The 13C-urea breath test (UBT) is the most widely used non-invasive diagnostic test for Helicobacter pylori. Debate continues to surround the possible interference of antacid intake on its result. This study aims to confirm the non-interference of almagate in the determination of H. pylori by UBT.
Patients and methodsObservational, multicentre study in adult patients treated with almagate in whom a UBT (TAUKIT®) was indicated. When the UBT result was negative, use of almagate was stopped for 30 days and the UBT was repeated. When the result was positive, no further determinations were made. The primary endpoint was the percentage of patients who, having had a negative result in the first breath test, were positive in the second after having stopped taking almagate (UBT false negatives, possibly attributable to almagate).
ResultsOf the 167 evaluable patients, 59% were female, average age was 49 and 97% had gastrointestinal symptoms. The result of the first UBT was negative in 71% of cases. Of these, in the second UBT test after stopping the almagate, the negative result was confirmed in 97.5%. Out of the total number of cases evaluated, the rate of false negatives was 1.8%.
ConclusionsTaking almagate has minimal or no interference in the result of UBT for the diagnosis of H. pylori infection. It can therefore be used in the weeks prior to a UBT.
El test del aliento con 13C-urea (TAU) es la prueba no invasiva más utilizada para diagnosticar Helicobacter pylori (H. pylori). La posible interferencia de la toma de antiácidos en su resultado es aún controvertida. El estudio se dirige a confirmar la no interferencia del almagato en la determinación de H. pylori mediante el TAU.
Pacientes y métodosEstudio observacional, multicéntrico, en pacientes adultos en tratamiento con almagato y a los que se indicó un TAU (TAUKIT®). Cuando el resultado del TAU fue negativo, se suprimió la toma de almagato durante 30 días y se repitió un segundo TAU. En los pacientes cuyo resultado fue positivo, no se realizaron más determinaciones. La variable principal a estudio fue el porcentaje de pacientes que teniendo resultado negativo en la primera prueba de aliento, tras suprimir la toma de almagato y repetirla, ésta se positivizó (falsos negativos del TAU, posiblemente atribuibles a almagato).
ResultadosDe los 167 pacientes evaluables, 59% fueron mujeres, la media de edad fue de 49 años y 97% de los casos presentaban sintomatología digestiva. El resultado del primer TAU fue negativo en un 71% de casos. De éstos, en la segunda prueba de TAU tras suprimir almagato, este resultado se confirmó en el 97,5%. El porcentaje de falsos negativos sobre el total de casos evaluados fue del 1,8%.
ConclusionesLa toma de almagato tiene una interferencia mínima o nula en el resultado del TAU para el diagnóstico de la infección por H. pylori; por tanto, se puede utilizar en las semanas previas a la realización del TAU.
Although recent data indicate that infection with Helicobacter pylori (H. pylori) has been waning in recent years, it is still highly prevalent globally1, affecting one third of the population of North America and Europe and up to 50% of the population of Southern and Eastern Europe and Asia.2
In Spain, approximately 50% of the general population is believed to be infected3, the prevalence being higher in some communities (up to 60% in the Community of Madrid)4, and especially in patients who consult for dyspepsia (67%).5
Diagnostic tests for H. pylori infection can be invasive, i.e. requiring an endoscopy, or non-invasive. The most widely used non-invasive test is the 13C-urea breath test (UBT), based on the urease activity of H. pylori and offering high diagnostic precision.5
However, previous studies have revealed frequent UBT false negatives, the most common causes being that the patient has received treatment with antibiotics6 or proton pump inhibitors (PPIs).7 Therefore, PPI treatment should be suspended two weeks before the test.7 The results regarding histamine H2-receptor antagonists (H2 blockers) are controversial, although they mostly show evidence of not affecting, or minimally affecting, the UBT's accuracy.8
PPIs are widely used for the treatment of dyspepsia and reflux9, although for symptomatic management (heartburn), antacids such as almagate10, indicated in adults and children over 12 years11 are often used. Understanding the possible interference of antacids in the UBT is important, both so that they can be avoided before the test is performed, in case they interfere, and so that they can be used as a possible alternative to PPIs in the weeks prior to the UBT if they do not interfere.
