The aim of this study was to test the encoding deficit hypothesis in Alzheimer disease (AD) using a recent method for correcting memory tests. To this end, a Spanish-language adaptation of the Free and Cued Selective Reminding Test was interpreted using the Item Specific Deficit Approach (ISDA), which provides three indices: Encoding Deficit Index, Consolidation Deficit Index, and Retrieval Deficit Index.
MethodsWe compared the performances of 15 patients with AD and 20 healthy control subjects and analysed results using either the task instructions or the ISDA.
ResultsPatients with AD displayed deficient encoding of more than half the information, but items that were encoded properly could be retrieved later with the help of the same semantic clues provided individually during encoding. Virtually all the information retained over the long term was retrieved by using semantic clues. Encoding was shown to be the most impaired process, followed by retrieval and consolidation. Discriminant function analyses showed that ISDA indices are more sensitive and specific for detecting memory impairments in AD than are raw scores.
ConclusionsThese results indicate that patients with AD present impaired information encoding, but they benefit from semantic hints that help them recover previously learned information. This should be taken into account for intervention techniques focusing on memory impairments in AD.
El presente trabajo tiene como objetivo comprobar la hipótesis del déficit de codificación en la enfermedad de Alzheimer (EA) mediante el uso de una reciente metodología de corrección de test de memoria. Para ello, una adaptación española del Free and Cued Selective Reminder Test fue interpretada mediante el Item Specific Deficit Approach (ISDA), el cual proporciona 3 índices: Índice de déficit de codificación, Índice de déficit de consolidación e Índice de déficit de recuperación.
MétodosSe comparó el rendimiento de 15 pacientes con EA y 20 sujetos sanos, y los resultados se analizaron mediante las instrucciones originales de la prueba y mediante el enfoque ISDA.
ResultadosLos participantes con EA codificaron de manera deficitaria más de la mitad de la información, pero aquella bien codificada fue recordada posteriormente utilizando las claves semánticas proporcionadas individualmente durante la codificación. Prácticamente la totalidad de la información recordada a largo plazo fue la recuperada con claves semánticas. La codificación fue el proceso más alterado, seguido de la recuperación de la información y del almacenamiento. Los análisis discriminantes mostraron que los índices ISDA son más sensibles y específicos que las puntuaciones brutas para la detección de alteraciones mnésicas en la EA.
ConclusionesLos resultados indican que las personas con EA presentan alteraciones en la codificación de la información, pero se benefician de ayudas semánticas para la recuperación a largo plazo de la información previamente aprendida, lo que debería ser utilizado en las intervenciones centradas en las alteraciones de memoria en la EA.
Memory changes constitute one of the most frequent and incapacitating consequences of brain damage.1 They may present as the first and most important symptom of a degenerative disease such as Alzheimer disease (AD).2,3 Patients with AD present changes in delayed memory that result in significantly poorer performances than those of healthy subjects on both episodic and semantic memory tests.4 Patients with AD may even perform at floor level during the early years of the disease.5 For this reason, they require meticulous assessment of all processes involved in learning and memory in order to identify which of these processes has changed, thus permitting clinicians to plan the most appropriate intervention. Neuropsychological tests are mainly interpreted based on differences between performance in the last learning series and performance in a long-term memory trial after a 20- to 30-minute interval during which subjects will rapidly forget previously learned information. On this basis, AD came to be known as a disease characterised by lack of long-term recall of verbal information with no changes in short-term memory, at least in early stages, as shown by the backward digit span task used in the Wechsler scales.5 Another finding that confirms this concept is the fact that patients with AD do not seem to benefit from semantic cues in the recognition phase in such tests as the Rey Auditory Verbal Learning Test or the California Verbal Learning Test (CVLT). These data suggest a pattern of changes in data storage that differs from the patterns displayed by other diseases such as vascular dementia,6 Parkinson's disease, or dementia with Lewy bodies. In the entities named above, patient performance improves with the aid of semantic cues.7,8
The Item Specific Deficit Approach (ISDA)Wright et al.9 recently developed a scoring system that differs from the one habitually used to specifically identify the memory process that has suffered changes. The study used the CVLT and examined memory in patients with HIV or traumatic brain injury.9–11 This procedure, named the ISDA provides 3 indices: (a) the Encoding Deficit Index (CodDI); (b) the Consolidation Deficit Index (ConsDI), and (c) the Retrieval Deficit Index (RetDI). The higher the CodDI, the more severe the encoding alteration. Both the ConsDI and RetDI are scored from 0 to 1, and higher scores indicate more severe impairment. Clinicians may use the ISDA to interpret patient performance more specifically since it resolves the problems derived from interpreting a global test score; the ISDA calculates performance by analysing each item individually.
