Adherence to guidelines on the periendoscopic management of antiplatelet therapy (APT) has not been analyzed in detail. Our aim was to assess adherence to guidelines in patients referred to our Endoscopy Unit on a case-by-case basis, describing in detail the detected deviations and identifying areas of improvement.
Patients and methodsCross-sectional study of outpatients consecutively scheduled for an unsedated upper or lower gastrointestinal endoscopy between January and June 2015. Patients on anticoagulant therapy were excluded.
Results675 patients were evaluated, including 91 (13.5%) patients on APT [upper GI endoscopy 25 (27.5%), lower GI endoscopy 66 (72.5%)]. Contrary to the clinical guidelines, aspirin was discontinued in 25 of the 77 patients previously prescribed the drug (32.5%) but this modification was patient's own decision in 11 cases. Most of the apparent deviations in the management of clopidogrel and dual antiplatelet therapy (DAPT) were not true non-adherence cases. The Primary Care physician modified an APT prescribed by another physician in 8 of 9 cases (88.9%), always in cases with aspirin. No relationship was found between the endoscopic procedure's predicted risk of bleeding or the patient's thrombotic risk and modification of therapy.
DiscussionIn many patients, the peri-procedural management of APT goes against current guidelines, but some of these inconsistencies cannot be considered true deviations from practice. Identified areas for improvement are increasing patient awareness about APT, disseminating the guidelines in Primary Care, and underscoring the significance of thrombotic risk related to APT withdrawal.
El cumplimiento de las guías clínicas sobre el manejo periendoscópico del tratamiento antiagregante plaquetario (TAP) no se ha analizado con detalle. Nuestro objetivo fue analizar caso por caso el cumplimiento de las guías en los pacientes que acuden a nuestra Unidad de Endoscopia, describiendo con detalle las desviaciones detectadas e identificando áreas de mejora.
Pacientes y métodosEstudio transversal sobre pacientes consecutivos programados para gastroscopia o colonoscopia realizadas sin sedación entre enero y junio de 2015. Se excluyeron los pacientes en tratamiento anticoagulante.
ResultadosSe evaluaron 675 pacientes de los que se incluyeron 91 (13,5%) por estar en tratamiento con antiagregante plaquetario (gastroscopias 25 [27,5%], colonoscopias 66 [72,5%]). La aspirina se interrumpió contrariamente a las guías clínicas en 25 de los 77 pacientes que la llevaban (32,5%), pero esta modificación fue una decisión del propio paciente en 11 casos. Muchas de las aparentes desviaciones en el manejo del clopidogrel y del tratamiento antiagregante plaquetario doble (TAPD) no eran verdaderos casos de no cumplimiento. El médico de Atención Primaria modificó el TAP prescrito por otro especialista en 8 de 9 casos (88,9%), siempre en casos de aspirina. No se encontró relación entre el riesgo de sangrado del procedimiento endoscópico o el riesgo de trombosis del paciente y la modificación del tratamiento.
DiscusiónEn una proporción significativa de pacientes el manejo periprocedimiento del TAP va en contra de las guías clínicas, pero algunas de estas desviaciones no pueden considerarse verdaderos incumplimientos. Áreas de mejora son aumentar la información al paciente sobre el TAP, extender la diseminación de las guías a atención primaria y resaltar la importancia del riesgo trombótico relacionado con la suspensión del TAP.
Antiplatelet agents are increasingly being prescribed in clinical practice to prevent stroke and cardiovascular thrombotic events. As a consequence, endoscopists are increasingly facing patients on antiplatelet therapy (APT) as it was shown in a Japanese study, in which 12.8% of patients undergoing endoscopy plus biopsy were on APT.1
A great variability among clinicians and endoscopists has been reported in the management of APT in the peri-endoscopic period despite the fact that several guidelines have been published to address this topic.2–5 For instance, a recent report that reviewed the colonoscopy preparation instruction sheets of 317 endoscopy units in the USA found that in about 50% of cases, instructions were against most of the guidelines’ recommendations.6 Lack of awareness, inertia to change policies, low grade of evidence of guideline's recommendations, variation between guidelines and medicolegal concerns are the most frequently alleged causes for non-adherence.6–8 Therefore, a great effort has been put on improving guidelines design and implementation.