In a preliminary study carried out by our group in 30 patients, the use of almagate did not interfere with the UBT results.12 This study’s primary variable was to confirm that the use of almagate does not interfere in the determination of H. pylori by UBT. Whether or not almagate interferes in certain clinical situations or subgroups of patients was also analysed.
Patients and methodsThis was an observational, multicentre, post-authorisation study carried out in 10 Spanish gastroenterology departments, under the usual medical and clinical practice conditions.
Patients of both sexes over 18 years of age who were scheduled to take the UBT test and were undergoing treatment with almagate for 30 days at a dose of 1.5 g of almagate oral suspension every 12 h and up to 24 h before the UBT participated. Pregnant or breast-feeding patients, those on treatment with PPIs or antibiotics in the 30 days before the UBT, or those who in the investigator’s opinion were unable to meet the study requirements were excluded.
The UBT was conducted with the 100 mg 13C-urea TAUKIT® kit (Isomed Pharma) containing a solution enriched with citric acid.5 The samples were analysed in a centralised reference laboratory in Madrid. The cut-off point was 5 per 1000; hence, when the difference of the value of the 13C/12C ratio between baseline and 30 min was >5°/00 it was considered positive for Helicobacter infection.
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practices and was approved by the Clinical Research Ethics Committee (CREC) of the Hospital Universitario de Bellvitge [Bellvitge University Hospital] [EPA010/14 approved on 08/05/2014)]. All patients provided their written consent.
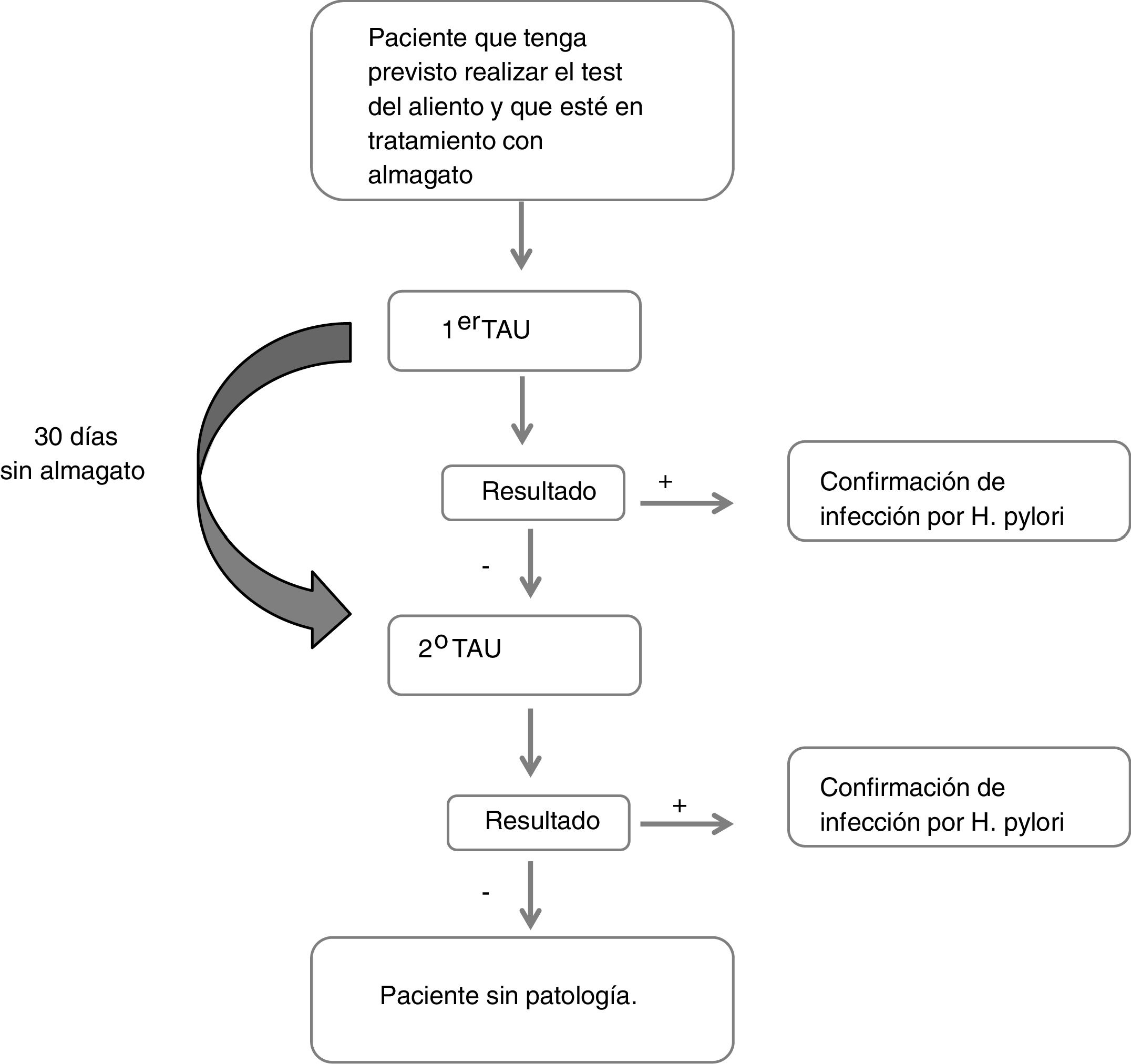
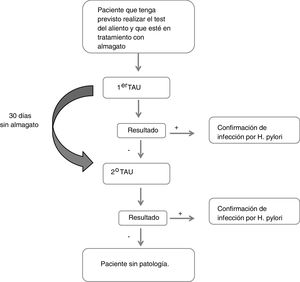
Description of the studyDuring the first visit in which the first UBT was performed, the reason for the indication of the UBT, the data regarding the treatment with almagate (administration date and dosage), and other treatments for digestive diseases or other concomitant pathologies were collected. Similarly, sociodemographic (sex, age, height, weight, BMI, race) and clinical data (gastric symptoms, history of gastric pathology and time since diagnosis, family history of gastric cancer) were collected. If the result of this first test was negative, the patient refrained from taking almagate and the test was repeated at a second visit 30 days later (Fig. 1). A patient was considered to have had a false negative result if they obtained a negative result in the first UBT and a positive result in the second.
