To evaluate the factors associated with false negatives in RT-qPCR in patients with mild-moderate symptoms of COVID-19.
Materials and methodsThis was a cross-sectional study that used a random sample of non-hospitalized patients from the primary care management division of the Healthcare Area of Leon (58 RT-qPCR-positive cases and 52 RT-qPCR-negative cases). Information regarding symptoms was collected and all patients were simultaneously tested using two rapid diagnostic tests – RDTs (Combined – cRDT and Differentiated – dRDT). The association between symptoms and SARS-CoV-2 infection was evaluated by non-conditional logistic regression, with estimation of Odds Ratio.
ResultsA total of 110 subjects were studied, 52% of whom were women (mean age: 48.2±11.0 years). There were 42.3% of negative RT-qPCRs that were positive in some RDTs. Fever over 38°C (present in 35.5% of cases) and anosmia (present in 41.8%) were the symptoms most associated with SARS-CoV-2 infection, a relationship that remained statistically significant in patients with negative RT-qPCR and some positive RDT (aOR=6.64; 95%CI=1.33–33.13 and aOR=19.38; 95% CI=3.69–101.89, respectively).
ConclusionsRT-qPCR is the technique of choice in the diagnosis of SARS-CoV-2 infection, but it is not exempt from false negatives. Our results show that patients who present mild or moderate symptoms with negative RT-qPCR, but with fever and/or anosmia, should be considered as suspicious cases and should be evaluated with other diagnostic methods.
Evaluar los factores asociados con falsos negativos a RT-qPCR negativa y sintomatología leve o moderada de COVID-19.
Materiales y métodosEstudio transversal. Se utilizó una muestra aleatoria de pacientes no hospitalizados de la Gerencia de Atención Primaria del Área de Salud de León (58 con RT-qPCR positiva y 52 con RT-qPCR negativa). Se recogió información sobre síntomas, y a todos se les realizaron simultáneamente dos pruebas de diagnóstico rápido (PDR): combinada (PRD-C) y diferenciada (PRD-D). La asociación de los síntomas con la infección por SARS-CoV-2 se evaluó mediante regresión logística no condicional, con el cálculo de odds ratio (OR).
ResultadosSe estudiaron un total de 110 personas, y el 52% de ellas fueron mujeres (edad media: 48,2±11,0años). El 42,3% de las RT-qPCR negativas dieron positivo en algún PDR. La fiebre de más de 38°C (presente en el 35,5% de los casos) y la anosmia (presente en el 41,2%) fueron los síntomas más asociados a la infección por SARS-CoV-2, relación que se mantuvo estadísticamente significativa en pacientes con RT-qPCR negativa y algún PDR positivo (ORa: 6,64; IC95%: 1,33-33,13, y ORa: 19,38; IC95%: 3,69-101,89, respectivamente).
ConclusionesLa RT-qPCR es la técnica de elección en el diagnóstico de la infección por SARS-CoV-2, pero no está exenta de falsos negativos. Nuestros resultados ponen de manifiesto que los pacientes que presentan síntomas leves o moderados con RT-qPCR negativa pero con fiebre y/o anosmia deben ser considerados casos sospechosos y deben ser valorados con otros métodos diagnósticos.
One of the main strategies for the prevention and control of the COVID-19 pandemic is detecting and isolating of the infection sources.1 The reverse transcription quantitative polymerase chain reaction (RT-qPCR) is the technique of choice for detecting infection sources due to its high sensitivity and specificity.2 It is also a well-known and widespread technique in the clinical laboratories of our hospitals.3 This method, however, is not free of false negatives, the most frequent causes of which being theinadequate sample collection, delays in transport, labeling errors and the poor virus elimination in the patient.4,5 For these reasons, in the face of clinical suspicion, a negative RT-qPCR result must be contrasted against other diagnostics tests.4
The aim of this article is to understand the factors associated with false negatives in RT-qPCR in patients with mild-moderate symptoms of COVID-19.
Material and methodsStudy designA cross-sectional study was carried out. A random selection was made of 58 non-hospitalized patients with positive RT-qPCR and 52 negative RT-qPCR. Patients were selected from the register of confirmed or suspected COVID-19 cases from the primary care management of the Healthcare Area of Leon. More than 14 days had passed in all patients since the beginning of the symptoms.