Alzheimer disease: a consolidation problem or a retrieval problem?AD is regarded as an illness in which memory problems stem from changes in memory storage, given that delayed recall in patients with AD is especially poor for verbal memory tasks12 and performance does not improve during the recognition phase.13,14 Nevertheless, tests using free recall as a measure of storage, and recognition as a measure of information retrieval, do not provide an in-depth view of the processes involved in learning and memory. If memory changes in AD occurred in the consolidation process, performance would not be improved by semantic cues; rather, this would indicate problems with information retrieval. Other authors15,16 indicate that AD patients’ performance may be improved by semantic cues, given that deep encoding (semantic processing) improves learning compared to the superficial encoding that occurs when information is processed on the phonetic level. However, they found impairment and gradual loss of both free and delayed recall. Lipinska and Bäckman17 found better results for cued recall in patients with AD after semantic encoding; there were no improvements in free recall. Herlitz et al.18 indicate that patients with AD may use semantic support in memory tasks, but they will probably require more support than healthy subjects in order to recall information. Based on the above findings, if performance by AD patients on verbal memory tasks consists of poor free recall with higher scores for cued recall following semantic encoding, memory deficits could be present in the encoding and retrieval processes. This study was performed to test several hypotheses. Our objectives were as follows: (a) to identify the 3 ISDA indices in patients with AD using the Free and Cued Selective Reminding Test19 (FCSRT); and (b) to demonstrate that the primary alteration in AD is found in the information encoding process, followed by the retrieval process, and last of all, the consolidation process. This pattern will show that memory improves when patients are given appropriate cues and information is encoded deeply (semantic processing).
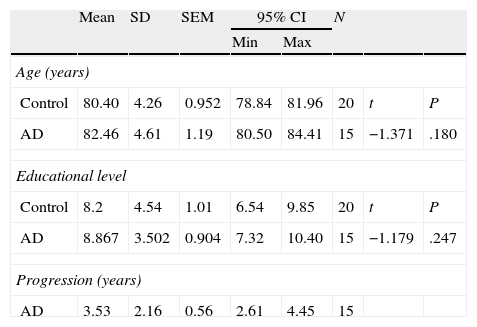
Subjects and methodsWe evaluated 15 subjects with AD, comprising 5 men (33.3%) and 10 women (66.7%) with ages ranging from 78 to 82 years. Participants in the AD group were patients treated at Hospital Centro de Cuidados Laguna in Madrid. Experienced neurologists diagnosed all patients according to NINCDS-ADRDA criteria for AD20,21; patients were also evaluated by an experienced neuropsychologist (RP-E). None of the participants displayed signs of neurological or psychiatric diseases other than AD that might explain their symptoms. The neuropsychological evaluation included in the study tests was performed as an aid to diagnosis; none of the participants was being treated with cognitive drugs.
Performance by these subjects was compared to performance by 20 controls, comprising 9 men (40%) and 11 women (60%) whose results were used to establish the normative data for a Spanish battery of neuropsychological tests for dementia (Exploración Neuropsicológica Mínima en Demencias), ENM.dem.22 This internationally used screening test battery assesses multiple cognitive functions, including orientation, executive functions, verbal memory, verbal fluency, abstraction, visual gnosis, and praxis. Exclusion criteria for control subjects, according to the ENM.dem, were prior or current central nervous system disease, substance abuse, systemic illness, or psychiatric illness. Subjects who had been treated with psychoactive drugs in the preceding 6 months were also excluded.
