Deep vein thrombosis (DVT) and pulmonary embolism (PE) are both complications linked with COVID-19. Lower limb point-of-care clinical ultrasound (POCUS) could detect occult clots, helping decide whom to treat with anticoagulation.
ObjectivesTo determine proximal DVT prevalence with POCUS screening among hospitalized COVID-19 patients.
Patients/MethodsLower limb POCUS was performed in all patients admitted either to the ward or intensive care unit (ICU) between April 22nd and 30th 2020. Clinical and laboratory features, prescriptions, thrombotic complications and outcomes were assessed.
Results87 patients were screened, of which 26 (29.8%) either had been discharged from ICU (19.5%) or were still in critical condition (10.3%). DVT was found in 4 patients (3 femoral, 1 popliteal), of which 1 had not received low molecular weight heparin (LMWH) prophylaxis. 21 CT pulmonary angiograms were performed, being positive for PE in 5 cases (23.8%); only 2 of these patients suffered DVT.
ConclusionsScreening lower extremities with POCUS did not find a high rate of DVT among patients receiving LMWH-prophylaxis. However, there was a noteworthy amount of PE without DVT.
La trombosis venosa profunda (TVP) y la embolia pulmonar (EP) son complicaciones relacionadas con la COVID-19. La ecografía clínica en el punto de atención (POCUS) de las extremidades inferiores podría detectar coágulos ocultos, ayudando a decidir a quién tratar con anticoagulación.
ObjetivosDeterminar la prevalencia de la TVP proximal con el cribado mediante POCUS entre los pacientes hospitalizados por COVID-19.
Pacientes/métodosSe realizó una POCUS de miembros inferiores a todos los pacientes ingresados en planta o en la Unidad de Cuidados Intensivos (UCI) entre el 22 y el 30 de abril de 2020. Se evaluaron las características clínicas y de laboratorio, las prescripciones, las complicaciones trombóticas y los resultados.
ResultadosSe examinaron 87 pacientes, de los cuales 26 (29,8%) habían sido dados de alta de la UCI (19,5%) o seguían en estado crítico (10,3%). Se detectó una TVP en cuatro pacientes (tres femoral, uno poplítea), de los cuales uno no había recibido profilaxis con heparina de bajo peso molecular (HBPM). Se realizaron 21 angiografías pulmonares por TC, siendo positivas para EP en cinco casos (23,8%); solo dos de estos pacientes sufrieron TVP.
ConclusionesEl cribado de las extremidades inferiores con POCUS no encontró una tasa elevada de TVP entre los pacientes que recibían profilaxis con HBPM. Sin embargo, hubo una cantidad notable de EP sin TVP.
Coronavirus disease-2019 (COVID-19) pneumonia may increase events such as venous thrombosis (VT).1,2 Different research has been conducted to prove an increase of deep vein thrombosis (DVT) and pulmonary embolism (PE) prevalence in those patients admitted to the hospital, despite proper prophylaxis. Additionally, there seems to be a gap between deep venous thrombosis (DVT) and pulmonary embolism (PE), being the former higher.3,4 It has been hypothesized that this difference is due to local inflammation, known as endotheliitis, which lead to the formation of thrombi without distal embolism.5
Following the hypothesis, COVID-19 disease is associated with elevated D-dimer values. In fact, higher levels of D-dimer lead to a poorer prognosis.6 Moreover, there is a special concern in determining which D-dimer level could predict thrombotic phenomena.7
Screening lower extremities with an ultrasound exam is an option to detect clots. The use of point-of-care ultrasound (POCUS) by properly trained physicians is highly accurate and an alternative option, especially when the critical situation of many patients dramatically limits feasibility of performing CT pulmonary angiogram (CTPA) to diagnose PE.
ObjectivesTo determine proximal deep vein thrombosis prevalence with POCUS screening among hospitalized COVID-19 patients.
MethodsWe performed an observational retrospective study conducted at a hospital in Granada, Spain. The inclusion criteria were: patients older than 18 years with a confirmed diagnosis of COVID-19 pneumonia who were hospitalized either in the general ward or ICU. Diagnosis of COVID-19 pneumonia was defined as a positive reverse transcription polymerase chain reaction (RT-PCR) test in either nasopharyngeal swab or sputum sample along with radiological findings consistent with pneumonia. No exclusion criteria were made on the basis of median hospital stay, clinical or analytical features.
