In the present study, anticonvulsant effects of aqueous extract (AE), hydro-alcoholic crude extract (HE), and its fractions (F-CHCl3, F-EtOAc, F-MeOH) of Paeonia daurica subsp. macrophylla (P. daurica ssp. macrophylla) root examined by using a pentylenetetrazol-induced model (PTZ) on mice.
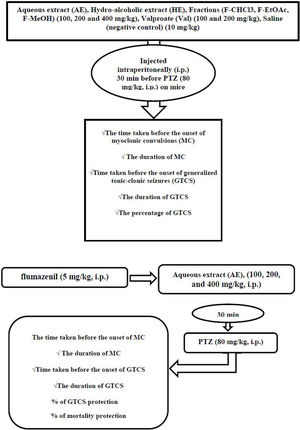
MethodsHE and its fractions as well as AE, in concentrations of (100, 200 and 400mg/kg), valproate (Val) (100 and 200mg/kg), and saline (negative control) (10mg/kg) were injected intraperitoneally (i.p.) 30min before PTZ (80mg/kg, i.p.). The time taken before the onset of myoclonic convulsions (MC), MC duration, time taken before the onset of generalized tonic-clonic seizures (GTCS), the duration of GTCS, and the percentage of GTCS and mortality protection recorded. The plant's anticonvulsant mechanisms were assessed using flumazenil (5mg/kg, i.p.) before AE (100, 200, and 400mg/kg, i.p.) injection. GraphPad Prism software was used to compare the differences between various treatment groups with one-way analysis of variance (ANOVA) followed by Tukey–Krammer multiple comparison tests.
ResultsAll the plant samples except F-EtOAc significantly delayed the onset and decreased the duration of PTZ-induced MCS and GTCS, and significantly reduced the GTCS and mortality rate. Pretreatment with flumazenil diminished the significant anticonvulsant effects of AE against PTZ-induced seizures.
ConclusionsIt can report that extract of P. daurica ssp. macrophylla might be a helpful guide for future studies in the treatment of epilepsy.
Epilepsia es el término usado para un grupo de trastornos caracterizado por las convulsiones espontáneas recurrentes. Un estudio enfocado en los productos naturales de los recursos tradicionales ofrece ventajas significativas que se están utilizando de manera más amplia en modelos animales de epilepsia y candidatos a mayor desarrollo clínico y sus fracciones (F-CHCl3, F-EtOAc, F-MeOH) de Paeonia daurica subsp. macrophylla (P. daurica ssp. macrophylla) raíz examinada utilizando un modelo inducido por pentilentetrazol (PTZ) en ratones.
MétodosLa maceración dinámica utilizada para extraer HE de la planta y técnica de cromatografía en columna de sílice utilizada para obtener F-CHCl3, F-EtOAc, así como fracciones de F-MeOH. La extracción de raíces secas se utilizó con agua destilada y se provocó AE. Las muestras de plantas (100, 200 y 400mg/kg), valproato (Val) (100 y 200mg/kg) y suero (control negativo) se inyectaron por vía intraperitoneal (ip) 30min antes de PTZ (80mg/kg, ip). El tiempo transcurrido antes del comienzo de convulsiones mioclónicas (MC), duración de las MC, tiempo transcurrido antes del comienzo de convulsiones tónico-clónicas generalizadas (GTCS), la duración de GTCS, así como el porcentaje de GTCS y protección contra la mortalidad registrada. Los mecanismos anticonvulsivos de planta fueron evaluados mediante el uso de flumazenil (5mg/kg, ip) antes de AE (100, 200 y 400mg/kg, ip) inyección. Se utilizaba el software GraphPad Prism® comparando las diferencias entre varios grupos de tratamiento con un análisis unilateral de variación (ANOVA) seguido por las pruebas de comparación múltiple de Tukey's Krammer.
ResultadosTodas las muestras de plantas, excepto F-EtOAc, retrasaron de manera considerable el inicio, y disminuyeron la duración de PTZ inducidos por MCS y GTCS, y redujo significativamente el GTCS, así como la tasa de mortalidad. El tratamiento previo con flumazenil disminuyó los efectos anticonvulsivos importantes AE contra convulsiones inducidas por PTZ.
ConclusionesPuede concluirse que el extracto de P. daurica ssp. macrophylla podría ser útil en el tratamiento de la epilepsia en humanos.
