visual evoked potentials and visual field) diagnostic techniques.11 Over the last decade, the assessment protocol for ON includes OCT for the study of the retina, especially of the retinal nerve fibre layer and the ganglion cell layer. Visual evoked potentials are of little help in acute episodes; therefore, in our protocol, they are only used in uncertain cases or when conversion disorder is suspected.
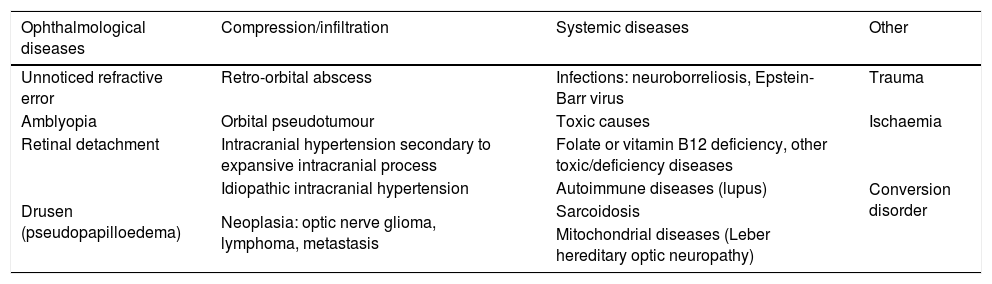
Table 2 lists a number of processes that should be included in the differential diagnosis of ON in children.
Differential diagnosis of optic neuritis.
| Ophthalmological diseases | Compression/infiltration | Systemic diseases | Other |
|---|---|---|---|
| Unnoticed refractive error | Retro-orbital abscess | Infections: neuroborreliosis, Epstein-Barr virus | Trauma |
| Amblyopia | Orbital pseudotumour | Toxic causes | Ischaemia |
| Retinal detachment | Intracranial hypertension secondary to expansive intracranial process | Folate or vitamin B12 deficiency, other toxic/deficiency diseases | Conversion disorder |
| Drusen (pseudopapilloedema) | Idiopathic intracranial hypertension | Autoimmune diseases (lupus) | |
| Neoplasia: optic nerve glioma, lymphoma, metastasis | Sarcoidosis | ||
| Mitochondrial diseases (Leber hereditary optic neuropathy) |
A gadolinium-enhanced MRI scan of the brain and optic pathway confirms diagnosis in most cases; in our series, however, 50% of MRI scans revealed no alterations, which may be due to the fact that some of the cases are very old and imaging techniques were less advanced than today. MRI may also help establish the risk of MS by detecting characteristic lesions (oval, periventricular white matter lesions measuring over 3mm). The prevalence of white matter alterations in adults with ON ranges from 23% to 75%.12 These patients are at greater risk of developing MS; evidence from children seems to support the same hypothesis.13,14
Blood and CSF analyses help rule out other causes, which are mainly infectious; antibody testing provides prognostic information and assists in treatment decision making. Presence of oligoclonal bands in the CSF indicates increased risk of MS, although it has also been associated with MRI white matter lesions; therefore, oligoclonal bands are not of clear prognostic importance.15 Presence of anti-AQP4 antibodies in the serum is associated with increased risk of NMO, particularly in patients with normal brain MRI results and those presenting recurrent ON or severe vision loss.16–18 For this reason, our protocol includes anti-AQP4 antibody testing as a routine procedure. Our protocol does not include determination of serum anti-MOG antibody levels; this information is available for patient 14 as he was included in another research project requiring blood samples. There is evidence that anti-MOG antibodies may appear transiently in nearly 50% of children with ADEM, although they may also be detected in other demyelinating processes; tests for anti-MOG antibodies are not readily available in most hospitals.19
All clinical trials of treatments for ON have included adult patients; these studies recommend intravenous methylprednisolone in patients with severe vision loss and/or bilateral involvement, since the drug may accelerate recovery. Methylprednisolone is also recommended for patients showing brain MRI alterations, as it seems to delay onset of MS.20 Oral prednisone is not recommended for ON as it increases the risk of recurrence. Although the association between oral prednisone and recurrent ON is reported in a single clinical trial,21 this finding was unexpected and its pathophysiological basis is not understood.