All the analyses were carried out from a single sample of evaluable patients that included all those who met the selection criteria and the protocol in order to be able to analyse the study's primary variable. This variable was defined as the percentage of patients with a negative result in the first UBT who were positive in the second after having stopped taking almagate (false negatives in the first UBT). These patients were classified according to the result obtained in the second test.
The analysis of the secondary variables allowed the patients to be characterised according to the UBT result. The aforementioned descriptive biodemographic and clinical variables, the details of the almagate treatment, pathologies and concomitant treatments were compared using percentages or means depending on the nature of the variable.
Statistical analysisThe calculation of the sample size was based on the study's main hypothesis, which was to evaluate the non-interference of almagate in the 13C-urea breath test result. A pilot study12 carried out in a short series of patients detected a negative result in 51.9% of cases; the negative result was confirmed in all of them (100%) when the test was repeated one month after the withdrawal of almagate. The proportion of patients with UBT false negatives performed in two tests was estimated from the binomial distribution, with a 95% confidence interval estimated. A sample size of 182 patients would yield a precision of ±7.5% to estimate the proportion of patients with UBT false negatives performed in two tests with a 95% confidence interval. Assuming 10% of patients that were not valid for analysis, approximately 200 patients needed to be recruited. The calculations were made with the help of the PASS program, 2011 version.
192 patients were recruited, 25 of whom were excluded: one for having been treated with a PPI in the 30 days before the UBT, seven for not having taken almagate as per the protocol (minimum of 25 days before the UBT), and 17 for not having performed the second breath test in the cases required by the protocol. Thus, the final evaluable sample was 167 patients (87% of the patients recruited).
Since there were ultimately 167 evaluable patients for the analysis, the proportion of patients with UBT false negatives was estimated with a precision of ±7.8%, considering that the proportion of negatives is equal to the proportion calculated in the sample size (51.9%).
The confidence intervals (95% CI) for the proportions of patients were calculated with the Wilson method.
The qualitative variables were described using absolute and relative frequencies and the continuous variables using the mean, standard deviation, median, minimum and maximum, including the total number of valid values.