Epilepsy is the third common neurological disorder after stroke and Alzheimer's disease.1 Over the last few decades, the incidence of epilepsy in the population has increased steadily, and this increase will add a socioeconomic burden on healthcare systems.2 The results of studies suggest that a considerable proportion of patients experience spontaneous remission. While seizures may be controlled with antiepileptic drugs in some patients, drug resistance may be seen in others 3.Furthermore, undesirable side effects of the clinically used drugs often render treatment difficult, so demand for new types of anticonvulsants exists.4 The medicinal effects of natural products are still considered a helpful subject for designing new drugs for human health.5 Among these, the biological effects of natural products, such as slowing down the progress of neurological diseases, as a significant source for drug discovery, have revolutionized.6Paeonia daurica subsp. macrophylla (Paeoniaceae) is a perennial plant with white and pink flowers growing in northern parts of Iran.7 About 25–40 Paeony species have been found in Asia, Southern Europe, and Eastern North America.8
The bioactivity of compounds from different parts of Paeonia species had been evaluated in different studies. There have been several studies on the antioxidative effect of Paeonia species of Chinese origin. P. lactiflora and P.suffruticosa and DPPH free radicals scavenging activity.9 Also, the study of isolated compounds like resveratrol from seeds of P. lactiflora on different cancer cell lines as well as six stilbenes had shown potent cytotoxic activity in a dose-dependent manner against C6 (mouse glioma) cancer cells and significant cytotoxic activity against HepG2 (liver hepatoma) and HT-29 (colon) human cancer cell lines respectively.10 Paeoniflorin also inhibited mitochondrial membrane potential dissipation, ATP loss, inactivation of complexes I and IV, cytochrome c release. In addition, paeoniflorin in one study prevented antimycin A-induced ROS release and nitrotyrosine increase. These results imply that paeoniflorin protects osteoblasts from antimycin A-induced cell death via improved mitochondrial function.11 Triterpenoids for their cytotoxic activities against human leukemia (HL-60), human hepatocellular carcinoma (HepG2), and human ovarian (SK-OV-3) cell lines were assessed.11,12 The results showed different cytotoxic activities against cell lines. A new terpenoid, palbinone, from Paeonia albiflora, had been found to have a strong inhibitory activity on the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH)-linked 3α-hydroxysteroid dehydrogenase (3α-HSD) of rat liver cytosol.13 Galloylpaeoniflorin, galloyloxypaeoniflorin, and suffruticosides A–D. showed more potent radical-scavenging effects on DPPH than a-tocopherol, and oxypaeoniflorin was found to exhibit a weak radical-scavenging.14
Roots of the different species of Paeony have been used as a traditional medicine for epilepsy since long back in the world.15Paeonia officinalis (P. officinalis), known as “Oode-Saleeb” in traditional Persian medicine, have been used for some diseases, especially epilepsy and brain disorders.16 Several components from this genus comprising monoterpenoids (pinane skeleton), triterpenoids, flavonoids, phenols, and tannins exhibit significant biological and pharmacological activities.17 In some species of Paeonia, anticoagulative, anti-inflammatory, hypoglycemic, and antiosteoporotic effects have been identified.18 According to past researches, the roots of the peony used as an anticonvulsant and antispasmodic as well as antimigraine agent in European, China and Japan medical systems.19,20 According to our knowledge, there is not enough information available regarding anticonvulsant effects of P. daurica subsp. macrophylla.
In this study, the anticonvulsant effects of aqueous extract (AE), hydro-alcoholic extract (HE), and its partitioned fractions ethyl-acetate (F-EtOAc), methanol (F-MeOH), and chloroform (F-CHCl3) from P. daurica subsp. macrophylla root induced by pentylenetetrazol (PTZ) animal model of mice was investigated.
Materials and methodsCollection and identification of the plantThe roots of P. daurica subsp. macrophylla was collected by the authors in July 2015 from Mazandaran province, Iran. The sample was identified by Prof. Gholamreza Amin and deposited at the herbarium of the pharmacy faculty, Tehran University of Medical Sciences, with the voucher number 6620-TEH.
Preparation of P. daurica subsp. macrophylla hydro-alcoholic extract (HE) and fractionsThe air-dried roots (430g) were powdered with a grinder and macerated three times with aqueous ethanol (EtOH) (80%) (Merck, Germany, each time for 48h). All hydro-alcoholic extracts were combined, filtrated, and concentrated to dryness by a rotary evaporator (Heidolph Laborota, Germany) at 40°C to give (89g, HE). The yield of the crude HE was about 20% (w/w). The HE (80g) was further partitioned by the column chromatography (1.5×22cm) method. The silica was used as an adsorbent (Mesh size 230–400 ASTM, Merck, Germany) and, the column was eluted with solvents comprising gradient volumes of n-hexane, EtOAc, CHCl3 and, MeOH (Merck, Germany). Then the residue was successively submitted to n-hexane (0.1g), F-EtOAc (2.26g), F-CHCl3 (2.06g), and F-MeOH (30.8g) fractions, respectively, according to their TLC profile and visualized spots using a sulfuric acid-anisaldehyde reagent. F-EtOAc, F-CHCl3, and F-MeOH fractions were selected along with HE for further evaluation and were taken up in saline containing Tween 80 (5%, v/v).
Preparation of P. daurica subsp. macrophylla aqueous extract (AE)The dried roots were suspended in distilled water (190×g dried roots per 2L water), and the mixture boiled for 60min at 70°C with occasional stirring. Aqueous extracts were combined, filtrated, and concentrated to dryness by a rotary evaporator (Heidolph Laborota, Germany) at 70°C. The obtained residue was cooled, filtered, and lyophilized (Virtis™ mobile freeze-dryer, model 125L) to give (29g, AE). The yield of the crude AE was about 15% (w/w). In addition, the final volume is stored until further evaluation. The AE was taken up in saline containing Tween 80 (5%, v/v).