Patients’ data were collected through their electronic health record (EHR). These consisted of baseline characteristics (mainly those predisposing for VT), analytical features (maximum values of D-dimer, C protein reactive (CPR) and ferritin), clinical factors related either to COVID-19 (time length from symptoms onset to admission, disease severity and complications, use of immunosuppressive therapy [IST], use of LMWH prophylaxis schedule or other anticoagulant therapy) or to VT and its diagnosis. Clinical severity was categorized as mild, moderate, severe and critical, depending on the category achieved during the admission, according the Handbook of COVID-19 of Zhejiang University School of Medicine.
Lower extremity ultrasound (US) was performed in all patients admitted to our hospital due to COVID-19 between April 22nd and 30th 2020 by Internal Medicine specialists trained in POCUS alongside with a radiologist who carried out the exams in all ICU patients and some ward patients in case of discordant findings. The ultrasound device used was a Sonosite X-Porte with a linear probe. The exams were performed at bedside, using a 2-point technique consisting of assessing compressibility of common femoral vein (CFV) and popliteal vein (PV) in search for clots. Additionally, we reviewed the CTPA performed according to physician criteria among our patient series in order to rule out PE.
Categorical variables were presented as frequency and percentage. Continuous quantitative variables were presented whether as mean and its standard deviation (SD) in the case of normal distribution or as median and interquartile range (IQR) otherwise. For comparison, either the Student's t test or the Mann-Whitney U test were performed according to the normality of the variable. * IBM SPSS statistics software version 20.0 (IBM, Chicago, IL, USA) was used for all calculations.
Borrado: A two-tailed alpha error of less than 0.05 was considered statistically significant.
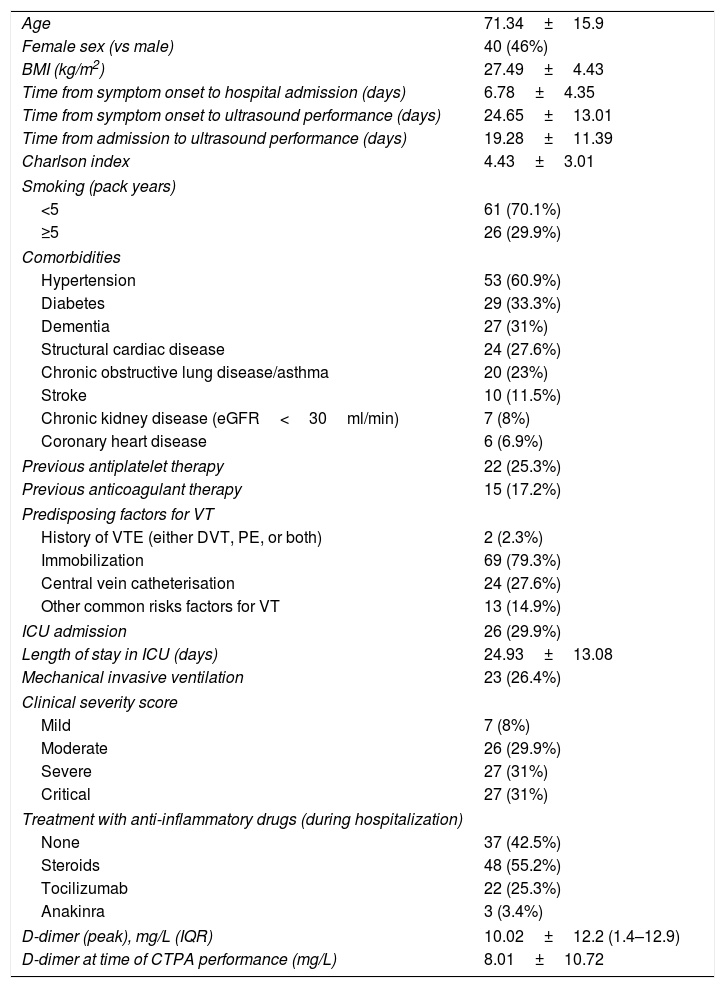
ResultsOur patients’ baseline features are summarized in Table 1. Eighty-seven (87) patients were included. At least one baseline risk factor for thrombosis was found in 14.9% of patients, apart from immobilization. Precisely 17.2% were on anticoagulant therapy prior to admission.