For the comparison of subgroups of patients in the quantitative variables, the parametric Student's t test or the non-parametric Mann-Whitney U test was used according to characteristics of normality of the variables. For the qualitative variables, the χ2 test was performed or, if the necessary conditions were not fulfilled, Fisher's exact test. A level of statistical significance of 0.05 was applied in all the statistical tests.
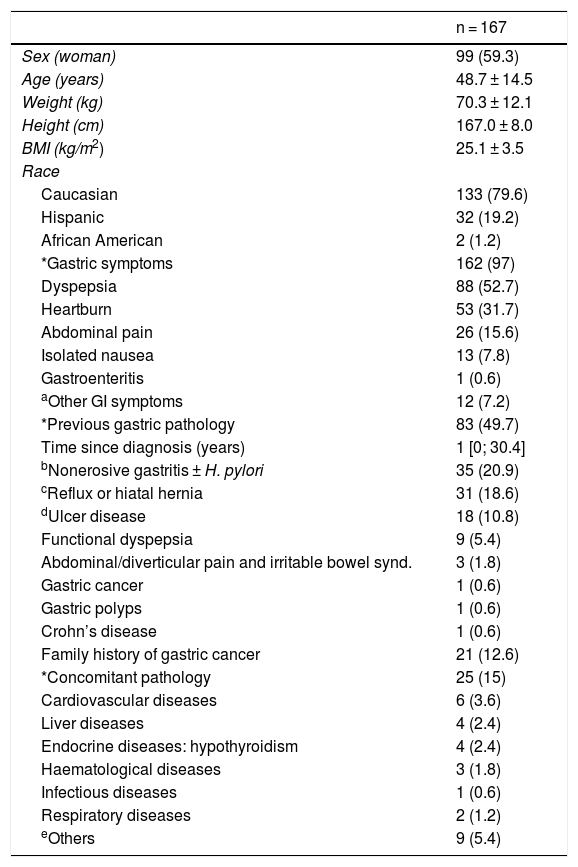
ResultsDemographic and clinical dataThe data are shown in Table 1. Mean age was 48.7 years, 59.3% were women and 79.6% were Caucasian.
Sociodemographic and clinical characteristics.
| n = 167 | |
|---|---|
| Sex (woman) | 99 (59.3) |
| Age (years) | 48.7 ± 14.5 |
| Weight (kg) | 70.3 ± 12.1 |
| Height (cm) | 167.0 ± 8.0 |
| BMI (kg/m2) | 25.1 ± 3.5 |
| Race | |
| Caucasian | 133 (79.6) |
| Hispanic | 32 (19.2) |
| African American | 2 (1.2) |
| *Gastric symptoms | 162 (97) |
| Dyspepsia | 88 (52.7) |
| Heartburn | 53 (31.7) |
| Abdominal pain | 26 (15.6) |
| Isolated nausea | 13 (7.8) |
| Gastroenteritis | 1 (0.6) |
| aOther GI symptoms | 12 (7.2) |
| *Previous gastric pathology | 83 (49.7) |
| Time since diagnosis (years) | 1 [0; 30.4] |
| bNonerosive gastritis ± H. pylori | 35 (20.9) |
| cReflux or hiatal hernia | 31 (18.6) |
| dUlcer disease | 18 (10.8) |
| Functional dyspepsia | 9 (5.4) |
| Abdominal/diverticular pain and irritable bowel synd. | 3 (1.8) |
| Gastric cancer | 1 (0.6) |
| Gastric polyps | 1 (0.6) |
| Crohn’s disease | 1 (0.6) |
| Family history of gastric cancer | 21 (12.6) |
| *Concomitant pathology | 25 (15) |
| Cardiovascular diseases | 6 (3.6) |
| Liver diseases | 4 (2.4) |
| Endocrine diseases: hypothyroidism | 4 (2.4) |
| Haematological diseases | 3 (1.8) |
| Infectious diseases | 1 (0.6) |
| Respiratory diseases | 2 (1.2) |
| eOthers | 9 (5.4) |
BMI: body mass index; GI: gastrointestinal; H. pylori: Helicobacter pylori.
Quantitative data expressed as mean ± SD or median [min; max] and qualitative as n (%).