On the other hand, practical issues may also hamper state-of-the-art management of APT prior to an endoscopic procedure. These issues may stay undetected if daily practice is not considered, hampering guidelines implementation and negatively influencing in patient's care.9 However, there is a lack of studies evaluating in detail the APT management on daily practice. A Japanese retrospective study reviewing medical records of patients undergoing an endoscopic procedure during one year confirmed that guidelines were not followed in about 50% of cases. However, possible causes for deviations were not investigated.10
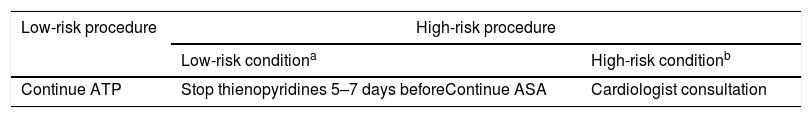
In our institution there is a consensus guideline, developed by gastroenterologists and hematologists, concordant with most of the main available Society Guidelines, which is available at the hospital's intranet. Generally speaking, this guideline recommends not modifying ASA or thienopyridines before low-risk procedures (diagnostic endoscopy±biopsies) and stopping thienopyridines 5–7 days before the procedure without modifying ASA if it is already prescribed for high-risk procedures (Table 1). Our aims were to assess adherence to this guideline at a patient's level describing in detail the detected deviations and identifying areas of improvement.
Patients and methodsData collectionProspective cross-sectional study based on surveys, in which consecutive outpatients on APT therapy and scheduled for unsedated gastroscopy or colonoscopy between January and June 2015 were included. Exclusion criteria were patients scheduled for a sedated endoscopic procedure (deep sedation) or for a complex therapeutic procedure (e.g. ERCP, EUS, EMR) because they receive specific or tailored instructions about APT from the anesthesiologist or the endoscopy unit staff. Patients on anticoagulation therapy were also excluded.
On the day of the examination, the endoscopist collected the following data from the referral note of every included patient: age, gender, procedure, indication for the endoscopic examination and referral doctor's specialty. Then, the patient was asked about APT type and indication, modification of APT prior to the procedure (discontinuation/change), person responsible for this change, and days of withdrawal if applicable. In Spain two formulations of ASA are available: 100mg and 300mg. As there is no clear evidence of a different antiplatelet profile between these two doses11 both were considered as ASA for the aims of our analysis.
Polypectomy, esophageal varices surveillance (with the possibility of band ligation) and stenosis dilation were considered high-risk procedures for bleeding. Most therapeutic and complex procedures, which are more prone to bleeding, are performed under deep sedation and were excluded from the analysis. APT indication was considered primary prophylaxis if the patient did not have a past medical history of thrombotic events. Otherwise, APT indication was considered secondary prophylaxis.
All data were included in an anonymous database. The Institutional Review Board of our center approved the study, and informed consent was obtained from every patient.
EndpointsThe primary endpoint was the non-adherence rate. Non-adherence was considered any deviation from our center approved guidelines (Table 1) not justifiable by the specific characteristics of the patient (e.g. stopping aspirin when it is not indicated or maintaining thienopyridines before a polypectomy in an average risk patient for a thrombotic event) Secondary endpoints were the adequacy to guidelines of the APT modification pattern, responsible for this modification and the description of possible factors related to modification. Medical records were reviewed to assess the adequacy of periendoscopic management of APT taking into account patient's characteristics.
Statistical methodsBaseline characteristics of patients were summarized using descriptive statistics. Categorical variables were described using frequencies and percentages. Continuous variables were summarized using median and range. Distribution of the variables among the groups was compared with the chi-square test, the Fisher exact test or the Mann–Whitney test when appropriate. The significance threshold was 0.05 for all the analyses. Stata software (Stata 14.2, Stata Corp., Texas, USA) was used for statistical analysis.