ProcedureParticipation in the study was voluntary. The invitation to participate in the study was extended via a telephone call, in which the participants were summoned for a biological sample and information collection. All protection regulations were followed during the collection process and the project was approved by the Ethics Committee of the Healthcare Area of León and the Bierzo (reference: 2073). After signing an informed consent, each participant completed a brief ad hoc questionnaire that collected information on socio-demographic data, symptoms, date of onset and end of symptoms and date of the RT-qPCR.
All patients were tested simultaneously with two RDTs6:
- -
Combined (c-RDT) (one band): Wondfo® SARS-COV-2 Antibody test (Lateral Flow Method) of GUANGZHOU WONDFO BIOTECH CO LTD,
- -
Differentiated (d-RDT) (two bands): Allowing differentiation between IgG and IgM. All Test® 2019-nCoV IgG/IgM Rapid Test Casette of HANGZHOU ALL TEST BIOTECH CO LTD.
Both tests were performed by two nurses using a finger-stick whole blood sample. The first test jointly determines the presence of IgM and IgG, while the second test makes a differentiated measurement of both antibody subtypes. The results of the tests were read 10–15min after they were carried out.
We considered as a case of COVID19 those patients with a positive result to at least one of the RDTs or RT-qPCR.
Statistical analysisCentral and dispersion measures were calculated in quantitative variables (mean and standard deviation (SD)) and frequencies with their 95% confidence intervals in qualitative variables. Using non-conditional logistic regression models, adjusted by age and sex at minimum, we obtained the Odds Ratio (aOR) to be considered COVID-19 case, performing stratified analysis according to the results of the RDTs and the RT-qPCR. The characteristics of those patients with negative RT-qPCR and positive PDR were examined. All analyses were performed with the STATA 15 statistical package.7
ResultsA total of 110 subjects were studied, of whom 51.8% were women. The age range was between 22 and 78 years, with a mean of 48.2±11.0 years. The number of days from the onset of symptoms to the performance of the RDTs ranged from 14 to 40 days, with a median of 26 days.
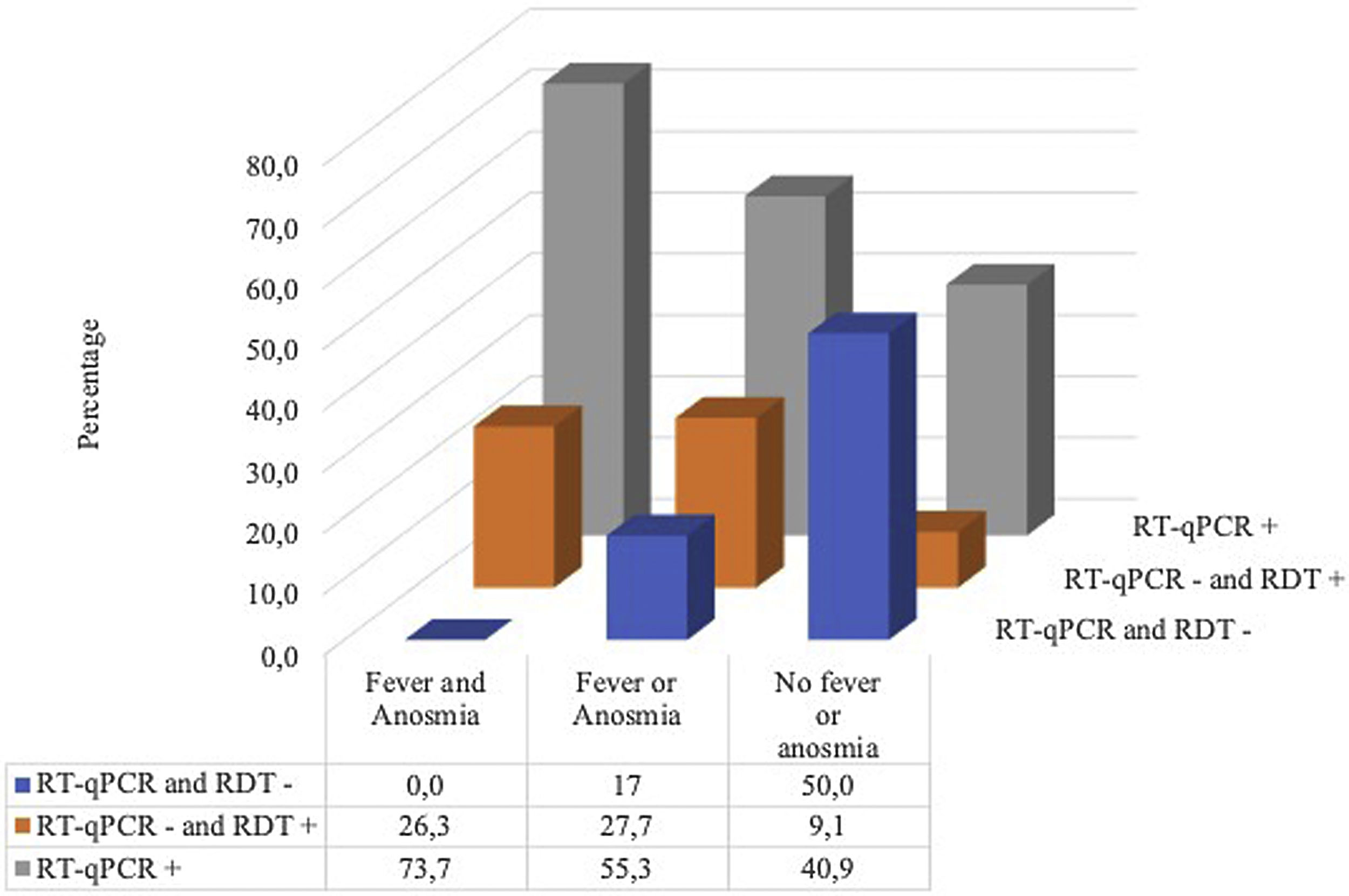
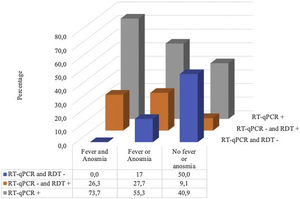
Of the 110 patients, 80 (72.7%) had either RT-qPCR or a positive RDT for SARS-CoV-2 (20 were IgM positive, 64 were IgG positive, 64 were IgM or IgG positive, 46 were combined, 65 were RDT positive, and 58 were RT-qPCR positive). Of the 52 with negative RT-qPCR, 22 (42.3%) were positive for some RDT; there were 14 positives for the two RDTs and 8 only for RDTd. (Fig. 1)
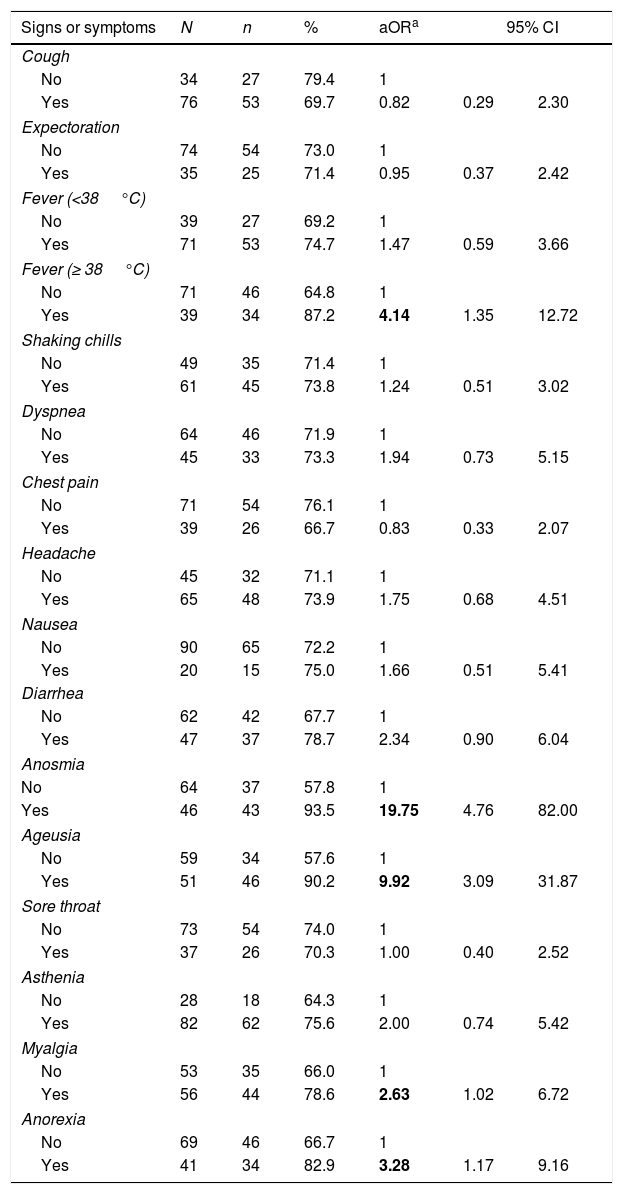
Table 1 shows the distribution of symptoms and their association with being a COVID-19 case. A statistically significant association with fever, anosmia, ageusia, myalgia and anorexia were observed in the model adjusted for age and sex. Adjusting for age, sex and the signs and symptoms statistically associated with COVID-19, the significant association with anosmia (aOR=20.39; 95%CI=4.74–87.73) and fever (aOR=4.33; 95%CI=1.24–15.11) was maintained.