Baseline and clinical characteristics of patients (n=87).
| Age | 71.34±15.9 |
| Female sex (vs male) | 40 (46%) |
| BMI (kg/m2) | 27.49±4.43 |
| Time from symptom onset to hospital admission (days) | 6.78±4.35 |
| Time from symptom onset to ultrasound performance (days) | 24.65±13.01 |
| Time from admission to ultrasound performance (days) | 19.28±11.39 |
| Charlson index | 4.43±3.01 |
| Smoking (pack years) | |
| <5 | 61 (70.1%) |
| ≥5 | 26 (29.9%) |
| Comorbidities | |
| Hypertension | 53 (60.9%) |
| Diabetes | 29 (33.3%) |
| Dementia | 27 (31%) |
| Structural cardiac disease | 24 (27.6%) |
| Chronic obstructive lung disease/asthma | 20 (23%) |
| Stroke | 10 (11.5%) |
| Chronic kidney disease (eGFR<30ml/min) | 7 (8%) |
| Coronary heart disease | 6 (6.9%) |
| Previous antiplatelet therapy | 22 (25.3%) |
| Previous anticoagulant therapy | 15 (17.2%) |
| Predisposing factors for VT | |
| History of VTE (either DVT, PE, or both) | 2 (2.3%) |
| Immobilization | 69 (79.3%) |
| Central vein catheterisation | 24 (27.6%) |
| Other common risks factors for VT | 13 (14.9%) |
| ICU admission | 26 (29.9%) |
| Length of stay in ICU (days) | 24.93±13.08 |
| Mechanical invasive ventilation | 23 (26.4%) |
| Clinical severity score | |
| Mild | 7 (8%) |
| Moderate | 26 (29.9%) |
| Severe | 27 (31%) |
| Critical | 27 (31%) |
| Treatment with anti-inflammatory drugs (during hospitalization) | |
| None | 37 (42.5%) |
| Steroids | 48 (55.2%) |
| Tocilizumab | 22 (25.3%) |
| Anakinra | 3 (3.4%) |
| D-dimer (peak), mg/L (IQR) | 10.02±12.2 (1.4–12.9) |
| D-dimer at time of CTPA performance (mg/L) | 8.01±10.72 |
BMI=body mass index; eGFR=estimated glomerular filtration rate; NOAC=novel oral anticoagulants; VT=venous thrombosis; VTE=venous thromboembolism; PE=pulmonary embolism; DVT=deep venous thrombosis; ICU=intensive care unit; LMWH=low-molecular-weight heparin; IQR=interquartile range; CTPA=computed tomography pulmonary angiogram.
Quantitative variables are expressed in mean±standard deviation, except those related to time, which are expressed in median.
At the time the screening was conducted, 9 (10.3%) were still in critical condition being at the ICU, and 17 patients (19.5%) had previously been there.
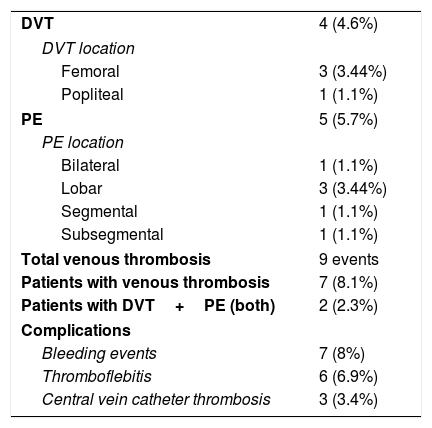
As seen in Table 2, DVT was found in 4 (4.6%) asymptomatic patients: 3 femoral and 1 popliteal location. Among these cases, 3 of them took place despite the use of any kind of prophylaxis strategy or anticoagulant dose. All of them had VT risk factors apart from COVID-19. Median time from the onset of symptoms and hospital admission to US screening resulted in 19.2 (±11.3) and 24.6 (±13.1) days each.