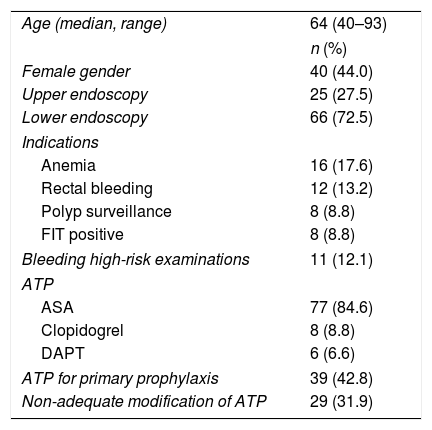
ResultsA total of 668 patients were consecutively evaluated between January and June 2015. Seven patients underwent both an upper gastrointestinal endoscopy and a colonoscopy during the study period. In these cases, because the APT status and management of the same patient could differ depending on the time of the examination, each appointment was considered as an individual patient for analysis purposes. In this way, 675 patients were considered for inclusion [278 (41.2%) upper GI endoscopies and 397 (58.8%) colonoscopies]. According to the referral note, 91 of the 675 patients (13.5%) were on APT, and therefore were included in the study (Table 2). [77 (11.4%) acetylsalicylic acid (ASA), 8 (1.2%) clopidogrel and 6 (0.9%) dual antiplatelet therapy (DAPT)]. Despite the existence of a specific check box asking for antiplatelet agents, 15 referral notes (2.2%) provided no information about APT status. Anemia [6 (24%)] and rectal bleeding [12 (18.2%)] were the most frequent indications for upper and lower endoscopy respectively. Two indications for upper endoscopies (varices surveillance) and 9 indications for lower endoscopy (polypectomy) were considered as high-risk for bleeding.
Characteristics of the 91 patients included.
| Age (median, range) | 64 (40–93) |
| n (%) | |
| Female gender | 40 (44.0) |
| Upper endoscopy | 25 (27.5) |
| Lower endoscopy | 66 (72.5) |
| Indications | |
| Anemia | 16 (17.6) |
| Rectal bleeding | 12 (13.2) |
| Polyp surveillance | 8 (8.8) |
| FIT positive | 8 (8.8) |
| Bleeding high-risk examinations | 11 (12.1) |
| ATP | |
| ASA | 77 (84.6) |
| Clopidogrel | 8 (8.8) |
| DAPT | 6 (6.6) |
| ATP for primary prophylaxis | 39 (42.8) |
| Non-adequate modification of ATP | 29 (31.9) |
ATP=antiplatelet therapy; DAPT=dual antiplatelet therapy; FIT=fecal immunochemical test.
APT modification: Of the 91 patients whose referral note stated that he/she was taking APT, said therapy was modified in 38 cases (41.3%). A detailed description of modifications is summarized in Fig. 1. ASA was stopped in 26 cases (33.8%) always against guidelines. Given patient's characteristics, all cases of DAPT modification could be considered as adequate. On the other side, clopidogrel was not temporarily withdrawn in 3 patients, a decision that could be also considered against guidelines. Therefore, periendoscopic management of APT could be considered against guidelines in 29 cases (31.9%).
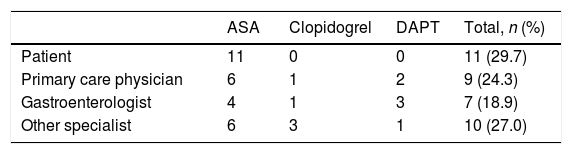
The decision to modify APT was taken by the patient in 11 cases (29.7%), all of whom were on ASA. A physician ordered the modification in the other 26 cases (70.3%), including 15 patients treated with ASA (Table 3). The decision to modify clopidogrel or DAPT was taken only by physicians. Specialists tended to modify their own patient's APT except Primary Care physicians (PCP), who modified other physician's patient APT in 8 of 9 cases (88.9%).
Person responsible for the modification of APT.a
| ASA | Clopidogrel | DAPT | Total, n (%) | |
|---|---|---|---|---|
| Patient | 11 | 0 | 0 | 11 (29.7) |
| Primary care physician | 6 | 1 | 2 | 9 (24.3) |
| Gastroenterologist | 4 | 1 | 3 | 7 (18.9) |
| Other specialist | 6 | 3 | 1 | 10 (27.0) |
In the case of ASA, 6 patients (54.5%) and 12 physicians (80.0%) discontinued administration more than five days before the procedure. Clopidogrel and DAPT were modified more than five days before the procedure in 4 of 5 cases and in 5 of 6 patients, respectively, and a physician made the decision in all cases. In 7 of these 11 patients the modification was not reversed because the physician in charge decided that the APT regimen was no longer necessary.