Animals and treatmentsAt the beginning of the study, 126 male NMRI albino mice weighing 25±5g (Tehran University of Medical Sciences, Iran) were housed under a 12-h light/dark cycle with free access to pellet food and tap water (22°C). The Ethics and Animal Care Committee approved the experiment of Tehran University of Medical Sciences (IR.TUMS.VCR.REC.1395.687). In addition, this study was in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals.21
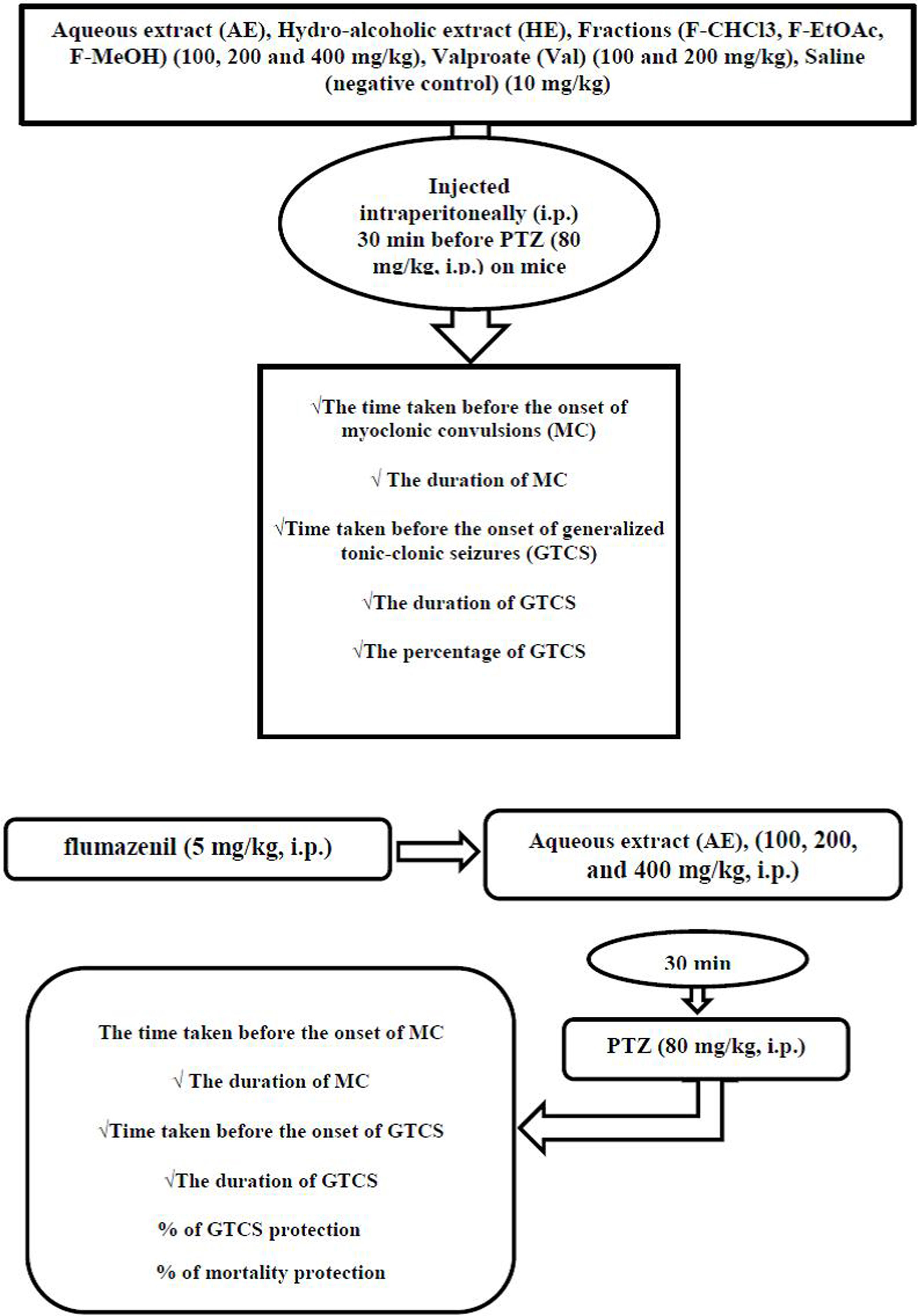
Induction of seizures and seizure observation proceduresAnimals were divided into eighteen groups of seven mice each. Group 1 was given 10mg/kg of sterile isotonic saline solution (negative control), and it was administered intraperitoneally (i.p.) 30min before PTZ administration. Groups 2 and 3 received 100mg/kg and 200mg/kg of valproate (Val) (Sigma, USA) as a positive control,22 diluted by the addition of sterile isotonic saline solution, and it was administered i.p. 30min before PTZ administration. The other groups (4–18) were given different doses of the treated extracts such as HE and fractions and AE (100, 200, and 400mg/kg; i.p.) 30min before each PTZ injection. Mice were observed for 30min after the last drug administration. Seizure intensity after PTZ injection evaluated using the six stages of Racine (RS) modified scale.23 (stage 0); “no response” (stage 1); “mouth and facial movements, hyperactivity and vibrissa twitching” (stage 2); “head nodding, head clonus and myoclonic jerk” (stage 3); “unilateral forelimb clonus” (stage 4); “rearing with bilateral forelimb clonus” (stage 5);”Generalized Tonic-Clonic Seizures (GTCS) with loss of righting reflex” and (stage 6); “mortality”. In the present study, 80mg/kg of PTZ (Sigma, USA) selected as a challenge dose produced convulsions (Tonic and Clonic Seizures (TCS)) and lethality. All groups tested for PTZ challenge dose (80mg/kg) induced seizures in mice.24 In this study, stages 1–4 of RS classification reported as onset and duration, latency time, of myoclonic seizures (MS), and stage 5 mentioned as onset and duration, latency time of GTCS. Otherwise, percent of GTCS protection and mortality rate recorded.
Anticonvulsant mechanism determinationIn order to investigate the probable involvement of GABAA receptors on the anticonvulsant effects of AE from P. daurica (the best anticonvulsant results), the flumazenil (a selective benzodiazepine receptor antagonist) and diazepam (a GABA receptor agonist as positive control) were used in 8 groups of 7 mice each.25 In the first four groups, mice were given AE (100, 200, and 400mg/kg; i.p.) and diazepam (2mg/kg; i.p.), respectively, 30min before the injection of PTZ (80mg/kg; i.p.). All the animals received flumazenil in the second four groups (5mg/kg; i.p.). After 5min, mice were given AE (100, 200, and 400mg/kg; i.p.) and diazepam (2mg/kg; i.p.), respectively, 30min before the injection of PTZ (80mg/kg; i.p.). Animals were observed for 30min after the last drug administration. Anticonvulsant factors such as onset and duration (30min observation) of MS, onset and duration (30min observation) of TCS, frequency, and the mortality rate in divided groups were assessed (Fig. 1).
Statistical analysisOne-way analysis of variance (ANOVA) followed by Tukey–Krammer multiple comparison tests compares the differences between various treatment groups using GraphPad Prism 5.01 (San Diego, CA). The statistical probability of p<0.05 was considered significant. Time measurements were reported as Mean ±SEM.