Radiological findings with POCUS or CTPA and complications during hospitalization.
| DVT | 4 (4.6%) |
| DVT location | |
| Femoral | 3 (3.44%) |
| Popliteal | 1 (1.1%) |
| PE | 5 (5.7%) |
| PE location | |
| Bilateral | 1 (1.1%) |
| Lobar | 3 (3.44%) |
| Segmental | 1 (1.1%) |
| Subsegmental | 1 (1.1%) |
| Total venous thrombosis | 9 events |
| Patients with venous thrombosis | 7 (8.1%) |
| Patients with DVT+PE (both) | 2 (2.3%) |
| Complications | |
| Bleeding events | 7 (8%) |
| Thromboflebitis | 6 (6.9%) |
| Central vein catheter thrombosis | 3 (3.4%) |
POCUS=point of care ultrasound; CTPA=computed tomography pulmonary angiogram; DVT=deep venous thrombosis; PE=pulmonary embolism.
CTPA was performed according to physician criteria in 21 patients in order to rule out PE, being detected in 5 cases (23.8%). There were 2 (40%) patients with PE who had concomitant DVT in lower limbs. All patients with PE were classified either as clinically severe or critical (40% and 60%) previously to the finding. Among the 26 ICU patients, 3 (11.6%) of them suffered PE.
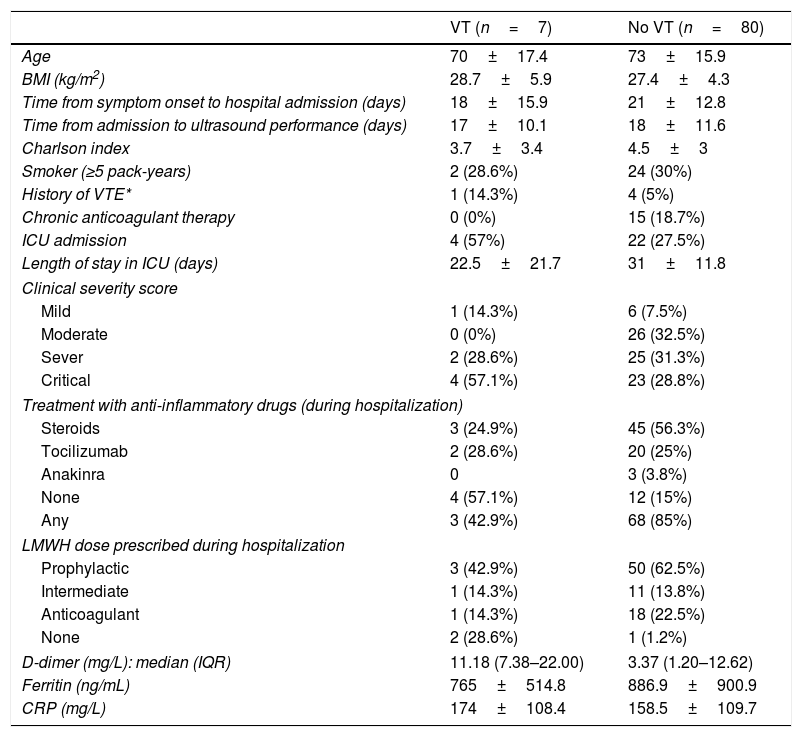
Globally, prevalence obtained for VT (DVT and PE) in our study was 8.1%. Separately, prevalence in critical care unit reached 15.4%, while at ward the rate was 4.9%. The main characteristics of those patients (VT) are shown in Table 3. Within them, we found a higher rate of patients admitted to ICU (57% in VT group vs 27.5% in non-VT group).
Clinical characteristics according to venous thrombosis findings.