Flatulence (n = 5), gastroesophageal reflux disease (n = 3), diarrhoea (n = 1), bloating (n = 1), upper gastrointestinal bleeding (n = 1), salivary hypersecretion (n = 1), halitosis (n = 1), vomiting (n = 1).
Gastritis (n = 11), chronic gastritis (n = 5), metaplasia (n = 1), isolated H. pylori infection (n = 18).
The indication for performing the breath test was a previous diagnosis of H. pylori infection in 38.9% (n = 65) of the patients. Of these 65 participants, the diagnosis had been made by breath test in 37 and by endoscopic biopsy in the remaining 28. In the rest, 63.5% (n = 106), the test was performed to ascertain whether or not they were infected for different clinical reasons.
97% of the evaluable patients had presented digestive symptoms, the most common symptoms being dyspepsia (52.7%) and reflux (31.7%). Five patients did not present any digestive symptoms.
In almost half of the patients (49.7%) there was a previous diagnostic certainty of digestive pathology, with a mean time since diagnosis of 2.4 years (±4.7), the most common diagnoses being gastritis (20.9%) and reflux or hiatal hernia (18.6%). The other half (50.3%) without a previous diagnosis of digestive disease was included to study uninvestigated dyspepsia more thoroughly or because they had a family history of gastric cancer (12.6% of cases) or H. pylori infection. A small number of patients were included because of their own desire to find out if they were carriers of the infection.
Concomitant pathologies were described in 15% of patients, the most common being cardiovascular disorders (3.6%), followed by liver disorders and endocrine disorders (2.4% in both cases).
TreatmentsThe mean time from the start of almagate treatment to the first UBT was 1.1 months (±1.0). All the evaluable patients received almagate for a minimum of 25 days prior to the UBT, with a mean of 29.9 days. 97% of the patients took the doses described in the protocol (1.5 g/12 h) and the remaining 3% took a lower (1.5 g/24 h) (four cases) or higher (1.5 g/8 h) (one case) dose.
Only two patients who had reported having a digestive disease were receiving any treatment for it at the time of the UBT or within the previous 30 days. One patient was taking loperamide and the other omeprazole (in this case before the second UBT).
Regarding other associated non-digestive pathologies, 22.2% of the patients evaluated stated that they were receiving concomitant treatment at the time of the UBT. Antihypertensive drugs (5.4%) and systemic hormonal preparations (levothyroxine 4.8%) were the most common, followed by lipid-lowering drugs (4.2%) and antidepressants (3.6%). It should be noted that three patients (1.8%) were taking ranitidine.
UBT resultsThe mean time between the first and second test was 48.5 days (± 26.4), with a median of 38.5 days.
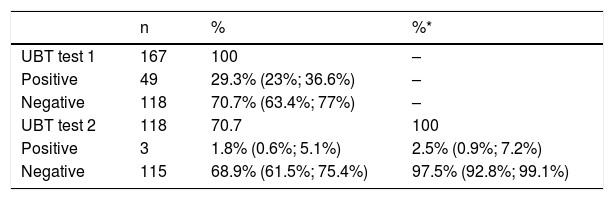
70.7% (95% CI: 63.4%; 77%) of the patients had a negative result in the first breath test. Of these, the second test confirmed the negative result in 97.5% (95% CI: 92.8%; 99.1%), and it was positive (false negatives) in 2.5% (95% CI: 0.9%; 7.2%) (Table 2). The proportion of false negatives out of the total number of patients evaluated (n = 167) was 1.8% (95% CI: 0.6%, 5.1%).
UBT results.
| n | % | %* | |
|---|---|---|---|
| UBT test 1 | 167 | 100 | – |
| Positive | 49 | 29.3% (23%; 36.6%) | – |
| Negative | 118 | 70.7% (63.4%; 77%) | – |
| UBT test 2 | 118 | 70.7 | 100 |
| Positive | 3 | 1.8% (0.6%; 5.1%) | 2.5% (0.9%; 7.2%) |
| Negative | 115 | 68.9% (61.5%; 75.4%) | 97.5% (92.8%; 99.1%) |
Data in brackets indicate the 95% confidence interval.
UBT: 13C-urea breath test.