Factors related to APT modification: No relationship was found between the procedure's predicted risk of bleeding and modification of therapy [modification in high risk procedures 5/11 (45.5%) vs. modification in low risk procedures 32/79 (40.5%): p=0.75]. Paradoxically, APT was modified more frequently in patients on secondary prophylaxis [26 of 41 (63.4%) vs. 8 of 29 (27.6%), p=0.003]. A physician recommended the modification in 21 of the 26 patients receiving secondary prophylaxis (80.8%). In the specific case of patients on ASA, a physician ordered the modification in 11 of those on secondary prophylaxis (68.7%).
DiscussionOur study confirms that the adherence to guidelines on the periendoscopic management of APT is far from good, particularly in the case of ASA. ASA therapy was stopped against guideline's recommendation in 32.5% of cases. Non-adherence to guidelines has been previously described in studies like the one of Lee et al.12 showing that only around 40% of endoscopists follow recommendations by maintaining aspirin before a polypectomy.
However two findings deserve a special comment. First, in 29.7% of cases the patient him/herself was responsible for the interruption of ASA. It is likely that a previous endoscopic procedure in which the patient was told to stop taking aspirin was responsible for this decision. Second, PCPs modified others patients’ APT most probably when patient went to the PCP office looking for advice. These findings identify two new areas of improvement to ensure guideline's implementation.
Clopidogrel and DAPT modification do not seem to follow the same pattern. Neither patients nor PCPs modified this APT. In 63.6% of cases the modification was not reversed after the endoscopic procedure because this therapy was no longer considered to be necessary. In the case of DAPT, 4 of 6 cases modification could have been considered to be against guidelines (2 complete and 2 ASA withdrawals), but in these patients either DAPT was no longer necessary or the other AP agent was indicated as a unique therapy (e.g. cilostazol for peripheral artery disease). Therefore, these deviations cannot be considered as true cases of non-adherence. This is a relevant issue because some studies have suggested that in some cases non-adherence to guidelines may be supported by valid reasons.9 With a trend to considere adherence to guidelines as a quality indicator13 a detailed evaluation of aparent deviation cases seems necessary to avoid missinterpretations.
We were not able to find any relationship between APT modification and the risk of bleeding of the endoscopic procedure and with the indication for APT (primary or secondary prophylaxis), and this is another area of improvement. Of note, physicians had modified treatment principally in patients on secondary prophylaxis, in whom the risk of bleeding was low, suggesting that they tended to fear bleeding more than the thrombotic events that can occur as a result of discontinuation of APT and which actually present an even greater challenge than bleeding.
Regarding the period of withdrawal, in our hospital, we recommend a period of 5 days discontinuation of clopidogrel, in line with most guidelines (Table 1).3,4 First to be noted is that in 48% of cases of ASA withdrawal indicated by a physician stopping was also longer than 5 days, in line with the results of Ono et al.10 In the cases in which clopidogrel was temporarely withdrawn following guidelines this stop was longer than 5 days in many cases. However, in 63.6% of cases clopidogrel or DAPT was definitively stopped once the case was evaluated by an specialist and APT was considered as no longer necessary.
This study is limited by the sample size, which is not large enough to analyze in detail the periendoscopic management of clopidogrel or DAPT. However, the high non-adherence rate in the case of ASA despite the relatively low number of patients included seems to be enough to prompt the development of improvement measures. Another limitation is that its results may be only applicable to outpatients and standard procedures, but this group represents the vast majority of the usual procedures and these patients are the ones to make the most of the identification of weaknesses in guidelines implementation. On the other hand, the strengths of the study are its prospective design at a patient's level and including both upper and lower gastrointestinal endoscopies; and the possibility of gathering information about patients referred to the endoscopy unit by different sources (primary care, gastroenterologists, etc.) with different knowledge about current guidelines.
In conclusion, in a significant proportion of patients the peri-procedural management of APT goes against current guidelines, but some of these inconsistencies cannot be considered as true deviations from practice. Possible areas of improvement for an efficient implementation of a guideline on APT management in the periendoscopic period are increasing patient information about APT, including the Primary Care Physician in the spreading of guidelines and underscoring the importance of the thrombotic risk related to APT withdrawal.
FundingThis research has not been granted by any public, private or non-profit agency or organization.
Conflicts of interestNone.