Risk of having at least one positive test according to different signs and symptoms.
| Signs or symptoms | N | n | % | aORa | 95% CI | |
|---|---|---|---|---|---|---|
| Cough | ||||||
| No | 34 | 27 | 79.4 | 1 | ||
| Yes | 76 | 53 | 69.7 | 0.82 | 0.29 | 2.30 |
| Expectoration | ||||||
| No | 74 | 54 | 73.0 | 1 | ||
| Yes | 35 | 25 | 71.4 | 0.95 | 0.37 | 2.42 |
| Fever (<38°C) | ||||||
| No | 39 | 27 | 69.2 | 1 | ||
| Yes | 71 | 53 | 74.7 | 1.47 | 0.59 | 3.66 |
| Fever (≥ 38°C) | ||||||
| No | 71 | 46 | 64.8 | 1 | ||
| Yes | 39 | 34 | 87.2 | 4.14 | 1.35 | 12.72 |
| Shaking chills | ||||||
| No | 49 | 35 | 71.4 | 1 | ||
| Yes | 61 | 45 | 73.8 | 1.24 | 0.51 | 3.02 |
| Dyspnea | ||||||
| No | 64 | 46 | 71.9 | 1 | ||
| Yes | 45 | 33 | 73.3 | 1.94 | 0.73 | 5.15 |
| Chest pain | ||||||
| No | 71 | 54 | 76.1 | 1 | ||
| Yes | 39 | 26 | 66.7 | 0.83 | 0.33 | 2.07 |
| Headache | ||||||
| No | 45 | 32 | 71.1 | 1 | ||
| Yes | 65 | 48 | 73.9 | 1.75 | 0.68 | 4.51 |
| Nausea | ||||||
| No | 90 | 65 | 72.2 | 1 | ||
| Yes | 20 | 15 | 75.0 | 1.66 | 0.51 | 5.41 |
| Diarrhea | ||||||
| No | 62 | 42 | 67.7 | 1 | ||
| Yes | 47 | 37 | 78.7 | 2.34 | 0.90 | 6.04 |
| Anosmia | ||||||
| No | 64 | 37 | 57.8 | 1 | ||
| Yes | 46 | 43 | 93.5 | 19.75 | 4.76 | 82.00 |
| Ageusia | ||||||
| No | 59 | 34 | 57.6 | 1 | ||
| Yes | 51 | 46 | 90.2 | 9.92 | 3.09 | 31.87 |
| Sore throat | ||||||
| No | 73 | 54 | 74.0 | 1 | ||
| Yes | 37 | 26 | 70.3 | 1.00 | 0.40 | 2.52 |
| Asthenia | ||||||
| No | 28 | 18 | 64.3 | 1 | ||
| Yes | 82 | 62 | 75.6 | 2.00 | 0.74 | 5.42 |
| Myalgia | ||||||
| No | 53 | 35 | 66.0 | 1 | ||
| Yes | 56 | 44 | 78.6 | 2.63 | 1.02 | 6.72 |
| Anorexia | ||||||
| No | 69 | 46 | 66.7 | 1 | ||
| Yes | 41 | 34 | 82.9 | 3.28 | 1.17 | 9.16 |
Fig. 2 shows that all the patients with fever and anosmia were positive in some test and in the case of patients with negative RT-qPCR but positive in some RDT, more than 80% (18/22) had either fever or anosmia or both.
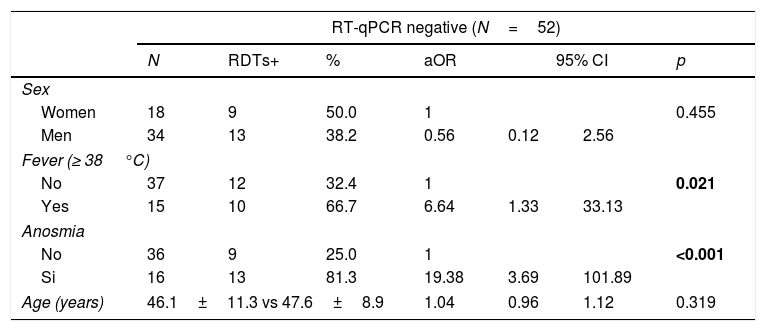
Table 2 shows how anosmia (aOR=19.38) and the presence of fever (aOR=6.64) are strongly and significantly associated with having COVID-19 in subjects who previously tested RT-qPCR-negative.