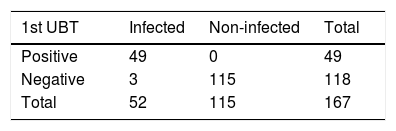
After the groups had been analysed and modified with the study's false negatives, the final results were 115, or 68.9%, uninfected patients (95% CI: 61.5%; 75.4%), and 52, or 31.1%, infected patients (95% CI: 24.6%; 38.5%) (Table 3).
Total infected and non-infected patients.
| 1st UBT | Infected | Non-infected | Total |
|---|---|---|---|
| Positive | 49 | 0 | 49 |
| Negative | 3 | 115 | 118 |
| Total | 52 | 115 | 167 |
UBT: 13C-urea breath test.
Sensitivity: 49/52 = 0.94; 94.2% (95% CI: 84.4%, 98.0%).
Specificity: 115/115 = 1; 100% 95% CI Not calculable.
Positive predictive value (PPV): 49/49 = 1; 100% 95% CI Not calculable.
Negative predictive value (NPV): 115/118 = 0.97; 97.5% (95% CI: 92.8%; 99.1%).
Of these 52 infected patients, 13 belonged to the group previously diagnosed with H. pylori, hence the prevalence of infection in this group was 20% (95% CI 12.1, 31.3) (13/65), and 39 belonged to the group that had not been diagnosed, hence the prevalence of infection in this other group was 38.2% (95% CI 29.4; 47.9) (39/102).
UBT result in terms of other variablesNo statistically significant differences were observed in the UBT result in relation to demographic characteristics, history of previous digestive pathology, other associated diseases or concomitant treatments.
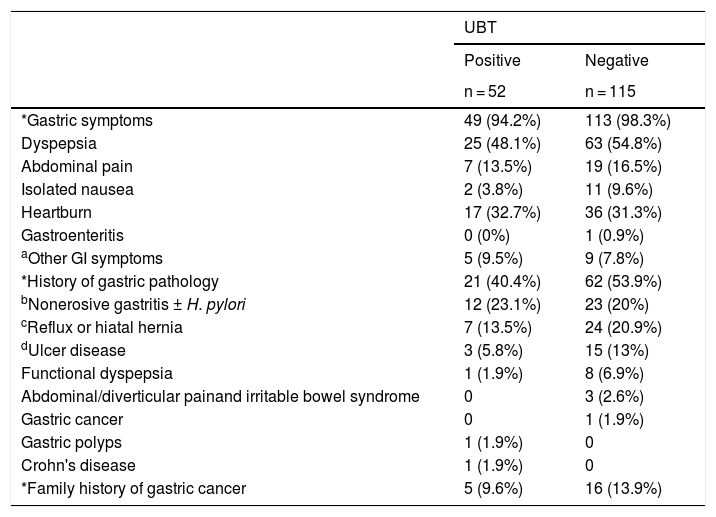
No statistically significant differences were observed for any of the digestive symptoms based on the UBT result (χ2 text; Fisher's exact test; p > 0.05) (Table 4). Five patients (three with a positive result and two with a negative result) did not present any symptoms of dyspepsia.
Gastric symptoms and history according to UBT result.
| UBT | ||
|---|---|---|
| Positive | Negative | |
| n = 52 | n = 115 | |
| *Gastric symptoms | 49 (94.2%) | 113 (98.3%) |
| Dyspepsia | 25 (48.1%) | 63 (54.8%) |
| Abdominal pain | 7 (13.5%) | 19 (16.5%) |
| Isolated nausea | 2 (3.8%) | 11 (9.6%) |
| Heartburn | 17 (32.7%) | 36 (31.3%) |
| Gastroenteritis | 0 (0%) | 1 (0.9%) |
| aOther GI symptoms | 5 (9.5%) | 9 (7.8%) |
| *History of gastric pathology | 21 (40.4%) | 62 (53.9%) |
| bNonerosive gastritis ± H. pylori | 12 (23.1%) | 23 (20%) |
| cReflux or hiatal hernia | 7 (13.5%) | 24 (20.9%) |
| dUlcer disease | 3 (5.8%) | 15 (13%) |
| Functional dyspepsia | 1 (1.9%) | 8 (6.9%) |
| Abdominal/diverticular painand irritable bowel syndrome | 0 | 3 (2.6%) |
| Gastric cancer | 0 | 1 (1.9%) |
| Gastric polyps | 1 (1.9%) | 0 |
| Crohn's disease | 1 (1.9%) | 0 |
| *Family history of gastric cancer | 5 (9.6%) | 16 (13.9%) |
UBT: 13C-urea breath test; GI: gastrointestinal; H. pylori: Helicobacter pylori.