Neuropsychological assessmentWe used the Spanish translation of the Mini-Mental State Examination (MMSE)23 to measure disease severity. The MMSE, used as a screening test for neurological diseases, provides information about such cognitive areas as orientation, attention, verbal memory, language, and constructive praxis. Scores range from 0 to 30, with values below 24 being indicative of cognitive dysfunction.
Free and Cued Selective Reminding TestWe used an adapted version of the FCSRT22 in which 12 written words are presented and each word belongs to a semantic category. Learning is tested throughout 2 recall trials, including one with free recall and another with cued recall in which the examiner provides the semantic cue for each item that was not recalled freely. Delayed memory was evaluated using both free and cued recall. The test was corrected according to the original instructions and ISDA indices were applied after that step. The version used here is similar to the Spanish-language version of the Brief Repeatable Battery of Neuropsychological Tests24 for multiple sclerosis, with slight modifications to ensure that all words were commonly used in Spanish.
Indices of the Item Specific Deficit ApproachThe ISDA analyses changes in performance using 3 independent indices. CodDI is the sum of all words recalled in less than half of the immediate-recall trials. ConsDI is obtained by dividing the total words recalled at least once during immediate-recall trials and not recalled in delayed recall trials (either free or cued) by the total words recalled at least once during learning trials (either free or cued). RetDI is obtained by dividing the total words recalled at least once during learning and recalled inconsistently in delayed recall trials (only with the aid of semantic cues) by the total words recalled at least once in learning trials (free or cued recall). Although the ISDA was first applied to the CVLT, it can be applied to any type of episodic memory test as long as the test has multiple learning trials and examines different types of delayed recall (free and cued), as is the case with the FCSRT. Indices cannot be calculated for tests that do not have different delayed recall modalities, for example, a test of free recall only.
Semantic fluencyWe included the semantic fluency test, which is sensitive to the memory changes present in initial stages of AD.25 Participants were asked to name as many animals as possible in a 60-second period.26 Repeated items were not counted. In the case of 2 terms designating different genders of the same animal (i.e. gato/gata), only one item was counted.
Statistical analysisMultivariate analysis (MANOVA) was used to identify potential sex effects on performance, with the variables ‘group’ and ‘sex’ as fixed factors. There was no significant sex effect on any of the study variables. Given that all differences were due to the ‘group’ factor, we used the t-test for independent samples to compare means of both demographic/neuropsychological variables and ISDA indices. This allowed us to calculate the effect size for significant effects.27 Values below 0.20 indicate a null effect size. Values of 0.20-0.50 indicate a small effect size. Values of 0.50-0.80 indicate a large effect size, and values above 0.80 indicate a very large effect size. For the AD group, we used the Pearson correlation to analyse the links between ISDA indices and performance on the FCSRT, and between different variables included in the FCSRT. To test the utility of different ISDA indices for identifying participants with memory dysfunction, we completed a series of discriminant analyses using performance in the semantic fluency task as the grouping variable. Since our sample size was small (N=35), we used a value of α=.01 for the different analyses.