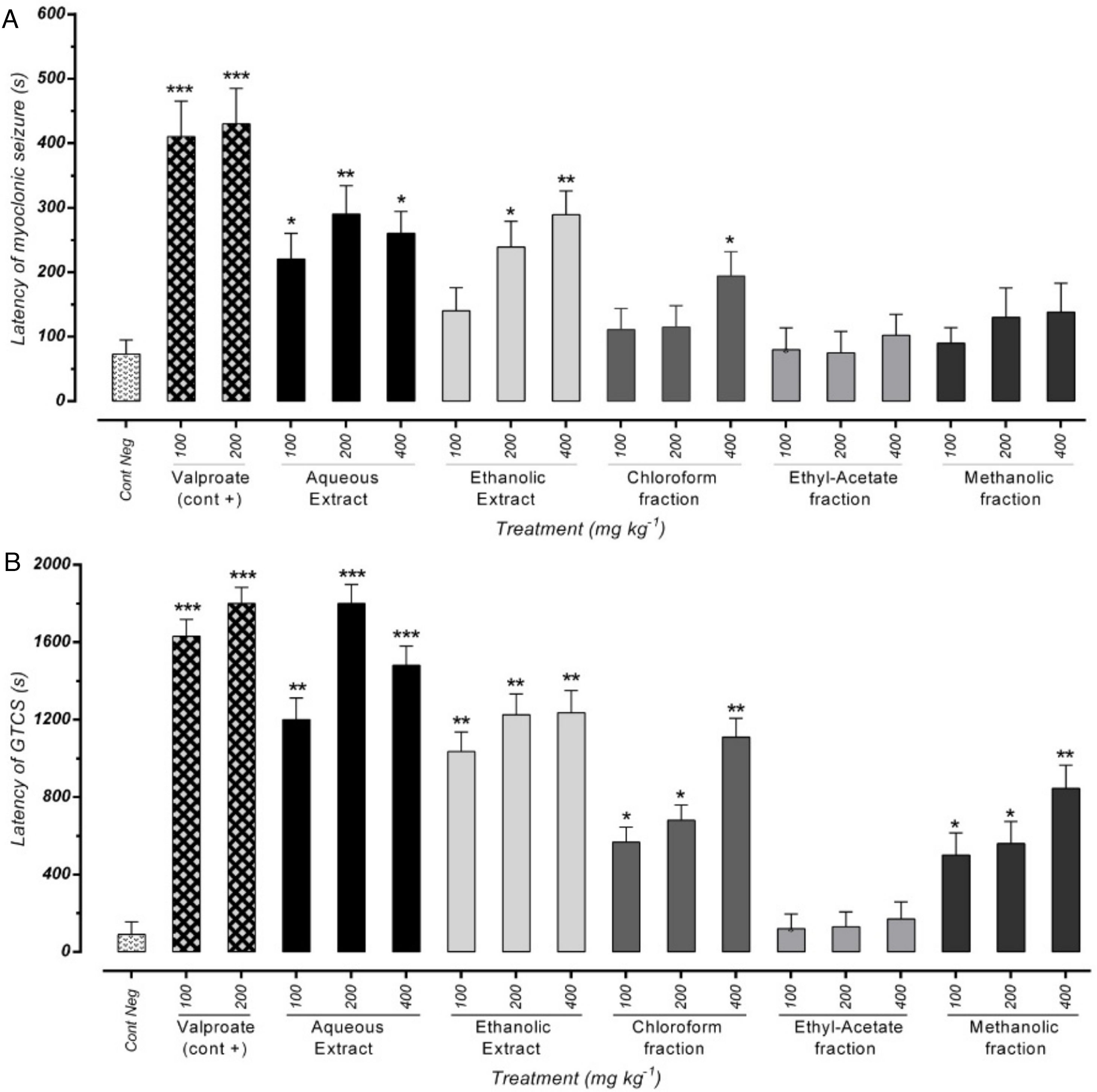
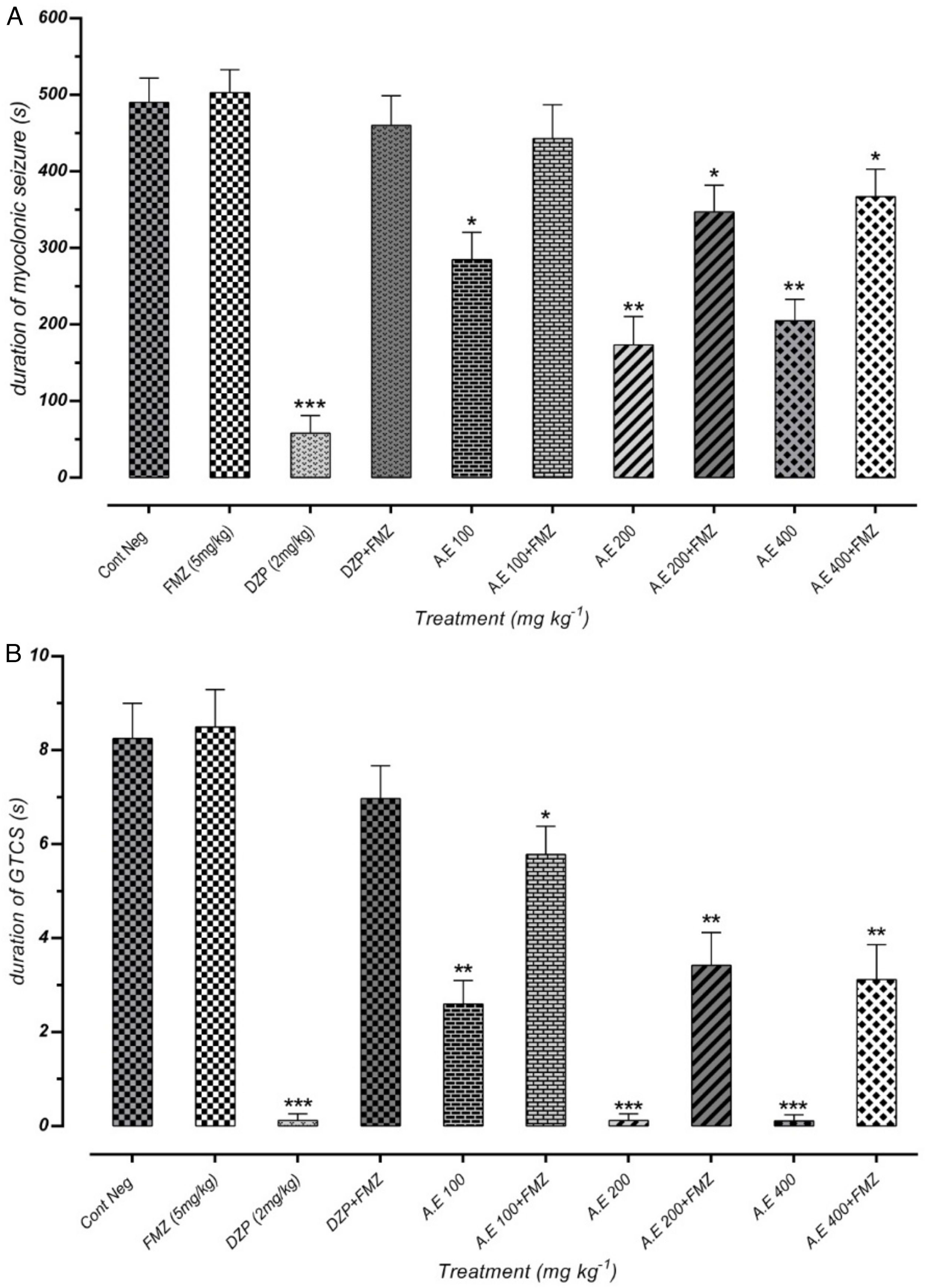
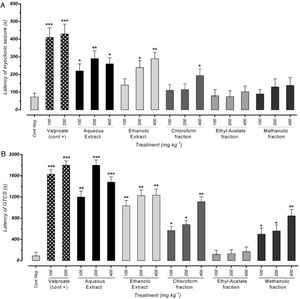
ResultsEffect of different doses of P. daurica subsp. macrophylla on the MS's latency time and GTCS induced by PTZThe first signs of the most mice in each group were myoclonic jerking at the end of the test period. Fig. 2(A) shows that pre-administration of Val at doses of 100mg/kg and 200mg/kg inhibits the latency of MS (p<0.05). In addition, pretreatment with 100 and 400mg/kg of AE and 400mg/kg of F-CHCl3 could significantly reduce the seizure scores (p<0.05) compared to the negative control test dose. The latency of AE (at a dose of 200mg/kg) and HE (at a dose of 400mg/kg) was more significant than the other drugs, and it significantly decreased mean seizure scores in periods relative to the PTZ group (p<0.01). The first time of GTCS and loss of balance was recorded as the onset of seizures (lack of TCS recorded equal to 1800s observation during 30min).
Effects of valproate, negative control and different doses of 100mg/kg, 200mg/kg and 400mg/kg from P. daurica extracts and fractions pretreatment on the onset of myoclonic seizures (MS) (A) and Generalized Tonic-Clonic Seizures (GTCS) (B) of mice. The values are presented as mean±SEM of 7 independent experiments (n=7) and analyzed by one-way analysis of variance (ANOVA) followed by Tukey's Multiple Comparison Post hoc Test to compare differences between various treatment groups (*p<0.05, **p<0.01, ***p<0.001 compared with control group).
Effectiveness of AE at doses of 200mg/kg and 400mg/kg were approximately equal to positive control doses (100mg/kg and 200mg/kg), and it significantly decreased GTCS periods (p<0.001). Moreover, pre-administration of 100mg/kg, all doses of HE, and 400mg/kg of F-CHCl3 and F-MeOH of P. daurica reduced the GTCS score in mice (Fig. 2(B)).