Children seem to have a lower risk of recurrent ON and MS.22,23 No clinical trial of treatments for ON has included paediatric patients; information about ON management in children is therefore scarce. As with adults, intravenous methylprednisolone seems to accelerate visual recovery in children.24–26 The published series suggest that visual prognosis is not altered, although determining the natural history of ON without treatment is not straightforward in children as corticosteroids are administered early in clinical practice.
Mild episodes not limiting the patient’s activity or interfering with school attendance do not require corticosteroids. Children usually receive intravenous infusion of methylprednisolone dosed at 20–30mg/kg (maximum of 1g per dose) for 3–5 days.1 If the patient recovers visual function after treatment, no oral treatment is needed. If the patient improves but does not fully recover vision, prednisone should be continued at 1–2mg/kg/day, reducing the dose by 5mg every 3–5 days for 14–21 days. The risk of adrenal suppression is minimal. Other treatments for neuroimmune diseases include intravenous immunoglobulins and plasmapheresis. The efficacy of these treatments for patients with ON has not been determined. Children showing no response to corticosteroids may respond to intravenous immunoglobulins, although most data come from studies of children with multiple symptoms of demyelination or ADEM.27–30 Published series use a total dose of 2g/kg, administered over 2 to 5 days.
Due to several atypical cases of tuberculosis detected recently, our centre routinely performs the Mantoux test at admission, as these patients may need high-dose corticosteroid therapy.31 Patients treated for ON at our unit receive intravenous methylprednisolone, followed by oral prednisone in some cases. Continuation with oral treatment was not correlated with incomplete symptom resolution after intravenous treatment and was established arbitrarily, given the lack of a pre-established treatment protocol. This is also reflected in the wide range of complementary tests performed.
Deciding whether to start long-term immunomodulatory treatment to prevent, delay, or mitigate subsequent MS is an important clinical decision in adult patients with ON. The results of 3 randomised clinical trials (Controlled High-Risk Avonex MS Prevention Study, Early Treatment of Multiple Sclerosis Study, and PreCISe Study Trial)32–34 support the use of interferon beta or glatiramer acetate in patients with MRI findings indicating high risk of developing MS.
Immunomodulatory treatment with interferon is not usually administered to younger children given that paediatric patients are at less risk of MS; children older than 12 years, however, are managed according to the same guidelines as those used for adults.35
The percentage of patients diagnosed with MS among children with ON varies greatly: 13% at 10 years of follow-up in a series of 79 patients,22 36% at 2 years in a series of 36 patients (68% of whom showed at least one MRI lesion in an area other than the optic nerve during ON),9 and 32% of a series of 41 patients (4 of whom showed no neurological signs other than optic nerve involvement at disease onset).7
In a study conducted in France, in which 296 children were followed up for a mean of 2.9 years after a single episode of acute CNS demyelination, diagnosis of MS was confirmed in 57% of cases at the end of the study period.36 The factors recorded in the initial episode that were associated with increased risk of presenting a second demyelinating event were: age older than 10 years, optic nerve lesions, and MRI patterns characteristic of MS (multiple well-circumscribed perivascular or subcortical lesions). Factors associated with lower risk of relapses were presence of spinal cord lesions and acute alterations in mental status.
According to our protocol, onset of immunomodulatory treatment must be agreed with the neurology department for children older than 10 years presenting risk factors for MS or NMO (brain MRI hyperintensities, oligoclonal bands, anti-NMO antibodies, recurrent ON).
Developing a management protocol minimises variability in patient management and facilitates diagnosis, follow-up, and treatment of this rare though potentially severe condition.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Monge Galindo L, et al. Neuritis óptica en pediatría: experiencia en 27 años y protocolo de actuación. Neurología. 2021;36:253–261.
This study was presented at the 40th Annual Meeting of the Spanish Society of Paediatric Neurology.