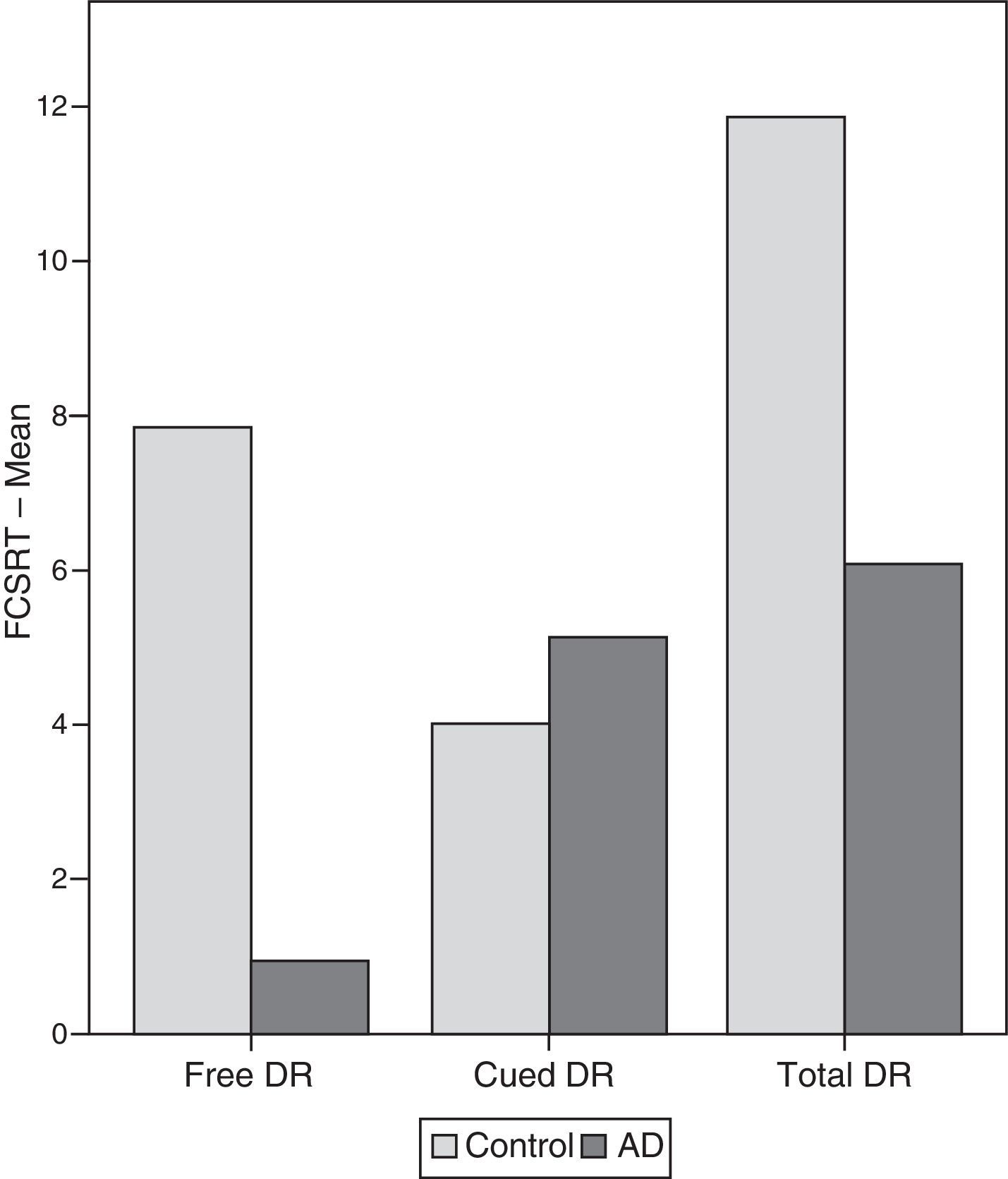
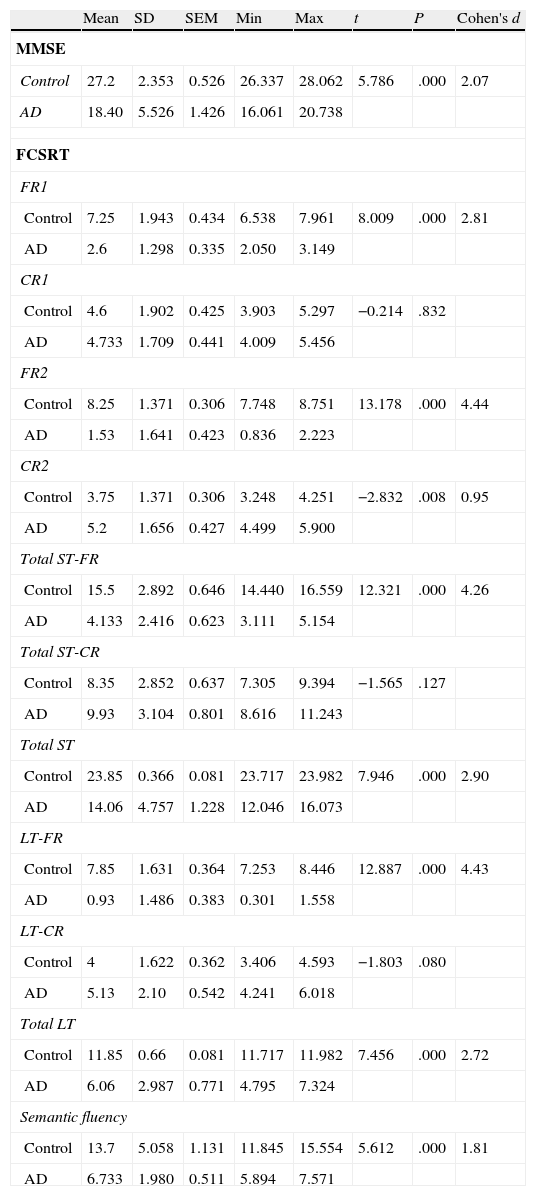
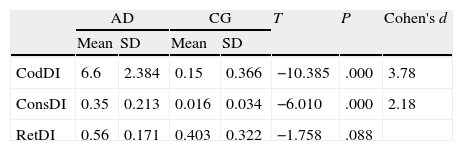
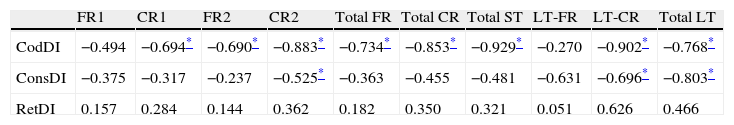
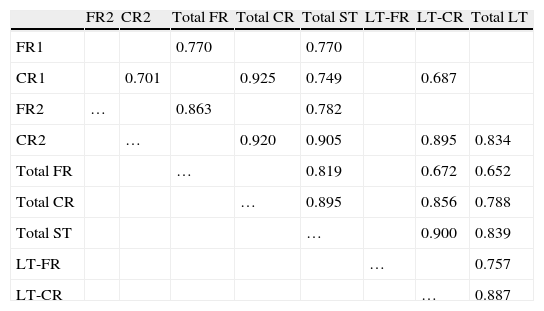
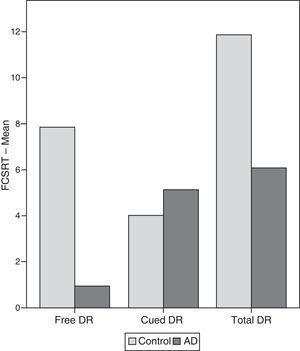
ResultsThere were no intergroup differences for age, years of schooling, or sex (Table 1), which shows that the groups were well matched. Regarding the MMSE and the FCSRT (Table 2 and Fig. 1), all groups differed for each of the neuropsychological measures except cued recall for trial 1 and total cued recall for both the immediate and delayed recall trials. MMSE scores were significantly lower in the AD group. The effect size for these differences was considerable (d=1.05-4.76) because mean scores varied greatly from group to group. The encoding and consolidation indices (Table 3) differed significantly between groups and the effect sizes were very large (d=3.78 and 2.18, respectively). However, there were no differences in the RetDI. CodDI in the AD group showed that 55% of the information was encoded insufficiently. ConsDI showed that 35% of the information encoded at some point during learning trials could not be recalled with either free recall or using semantic cues. Nevertheless, RetDI showed that 56% of the information encoded by subjects with AD at some point during learning and not accessed with free recall could be retrieved using semantic cues (Table 3). After analysing the relationship between different variables (Table 4), we observed that CodDI correlated with almost all variables related to learning and cued delayed recall, but not with free delayed recall. ConsDI was correlated with cued recall but not free recall, indicating that the smaller the consolidation deficit, the more successful the delayed cued recall. RetDI did not correlate to either immediate or delayed recall. Regarding performance on the FCSRT, Table 5 shows that the greatest correlations are between cued recall in the last learning trial and cued delayed recall (RFR2xLT-CR), and between cued delayed recall and total delayed recall (RLT-CRxTOTAL-LT). This indicates that most of the information that is recalled, whether in the short term or the long term, is information retrieved using semantic cues. As shown in Table 2, the rate of information retrieval in the short term using semantic cues is 2.4 times greater than that recalled freely; for delayed recall, this rate is 5.5 times greater than that for free recall. The percentage of the total information that was recalled (free or cued recall, respectively) amounted to 29.4% and 70.6% for short-term trials and 15.3% and 84.7% for long-term trials.