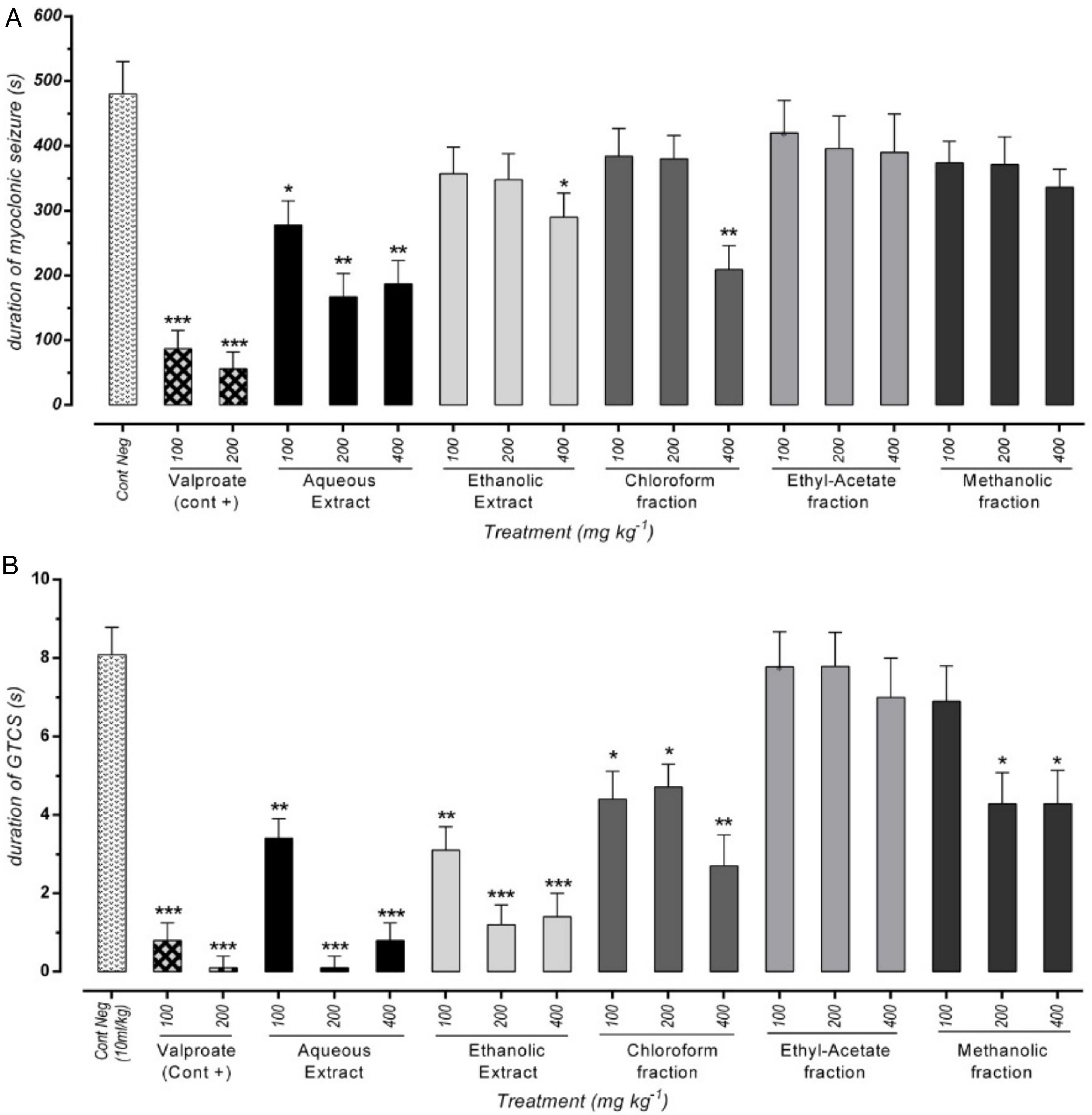
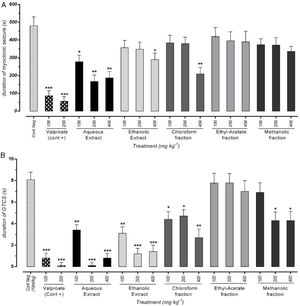
Effect of different doses of P. daurica subsp. macrophylla on the duration of MS and GTCS induced by PTZThe duration of MS assortment of mice, like face and nose movements, myoclonic jumps, and forearm muscles tonus reported. AE at doses of 200mg/kg and 400mg/kg and F-CHCl3 at a dose of 400mg/kg were significantly decreased the duration of MS (p<0.01) (Fig. 3(A)). The time combination spent with plant samples treatment on duration of GTCS in mice recorded. Administration of AE (200mg/kg) along with AE (400mg/kg), and HE (200 and 400mg/kg) reduced the duration time (p<0.001). Fig. 3(B) also demonstrated that pretreatment with 100mg/kg of AE and 400mg/kg of F-CHCl3 could significantly reduce the duration (p<0.01).
Effect of valproate, negative control and different doses of 100mg/kg, 200mg/kg and 400mg/kg from Paeonia daurica subsp macrophylla extracts and fractions pretreatment on the duration of myoclonic seizure (MS) (A) and Generalized Tonic-Clonic Seizures (GTCS) (B) of mice. The values are presented as mean±SEM of 7 independent experiments (n=7) and analyzed by one-way analysis of variance (ANOVA) followed by Tukey's Multiple Comparison Post hoc Test was used to compare differences between various treatment groups (*p<0.05, **p<0.01, ***p<0.001 compared with control group).
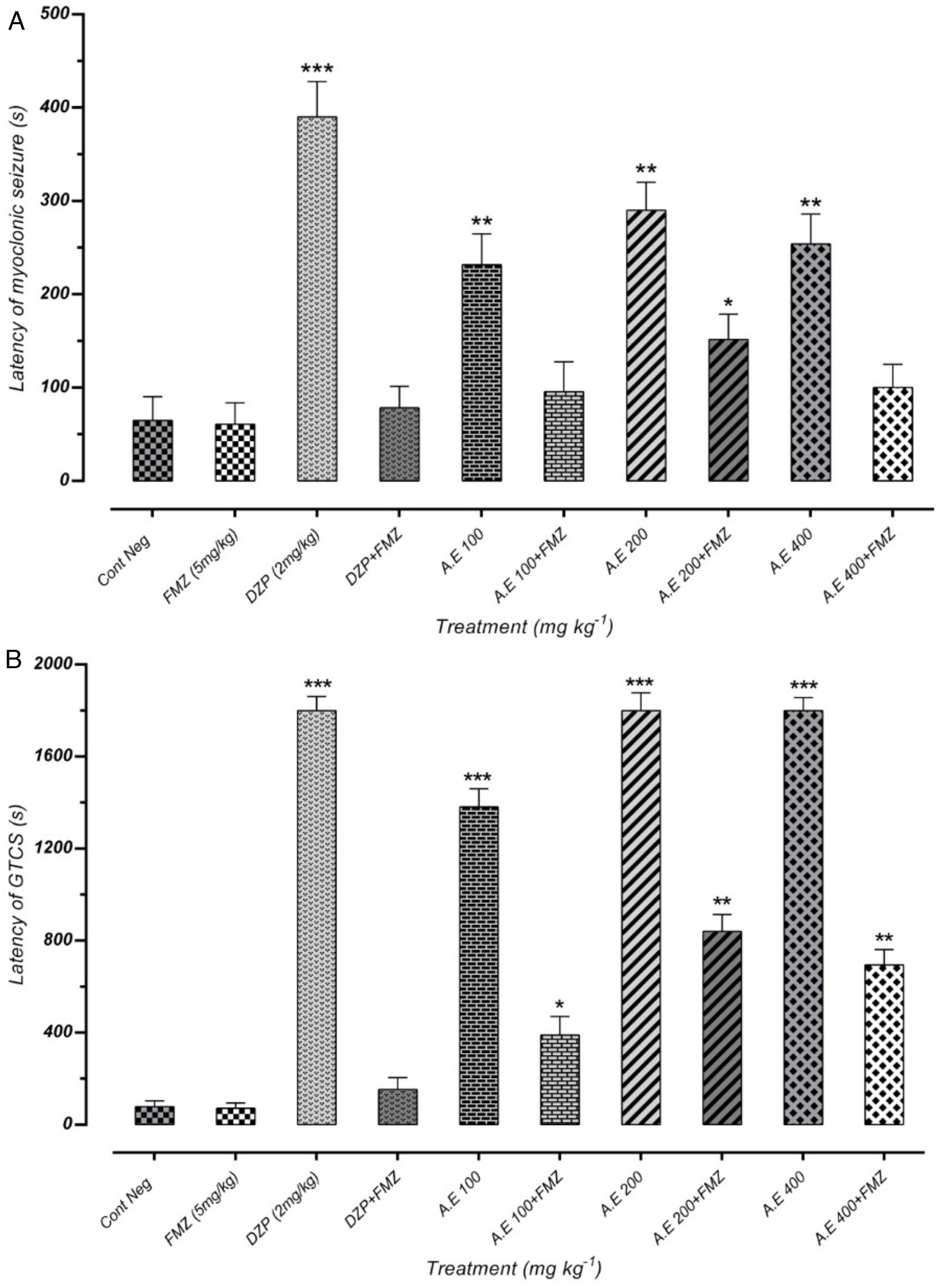
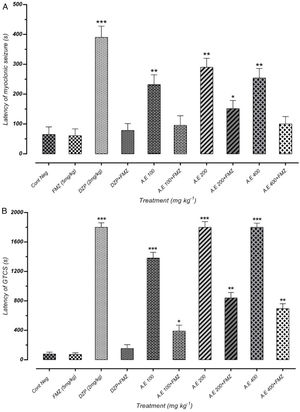
As represented in Fig. 4(A) and (B), pretreatment with flumazenil 5min before the AE administration inhibited the significant anticonvulsant effect of extracts on MS and GTCS onset postponement. Besides, flumazenil reversed the AE effect on the increment of MCS and GTCS latency and the protection percentage against MCS and GTCS. These results suggest the significant effect of the AE on the onset of convulsion delay, probably through GABAergic receptors. However, these findings must be confirmed by different in vitro binding assays. The results of our study indicated that AE significantly reduces the seizures duration. In addition, the latency duration of seizures induced by PTZ in mice increases in a dose-dependent manner.