| VT (n=7) | No VT (n=80) | |
|---|---|---|
| Age | 70±17.4 | 73±15.9 |
| BMI (kg/m2) | 28.7±5.9 | 27.4±4.3 |
| Time from symptom onset to hospital admission (days) | 18±15.9 | 21±12.8 |
| Time from admission to ultrasound performance (days) | 17±10.1 | 18±11.6 |
| Charlson index | 3.7±3.4 | 4.5±3 |
| Smoker (≥5 pack-years) | 2 (28.6%) | 24 (30%) |
| History of VTE* | 1 (14.3%) | 4 (5%) |
| Chronic anticoagulant therapy | 0 (0%) | 15 (18.7%) |
| ICU admission | 4 (57%) | 22 (27.5%) |
| Length of stay in ICU (days) | 22.5±21.7 | 31±11.8 |
| Clinical severity score | ||
| Mild | 1 (14.3%) | 6 (7.5%) |
| Moderate | 0 (0%) | 26 (32.5%) |
| Sever | 2 (28.6%) | 25 (31.3%) |
| Critical | 4 (57.1%) | 23 (28.8%) |
| Treatment with anti-inflammatory drugs (during hospitalization) | ||
| Steroids | 3 (24.9%) | 45 (56.3%) |
| Tocilizumab | 2 (28.6%) | 20 (25%) |
| Anakinra | 0 | 3 (3.8%) |
| None | 4 (57.1%) | 12 (15%) |
| Any | 3 (42.9%) | 68 (85%) |
| LMWH dose prescribed during hospitalization | ||
| Prophylactic | 3 (42.9%) | 50 (62.5%) |
| Intermediate | 1 (14.3%) | 11 (13.8%) |
| Anticoagulant | 1 (14.3%) | 18 (22.5%) |
| None | 2 (28.6%) | 1 (1.2%) |
| D-dimer (mg/L): median (IQR) | 11.18 (7.38–22.00) | 3.37 (1.20–12.62) |
| Ferritin (ng/mL) | 765±514.8 | 886.9±900.9 |
| CRP (mg/L) | 174±108.4 | 158.5±109.7 |
VT=venous thrombosis; BMI=body mass index; VTE=venous thromboembolism; ICU=intensive care unit; LMWH=low molecular height heparin; CRP=C-reactive protein; IQR=interquartile range.
Quantitative variables are expressed in mean±standard deviation, except those related to time, which are expressed in median.
Patients in the VT group were found to have higher maximum D-dimer values: median 11.18 (IQR 7.38–22.00) mg/L vs. 3.37 (IQR 1.20–12.62) mg/L in the non-VT group. * No other differences were observed in the rest of the analytical parameters.
Borrado p=0.034.
Heparin prophylactic doses were titrated according to centre protocols. Most subjects (62.1%) received LMWH standard doses; 3500 international units (IU) of bemiparin daily. A group of patients (13.8%) received intermediate doses (5000IU bemiparin daily). Up to 19 patients (21.8%) received anticoagulant LMWH doses because of previous indications and only 3 (3.4%) did not receive any LMWH scheme at all. Seven (7, 8%) bleeding events were found among our patients, yet only two of them were major haemorrhages.
DiscussionWhile DVT prevalence in a general inpatient setting has been a controversial topic, some series point out that DVT prevalence might be increased in the course of COVID-19.1–4 Moreover, it appears to be clear that DVT prevalence will be higher when LMWH-prophylaxis is obviated2 or distal DVT is considered.8
The results in our study seem to reveal that proximal DVT prevalence in admitted patients with COVID-19 (4.6%) is similar to that found previously in other series, provided LMWH-prophylaxis is prescribed.8–10
The prevalence of PE events in patients infected with SARS-COV-2 has been suggested to be increased, showing a gap between PE and DVT prevalence.3,4,7,9 Our results (5.7% PE in all patients) show a similar trend, nonetheless only 21 CTPA (24.1%) were performed globally. Among our 5 patients who had PE, only 2 of them were detected to have DVT at the same time.
Being known that COVID-19 disease is associated with elevated D-dimer values among hospitalized patients, our results show higher levels in the VT group, seeming to be at great use to predict thrombotic phenomena among COVID-19 patients.7,8
Antithrombotic prophylaxis among COVID-19 hospitalized patients is widely recommended. Up to 96.6% of our patients received some kind of antithrombotic scheme. Among the 3 patients who did not, 2 thrombotic events were found. The use of higher doses of HBPM are yet to be established.10
In our study DVT screening was performed long after COVID-19 symptoms onset (24.6 days) and patients’ admission (19.3 days). Therefore, chances of missing events that could be developed in the latest days of admission were considerably reduced.
ConclusionsPOCUS is a helpful tool detecting DVT in COVID-19 patients. Our series found a DVT prevalence of 4.6%. However, the absence of DTV did not exclude the presence of PE.
Conflict of interestsThe authors declare that they have no conflict of interest.