Demographic data
| Mean | SD | SEM | 95% CI | N | ||||
| Min | Max | |||||||
| Age (years) | ||||||||
| Control | 80.40 | 4.26 | 0.952 | 78.84 | 81.96 | 20 | t | P |
| AD | 82.46 | 4.61 | 1.19 | 80.50 | 84.41 | 15 | −1.371 | .180 |
| Educational level | ||||||||
| Control | 8.2 | 4.54 | 1.01 | 6.54 | 9.85 | 20 | t | P |
| AD | 8.867 | 3.502 | 0.904 | 7.32 | 10.40 | 15 | −1.179 | .247 |
| Progression (years) | ||||||||
| AD | 3.53 | 2.16 | 0.56 | 2.61 | 4.45 | 15 | ||
| N | % | |||
| Sex | ||||
| Control | ||||
| Men | 9 | 45 | ||
| Women | 11 | 55 | χ2 | P |
| AD | ||||
| Men | 5 | 33.3 | 0.486 | .486 |
| Women | 10 | 66.7 | ||
SD, standard deviation; AD, Alzheimer disease group; SEM, standard error of the mean; CI, confidence interval.
Neuropsychological data. CG×AD
| Mean | SD | SEM | Min | Max | t | P | Cohen's d | |
| MMSE | ||||||||
| Control | 27.2 | 2.353 | 0.526 | 26.337 | 28.062 | 5.786 | .000 | 2.07 |
| AD | 18.40 | 5.526 | 1.426 | 16.061 | 20.738 | |||
| FCSRT | ||||||||
| FR1 | ||||||||
| Control | 7.25 | 1.943 | 0.434 | 6.538 | 7.961 | 8.009 | .000 | 2.81 |
| AD | 2.6 | 1.298 | 0.335 | 2.050 | 3.149 | |||
| CR1 | ||||||||
| Control | 4.6 | 1.902 | 0.425 | 3.903 | 5.297 | −0.214 | .832 | |
| AD | 4.733 | 1.709 | 0.441 | 4.009 | 5.456 | |||
| FR2 | ||||||||
| Control | 8.25 | 1.371 | 0.306 | 7.748 | 8.751 | 13.178 | .000 | 4.44 |
| AD | 1.53 | 1.641 | 0.423 | 0.836 | 2.223 | |||
| CR2 | ||||||||
| Control | 3.75 | 1.371 | 0.306 | 3.248 | 4.251 | −2.832 | .008 | 0.95 |
| AD | 5.2 | 1.656 | 0.427 | 4.499 | 5.900 | |||
| Total ST-FR | ||||||||
| Control | 15.5 | 2.892 | 0.646 | 14.440 | 16.559 | 12.321 | .000 | 4.26 |
| AD | 4.133 | 2.416 | 0.623 | 3.111 | 5.154 | |||
| Total ST-CR | ||||||||
| Control | 8.35 | 2.852 | 0.637 | 7.305 | 9.394 | −1.565 | .127 | |
| AD | 9.93 | 3.104 | 0.801 | 8.616 | 11.243 | |||
| Total ST | ||||||||
| Control | 23.85 | 0.366 | 0.081 | 23.717 | 23.982 | 7.946 | .000 | 2.90 |
| AD | 14.06 | 4.757 | 1.228 | 12.046 | 16.073 | |||
| LT-FR | ||||||||
| Control | 7.85 | 1.631 | 0.364 | 7.253 | 8.446 | 12.887 | .000 | 4.43 |
| AD | 0.93 | 1.486 | 0.383 | 0.301 | 1.558 | |||
| LT-CR | ||||||||
| Control | 4 | 1.622 | 0.362 | 3.406 | 4.593 | −1.803 | .080 | |
| AD | 5.13 | 2.10 | 0.542 | 4.241 | 6.018 | |||
| Total LT | ||||||||
| Control | 11.85 | 0.66 | 0.081 | 11.717 | 11.982 | 7.456 | .000 | 2.72 |
| AD | 6.06 | 2.987 | 0.771 | 4.795 | 7.324 | |||
| Semantic fluency | ||||||||
| Control | 13.7 | 5.058 | 1.131 | 11.845 | 15.554 | 5.612 | .000 | 1.81 |
| AD | 6.733 | 1.980 | 0.511 | 5.894 | 7.571 | |||
ST, short term; SD, standard deviation; AD, Alzheimer disease group; SEM, standard error of the mean; FCSRT, Free and Cued Selective Reminding Test; CG, control group; LT, long term; MMSE, Mini-Mental Status Examination; FR, free recall; CR, cued recall.