Distribution of factors associated with being a COVID-19 case in patients with negative RT-qPCR or RDTs.
| RT-qPCR negative (N=52) | |||||||
|---|---|---|---|---|---|---|---|
| N | RDTs+ | % | aOR | 95% CI | p | ||
| Sex | |||||||
| Women | 18 | 9 | 50.0 | 1 | 0.455 | ||
| Men | 34 | 13 | 38.2 | 0.56 | 0.12 | 2.56 | |
| Fever (≥ 38°C) | |||||||
| No | 37 | 12 | 32.4 | 1 | 0.021 | ||
| Yes | 15 | 10 | 66.7 | 6.64 | 1.33 | 33.13 | |
| Anosmia | |||||||
| No | 36 | 9 | 25.0 | 1 | <0.001 | ||
| Si | 16 | 13 | 81.3 | 19.38 | 3.69 | 101.89 | |
| Age (years) | 46.1±11.3 vs 47.6±8.9 | 1.04 | 0.96 | 1.12 | 0.319 | ||
The results of our study reflect that 42.3% of respondents with negative RT-qPCR were positive for some RDT. The symptoms most commonly associated with SARS-CoV-2 infection were fever over 38°C (present in 35.5% of cases) and anosmia (present in 41.8%), a relationship that remained significant in individuals with negative RT-qPCR and some positive RDT (aOR=6.64; 95%CI=1.33–33.13 and aOR=19.38; 95% CI=3.69–101.89 respectively).
RT-qPCR is the most widely used technique in the diagnosis of SARS-CoV-2 infection, given its high reliability.3 However, it is not exempt from false negatives, which may be due to tests with fewer genes detected, mild symptoms or very early stages of the disease, upper respiratory tract samples, denaturation of the materials used and/or delay in transport or processing of the sample.4,5
Our data show that 42.3% of the subjects analyzed were classified as healthy according to the technique of choice (RT-qPCR), presenting infection after the results obtained in the RDTs. The Spanish Society of Intensive and Critical Medicine and Coronary Units (SEMICYUC) warned that false negatives by RT-qPCR can reach 30% in hospitalized patients, referring to a study carried out by Yang et al. in China.8,9 These results highlight the need for caution in diagnosis, especially in cases of negative RT-qPCR and high clinical suspicion.
With regard to symptoms associated with SARS-CoV-2 infection, fever and anosmia were significant in the false negatives. These symptoms associated with the infection were not surprising, given that from the beginning of the pandemic, fever, cough and difficulty breathing were the warning symptoms. According to data published in a recent review, fever is present in 83–98% of cases, but up to 17% of patients may have afebrile illness.5 The Centers for Disease Control and Prevention (CDC) has expanded the list of symptoms associated with the disease to include chills, muscle pain, sore throat, and recent loss of taste or smell.10–12 This last symptom, anosmia, is emerging strongly among the cases detected worldwide, having been described by several studies with a prevalence between 33 and 86%.13,14 Despite not being present in all cases, these are very characteristic symptoms that can arise in the early stage of the disease and should be taken into account as a clinical suspicion.
The results obtained in this study must be interpreted with caution, given that the main limitations are the descriptive nature of the study and the small sample size. However, it is one of the first research studies in Spain to highlight the factors associated with false negatives in the test of choice in the diagnosis of COVID-19, to ensure the diagnosis of the diasease.
ConclusionThe rapid spread of the COVID-19 pandemic requires the swift generation of knowledge to combat this disease. Our results reflect how patients with fever and anosmia and negative RT-qPCR should be considered as suspected COVID-19 cases in clinical practice, given the percentage of false negatives that this test of choice is presenting. Furthermore, it highlights the need to perform a combination of tests, such as the use of RDTs, to ensure the diagnosis of the diasease.
FundingThis research has not received specific support from public sector agencies, the commercial sector or non-profit organizations.
Conflict of interestThe authors declare no conflict of interest.
The authors would like to thank all patients for their participation in this study who have voluntarily agreed to collaborate in the evaluation of diagnostic techniques against COVID-19. Thank you to the primary care management division of the Healthcare Area of Leon and the University of Leon also, because their collaboration has been crucial in developing this study.