Effect of negative control and different doses of 100mg/kg, 200mg/kg and 400mg/kg from Paeonia daurica subsp. macrophylla aqueous extract (AE) pretreatment compared to the flumazenil (FMZ) on the onset of myoclonic seizures (MS) (A) and Generalized Tonic-Clonic Seizures (GTCS) (B). DZP: diazepam. The values are presented as mean±SEM of 7 independent experiments (n=7) and analyzed by one-way analysis of variance (ANOVA) followed by Tukey's Multiple Comparison Post hoc Test was used to compare differences between various treatment groups (*p<0.05, **p<0.01, ***p<0.001 compared with control group).
As represented in Fig. 5(A) and (B), there was a significant positive correlation between flumazenil injection in mice and reduction of seizure duration induced by AE due to GABAergic receptor activities. However, effectiveness reduction after flumazenil injection (benzodiazepine receptor antagonist) was lower than the positive control (diazepam). These results can demonstrate the anticonvulsant effects of aqueous plant extract via other probable mechanisms in addition to GABAergic pathways.
Effect of negative control and different doses of 100mg/kg, 200mg/kg and 400mg/kg from Paeonia daurica subsp. macrophylla aqueous extract (AE) pretreatment compared to the flumazenil (FMZ) on the duration of myoclonic seizures (MS) (A) and Generalized Tonic-Clonic Seizures (GTCS) (B). DZP: diazepam. The values are presented as mean±SEM of 7 independent experiments (n=7) and analyzed by one-way analysis of variance (ANOVA) followed by Tukey's Multiple Comparison Post hoc Test was used to compare differences between various treatment groups (*p<0.05, **p<0.01, ***p<0.001 compared with control group).
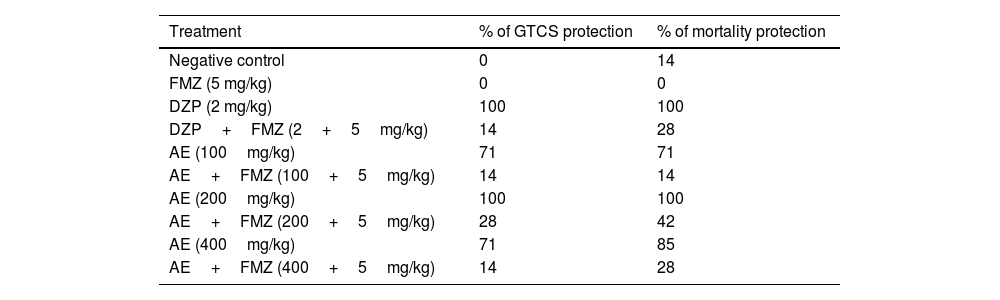
Percent of GTCS and mortality protection of AE in different doses of 100mg/kg and 200mg/kg compared to DZP were 70%. In higher concentrations (400mg/kg), the percent of GTCS and mortality protection decreased. Pretreatment with flumazenil 5min before AE (200mg/kg) injection significantly decreased mortality upon PTZ induced seizure. However, the difference between the percent of GTCS and mortality protection in mice that had received AE (200mg/kg) pretreated with flumazenil and the control group was statistically significant. Moreover, Flumazenil reduced seizure protection (%) in AE (400mg/kg) from 100% to 14%. In addition, flumazenil reversed the anticonvulsant action of AE to some extent. Also, flumazenil inhibited the anticonvulsant activity of diazepam (Table 1).