Flatulence (n = 5), gastroesophageal reflux disease (n = 3), diarrhoea (n = 1), bloating (n = 1), upper gastrointestinal bleeding (n = 1), salivary hypersecretion (n = 1), halitosis (n = 1), vomiting (n = 1).
One of the 118 patients with a negative result in the first test indicated that they had received omeprazole in the period before the second UBT; the negative result in this case was confirmed in the second breath test, which is why it was not excluded for evaluation.
Treatment with almagate according to the UBT resultNo statistically significant differences were observed in the UBT result (Mann-Whitney U test; p > 0.05) in terms of the duration of previous treatment with almagate or the dosage, according to the UBT result (Fisher's exact test; p > 0.05).
DiscussionThe prevalence of H. pylori infection in Spain is high and is similar to that of other southern European countries2, reaching 65% in patients with dyspepsia.13
Dyspepsia is a very prevalent condition that affects 20%–25% of western populations.14,15 In the population aged <50-55 years and without warning signs, the recommended initial management is the “test and treat” strategy, which involves a non-invasive test for H. pylori followed by eradication therapy in positive cases.16
In general, the diagnostic accuracy of the UBT is good, with a sensitivity and specificity >95% in most cases.5 Different techniques are used to perform it, and the one used in this study includes citric acid. The cut-off point established is five units, and its sensitivity is 96% (which indicates that there is a small percentage of false negatives). Its specificity is 100% and the positive (PPV) and negative (NPV) predictive values are 100 and 92%17, respectively.
The breath test without citric acid18 and stool antigen test for H. pylori19 have a lower sensitivity but good specificity for de novo diagnosis of H. pylori. However, the stool antigen test has a significant false positive rate when used for post-eradication monitoring.20
The data obtained in our study, with a sensitivity of 94.2% (95% CI: 84.4%; 98%), a specificity of 100% (95% CI not calculable) and high predictive values [PPV: 100% (95% CI not calculable) and NPV: 97.5% (95% CI: 92.8%; 99.1%)] (Table 3), as was to be expected in areas with a high prevalence of infection, are similar to those of the technique's reference study.17 The apparent greater sensitivity [96% (95% CI: 81%; 99%)] of the reference study may be due to the fact that in this case the UBT results were confirmed with two other diagnostic tests and, when they were discordant, which was the case in three patients, they were excluded from the sensitivity and specificity calculations.17 In any case, the confidence interval of the sensitivity of our study is included within the CI of the sensitivity of the reference study.
According to the NPV, the probability of not having H. pylori, if the UBT result is negative, is almost 98%, meaning that when the test result is negative there is still a 2% chance of actually being infected and going undiagnosed (NPV-1). The 95% CI range (92.8%; 99.1%) indicates that in the population treated with almagate the probability of going undiagnosed can vary from 0.9% to 7.2%.