ISDAxFCSRT correlations in the AD group
| FR1 | CR1 | FR2 | CR2 | Total FR | Total CR | Total ST | LT-FR | LT-CR | Total LT | |
| CodDI | −0.494 | −0.694* | −0.690* | −0.883* | −0.734* | −0.853* | −0.929* | −0.270 | −0.902* | −0.768* |
| ConsDI | −0.375 | −0.317 | −0.237 | −0.525* | −0.363 | −0.455 | −0.481 | −0.631 | −0.696* | −0.803* |
| RetDI | 0.157 | 0.284 | 0.144 | 0.362 | 0.182 | 0.350 | 0.321 | 0.051 | 0.626 | 0.466 |
ST, short term; CodDI, Encoding Deficit Index; ConsDI, Consolidation Deficit Index; RetDI, Retrieval Deficit Index; LT, long term; CR, cued recall; FR, free recall.
Correlations in the FCSRT AD group
| FR2 | CR2 | Total FR | Total CR | Total ST | LT-FR | LT-CR | Total LT | |
| FR1 | 0.770 | 0.770 | ||||||
| CR1 | 0.701 | 0.925 | 0.749 | 0.687 | ||||
| FR2 | … | 0.863 | 0.782 | |||||
| CR2 | … | 0.920 | 0.905 | 0.895 | 0.834 | |||
| Total FR | … | 0.819 | 0.672 | 0.652 | ||||
| Total CR | … | 0.895 | 0.856 | 0.788 | ||||
| Total ST | … | 0.900 | 0.839 | |||||
| LT-FR | … | 0.757 | ||||||
| LT-CR | … | 0.887 |
P<.01 for all data included in the table.
ST, short term; LT, long term; FR, free recall; CR, cued recall.
Discriminant analyses were performed by first taking semantic fluency scores from all participants and transforming them into standard scores (M=50, SD=10) using the mean and standard deviation of the control group. Standard scores higher than 40 were entered as a different variable with a value of 0 (no impairment), while scores of 40 or less were entered as a value of 1 (indicating impairment). Discriminant analysis using FCSRT scores was able to identify changes in performance on the semantic fluency task (Wilks λ=0.629, χ2=14.388, P=.006; canonical correlation [cc]=0.609). Free delayed recall contributed more than any other index to discriminant function (cc=1.288), followed by cued delayed recall (cc=0.694), total cued immediate recall (cc=0.241) and total free immediate recall (cc=−0.130). The discriminant function correctly classified 80% of the participants; sensitivity and specificity were 50% and 85%, respectively. Results from the discriminant analysis that identified impaired performance on the semantic fluency task based on ISDA indices showed that these indices are significant (Wilks λ=0.561, χ2=18.212, P<.001; cc=0.663). The RetDI was the index with the largest impact on discriminant function (cc=0.756), followed by ConsDI (cc=0.632) and CodDI (0.244). The discriminant function correctly classified 85.7% of the participants; sensitivity and specificity were 87.5% and 85.2%, respectively.