Effect of different doses of P. daurica subsp. macrophylla on percent of GTCS protection and percent of mortality protection.
| Treatment | % of GTCS protection | % of mortality protection |
|---|---|---|
| Negative control | 0 | 14 |
| FMZ (5 mg/kg) | 0 | 0 |
| DZP (2 mg/kg) | 100 | 100 |
| DZP+FMZ (2+5mg/kg) | 14 | 28 |
| AE (100mg/kg) | 71 | 71 |
| AE+FMZ (100+5mg/kg) | 14 | 14 |
| AE (200mg/kg) | 100 | 100 |
| AE+FMZ (200+5mg/kg) | 28 | 42 |
| AE (400mg/kg) | 71 | 85 |
| AE+FMZ (400+5mg/kg) | 14 | 28 |
Negative control, positive control and, different doses of 100mg/kg, 200mg/kg, and 400mg/kg from Paeonia daurica subsp. macrophylla aqueous extract (AE) pretreatment compared to the flumazenil on the percent of GTCS protection and percent mortality protection (n=7). FMZ, DZP, and AE indicate flumazenil, diazepam, and aqueous extract, respectively.
Epilepsy is a brain disorder, which affects the whole age ranges from neonates to older people with varied manifestations. At least 70% of patients achieve complete control with antiepileptic drug treatment.26 Some potential neurotransmitters such as opioids and nitric oxide are suggested as messengers. However, none of these explanations is entirely satisfactory.27,28 A sufficiently high dose of PTZ, a selective blocker of the chloride channel coupled to the GABAA receptor complex, can produce a continuum of seizure activity used for the evaluation of antiepileptic drugs.29,30 There are pieces of evidence about various natural products used for the treatment of epilepsy in different systems of traditional medicine when tested in modern bioassays for the detection of anticonvulsant activity.23 kinds of literature indicates that some species of the genus Paeonia (Paeoniaceae) may be considered as poorly studied plants. The pharmacological activities of constituents and mechanism of antiepileptic action, especially the paeoniflorin-related ones, are still under investigation.2,3 Some compounds identified in different anatomical parts of Paeonia like terpenoids, tannins, flavonoids, stilbenes, steroids, paeonols, and phenols, as well as anthocyanins, were reported.31 Recently, three compounds benzoic acid, veratric acid and oleanolic acid were isolated and identified from chloroform fraction of the root of P. daurica ssp. macrophyla.32 Vazirian et al. (2018) reported major constituents of the essential oil such as salicylaldehyde (20.32%), beta-pinene-oxide (13.35%) and thymol acetate (61.12%), obtained from the roots of P. daurica subsp. macrophylla.33
Some Paeonia plants (like P. albiflora) are still widely used in Europe, China, and Japan medicine against many nervous system diseases.34,35 In a previous study, screening extracts of the roots of three Greek Paeonia species resulted in the identifying of some interesting prophylactic anticonvulsant activity.36
This information led to the further phytochemical investigation of the most active extracts of P. daurica subsp. macrophylla. This plant is used in Iranian folk medicine as an analgesic, nerve sedative, anti-inflammatory agent, and remedy for cardiovascular and female genital diseases.25 In this study, anticonvulsant effects of aqueous extract, hydro-alcoholic crude extract, F-CHCl3, F-EtOAc, F-MeOH of P. daurica subsp. macrophylla (P. daurica ssp. macrophylla) was examined by using a PTZ-induced model on mice. All the plant samples (100, 200 and, 400mg/kg, i.p.) except F-EtOAc significantly delayed the onset and decreased the duration of PTZ-induced MCS and GTCS, and significantly reduced the GTCS and mortality rate. Pretreatment with flumazenil, 5min before the AE administration, diminished the significant anticonvulsant effect of AE against PTZ-induced seizure. However, the results showed a significant difference with the diazepam-treated group. Flumazenil did not entirely reverse the AE effect on the increment of MCS and GTCS latency time and the protection percentage against GTCS and mortality. Upon pretreatment with flumazenil, mortality was also increased; it might, therefore, be assumed that AE exerts some of its anticonvulsant effects through the GABAA benzodiazepine receptor complex. The mechanism by which Paeonia exerts these antiepileptic actions is uncertain. There is some evidence that Paeonia may act through potentiation of GABAergic inhibition, by stimulating the GABA synthetic enzyme Glutamate decarboxylase activity and inhibit GABA degrading enzymes in the kindling model.37,38 The pharmacological activities of the medicinal plants are due to the synergistic action and not because of any individual component. Therefore, mechanistic investigation and their pharmacokinetic data are also critical to have an insight into the exact mechanism of action. These findings confirmed previous knowledge based on the relationship between Paeonia plants that affect the animal model of PTZ-induced seizure in humans. Knowledge of potency of P. daurica ssp. macrophylla extract and constituents might be useful in treating epilepsy and will discover new insight into further investigations.
FundingThis study was supported by the Tehran University of Medical Sciences (TUMS) (Grant No.: 95-02-96-30724).
Conflict of interestWe declare that there is no conflict of interest.