Different drugs can interfere with the UBT result. Antibiotics can cause false negatives6, so it is recommended that they be avoided for at least four weeks before the UBT is performed.21
PPIs are the most widely used drugs in the treatment of dyspepsia and H. pylori.11 However, their use is associated with a decrease in the survival of the Helicobacter and its urease activity22, which can lead to false negatives. Therefore, the UBT test is not reliable if it is performed within two weeks of stopping treatment with PPIs.7,21,22 One of the patients with a negative result in the first test had received omeprazole in the period before it was performed; however, the negative result was confirmed. Omeprazole is documented as one of the PPIs that interfere with UBT22, although some works have suggested that the false negative rate is lower than with other PPIs.23
The impact of H2 blockers, such as ranitidine, on the UBT result is controversial24,25; however, it seems not to interfere, or any interference is of scant relevance.24,26 There are differences in the results between studies that analyse the possible interference of H2 blockers with UBT, which may be attributable to both the duration of the previous treatment with H2 blockers as well as to questions inherent in the actual UBT test (whether it includes citric acid, what the dose is and what the cut-off point is).26 Regarding antacids, their mechanism of action consists of alkalising and neutralising the acid accumulated in the stomach27 and they have no known activity against H. pylori. While it has been asserted that antacids do not interfere with the sensitivity of H. pylori28 breath tests, few studies have been performed to confirm this. Almagate is one of the most widely used antacids10 and is indicated for the relief and symptomatic treatment of stomach acidity and heartburn in adults and children over 12 years.11 In a pilot study in 27 patients, infection was ruled out in 14 in the initial UBT and negativity was confirmed in all cases following the suspension of almagate; i.e. no false negatives were observed. In this study, conducted with the same methodology but in an adequate sample of cases, found a false negative result of the UBT in 1.8% (n = 3) of the patients (n = 167). This datum demonstrates that the breath test is altered in a minimal percentage of patients who have received previous treatment with almagate at the usual recommended doses and confirms the results of the aforementioned pilot study.12
Other factors that cause the test to yield a small percentage of false negatives17 are fasting time (at least eight hours before the test) and the duration of the treatment with the drug potentially causing the interference.29
In our study, it should be noted that two other patients who had also been exposed to ranitidine were not false negatives since one of them tested positive in the first UBT and the other, negative, was confirmed as such in the second.
It is worth analysing the three patients classified as false negatives for almagate the present study who were from the same site. One of them had also taken ranitidine, which could have influenced the test result (although the kit used has citric acid and a high concentration of urea). Therefore, it is difficult to conclude to which treatment, the antacid or the ranitidine, we should attribute responsibility for the false negative. The second false negative can be accounted for by data from the medical history which showed that eradication therapy had been given a few years previously, although the eradication had not been verified and had therefore probably not been achieved. The third case had received eradication therapy some years previously, and the first UBT result was negative (with a delta of 4, limit) that turned positive (delta of 8) in the second UBT, bringing up the question of the limit values between 2 and 5. Although the test's cut-off point is 5, the positive and negative results of the UBT have been seen to lie outside the range of 2/1000 and 5/1000, indicating that the values between 2 and 5 should be confirmed.30
The study's limitations include its observational nature, rendering it more susceptible to possible interference from confounding factors. Furthermore, by including patients who had been previously diagnosed and who therefore had possibly already undergone treatment and eradication, the prevalence of infection in the sample is reduced. As prevalence diminishes, a positive result will not allow confirmation of the diagnosis, and these positives were not confirmed with any other reference test. This would affect the PPV, which may be lower than expected. However, in view of the results of this study, which are very similar to the pilot study, and considering that the sample size is adequate, we believe that it can be asserted that almagate does not significantly interfere with the result of the UBT.
ConclusionsIn conclusion, almagate does not interfere with or has a minimal interference in the result of the breath test for the diagnosis of H. pylori infection. Almagate is a pharmacological alternative for treating the symptoms that patients present in the weeks before the breath test, during which PPI treatment must be suspended a minimum of two weeks before the UBT test to assess whether or not they are infected with H. pylori.
We would also like to acknowledge the participation of the following principal investigators at their reference centres: Dr Josep Merlo Mas (Centre Medicoquirúrgic Servidigest), Dr Llúcia Titó (Hospital de Mataró [Mataró Hospital]), Dr Josep María Botargues (Hospital Bellvitge), Dr José A. Pajares Díaz (Hospital Gregorio Marañón [Gregorio Marañón Hospital]).
Esther Parramón Altarriba from Hospital CIMA Sanitas [Sanitas CIMA Hospital] in Barcelona carried out the UBT tests at that centre.
Almudena Pardo Mateos, PhD, contributed medical writing, which was financed by Almirall, S.A.
Please cite this article as: Pons C, Varas M, Gisbert JP, Barenys M, Pajuelo F, Fernández FJ. Ausencia de interferencia de almagato en los resultados de la prueba de aliento para el diagnóstico de Helicobacter pylori (estudio Almatest). Gastroenterol Hepatol. 2021;44:628–636.