DiscussionThe purpose of this study was to verify that the principal changes in learning and verbal memory in patients with AD occur in the information encoding and retrieval processes. In line with the studies by Lipinska and Bäckman,17 we aimed to show that patients with AD display better results for delayed recall if a deep encoding strategy is used. To this end, we use a novel method for interpreting performance on verbal memory tasks based on analysing individual items rather than the total score.9–11 Based on performance in an adapted version of the FCSRT, analyses showed that subjects with AD presented learning and verbal memory impairments compared to healthy subjects. These changes mainly reflected an information encoding impairment (55%), followed by changes in retrieval of correctly encoded information (56%), and lastly, changes in consolidation of information that had been encoded correctly during the learning phase. Results for using semantic cues to recover information encoded correctly during learning did not differ between groups, a finding that indicates that patients with AD can learn in a context of deep processing of semantic cues that are specific to each item of information. Lipinska and Bäckman17 found that participants with AD showed more pronounced improvement on delayed information retrieval tasks than control subjects when using semantic cues instead of superficial phonetic processing. There were no differences in cued recall between the 2 groups. Although the information retrieval deficit is more pronounced in the AD group in our study, differences are not significant and our findings coincide with those of Lipinska and Bäckman.17 These data confirm our hypothesis that the main deficit in learning and memory in AD patients resides in the encoding process. However, performance improves considerably when appropriate cues are provided during both the learning phase and the information retrieval phase. ISDA indices were examined for their ability to identify the memory deficits reflected by a verbal fluency test (such tests are known to show changes in the initial phases of AD).25 ISDA indices displayed higher sensitivity and specificity than traditional indices obtained from FCSRT results. Regarding memory impairments in subjects with AD, results showed that 55% of the information was encoded incorrectly in this group. ConsDI showed that 35% of the information encoded at some point during learning was forgotten over the long term, while 56% was evoked by the same semantic cues the participants used during the learning trials. These results were corroborated by overall performance on the FCSRT. Forty-five percent of the 12 words (the amount of information that was encoded correctly) yields a mean of 5.4 words on the adapted version of FCSRT. The mean of 5.13 for cued delayed recall and of 6.06 for total delayed recall indicate a much higher rate of information retrieval using semantic cues than with free recall only in patients with AD. However, several factors affect these results, beginning with the small sample size (N=35). To avoid type I errors, the α level was set at 1%, which lends validity to these results. The test of episodic memory used here is an adapted form of the FCSRT with fewer words and fewer learning trials than other varieties used in clinical practice.28,29 However, the test includes several learning trials and different types of delayed recall. This is no impediment to using the test in clinical practice, given that the test has been validated for evaluating memory functions in other clinical populations.24 Secondly, ISDA indices were originally applied to the CVLT, a test presenting 16 words to be learned over 5 learning trials, plus a deferred recall trial and a recognition trial. The adapted version of the FCSRT included in our study lacks a recognition trial. Recognition trials may modify data substantially, given that they provide a third opportunity for recovering information in addition to free and cued recall. Including a recognition test would yield results that would be similar, at the very least, to those presented in this study. However, this modification could also increase the number of correct answers if subjects identified items that they had been unable to recall using cues. This change would therefore increase values on the RetDI and decrease values on the ConsDI. In contrast, it would not affect CodDI since this index is independent of the number of correct responses in delayed recall. If this were the case, it would support our study's hypotheses by finding a deep encoding to be beneficial to long-term recall of words using recognition. Further studies should include recognition trials when ISDA indices are applied to memory tests such as the FCSRT. This test differs considerably from the CVLT in that it does not group multiple items within the same category, but rather assigns a specific semantic category to each item. Results from this study, interpreted directly from performance in learning and memory trials, show that AD patients are capable of learning words when deep coding is achieved using semantic cues and the same cues are used during both the learning and retrieval phases. Based on the above, and despite the limitations of this study, it is possible to conclude that cognitive interventions aimed at improving memory in patients with AD should include the levels of processing approach,30 which favours semantic encoding of material. Interventions should also include the transfer-appropriate processing approach,31 which postulates that the probability of recalling verbal information over the long term increases when the information is evoked using the same cues used during the encoding process. Future studies using tests that are more coherent with the ISDA may provide data on early identification of memory changes in AD. They may also shed light on the prognosis for these changes over the course of the disease and identify which of the processes involved in learning and memory32 have been altered. On this basis, clinicians will be able to plan the most appropriate cognitive interventions.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Oltra-Cucarella J, Pérez-Elvira R, Duque P. Beneficios de la codificación profunda en la enfermedad de Alzheimer. Análisis del rendimiento en una tarea de memoria mediante el Item Specific Deficit Approach. Neurología. 2014;29:286–293.