Immunomodulators and biologics are two of the main drugs used for the treatment of inflammatory bowel disease (IBD). Some of these agents have been associated with certain infections and lymphoproliferative disorders, including Epstein–Barr virus (EBV) infection. Our aim was to determine the influence of immunosuppression in the EBV viral load in patients with IBD.
Materials and methodsWe prospectively included naïve patients with IBD who were starting immunosuppressive therapy in four IBD Units. All patients were assessed at baseline and four months after starting immunosuppression for clinical disease activity, biomarkers, EBV serology (IgM VCA, IgG VCA and IgG EBNA) and viral load.
ResultsThirty-two patients were included. At baseline, all patients showed positive results for IgG VCA or IgG EBNA with undetectable EBV viral load. No patient showed detectable EBV viral load after starting the immunosuppressive therapy.
ConclusionImmunosuppression did not influence on EBV viral load in the short-term in naïve IBD patients.
Los fármacos inmunomoduladores y biológicos son algunos de los tratamientos usados con más frecuencia en la enfermedad inflamatoria intestinal (EII). Algunos de ellos se han relacionado con un mayor riesgo de infecciones o síndromes linfoproliferativos, entre los que se encuentra el virus de Epstein-Barr (VEB). Nuestro objetivo era determinar la influencia a corto plazo de la inmunosupresión sobre la carga viral en pacientes con EII.
Material y métodosIncluimos de forma prospectiva pacientes con EII en los que se iniciaba algún tratamiento inmunosupresor en 4 hospitales. Todos los pacientes fueron evaluados en el momento de iniciar el tratamiento y 4 meses después de iniciarlo, mediante la actividad clínica, los biomarcadores, la serología del VEB (IgM VCA, IgG VCA e IgG EBNA) y su carga viral.
ResultadosSe incluyeron 32 pacientes, observando en todos ellos una serología positiva para IgG VCA o IgG EBNA, con una carga viral indetectable. No se observó ninguna muestra con carga viral detectable durante el seguimiento.
ConclusiónLa inmunosupresión no influye sobre la carga viral del VEB a corto plazo en pacientes con EII.
Inflammatory bowel disease (IBD) is a chronic disorder of the gastrointestinal tract. This concept is divided into two different conditions, namely Crohn's disease (CD) and ulcerative colitis (UC).1 The natural history of the disease usually involves a relapsing and remitting course. The drugs that are currently approved for this disorder are directed towards reducing or blocking the key inflammatory mediators involved in the uncontrolled inflammatory activity in the gut. Due to their immunosuppressive effects, immunomodulators (thiopurines and methotrexate) and anti-tumour necrosis factor (TNF) agents are two of the main therapies prescribed in clinical practice. Both of them have shown its efficacy in inducing and maintaining clinical remission in patients with IBD.2,3 One of the main concerns about these drugs is the potential increased risk of some infections and malignancies.4,5
Epstein–Barr virus (EBV) is a member of the Herpesviridae family with worldwide distribution and has a prevalence of more than 90% in the adult population.6 Most of the primary infections occur early in childhood and are usually asymptomatic. During the adolescence it can also be asymptomatic or present as an infectious mononucleosis. After the primary infection this virus usually goes through a latent phase and thus it can escape from cytotoxic T cells for years.6 In immunocompetent individuals, the cellular immunity plays a central role in controlling the replication of the virus.6 Patients under immunosuppressive therapy are at increased risk of haematological malignancies, especially lymphomas. In this setting, the most common types of lymphoma are posttransplant-like lymphomas, that are usually related to a reactivation of a chronic EBV latent infection.7 In the early post-transplantation phase, the clinical onset of these lymphomas in hematopoietic stem cell recipients is usually preceded by a progressive rise in the circulating EBV viral load.8 Subjects receiving haematopoietic stem cell transplantation have been considered at high risk of reactivation of EBV, so close monitoring of EBV viral load has been recommended.9,10 Patients with IBD are also at increased risk of lymphoma, and specially those patients treated with thiopurines.4 The aim of our study was to determine the short-term influence of immunosuppression in the EBV viral load in patients with IBD.
Material and methodsWe designed a prospective and multicentric study in four IBD Units in Spain. Inclusion criteria comprised patients aged ≥18 years old with an established diagnosis of CD or UC, naïve to immunosuppressive therapy (thiopurines, methotrexate or biologic agents) and who were starting any immunosuppressive therapy for their underlying IBD. We excluded patients who have previously received antiviral drugs for Herpesviridae family in the last 12 months, with a previous diagnosis of any lymphoproliferative disorder or have received Rituximab before being included in the study.
Demographic data and the characteristics of the disease were compiled with the following set of variables: age, sex, weight, height, smoking habit, age at diagnosis, disease extent according to the Montreal classification, previous and concomitant medical or surgical treatments and the indication for the immunosuppressive therapy. All patients were assessed at baseline and after 4 months of starting immunosuppression. We determined at both visits the clinical disease activity with the partial Mayo score in UC patients and with the Harvey–Bradshaw index in CD.11,12 Clinical remission was defined by a partial Mayo score ≤2 or a Harvey-Bradshaw score ≤4. All patients were evaluated with EBV serology (IgM viral-capsid antigen [VCA], IgG VCA and IgG Epstein–Barr nuclear antigen [EBNA]) and serum EBV viral load at both visits. C-reactive protein (CRP) was determined at the same time points.
EBV serology was performed in whole blood after centrifugation at 2500rpm for 15min. We measured the titres of IgM and IgG antibodies by chemiluminescence with Liaison® XL (DiaSorin, Vercelli, Italy). EBV viral load was determined in all patients after extracting the DNA (QIAamp DNA Mini Kit, BioRobot® EZ1™, QIAGEN) with real-time PCR (RealStar® EBV PCR Kit, Altona Diagnostics). The lower limit of detection was 220copies/mL, considering the determinations under this value as undetectable. A significant viral load was contemplated if we detected more than 20,000EBVcopies/mL, independently of the serological status.8 Patients with negative results in all the serological examinations were considered as seronegative. Acute infection was considered in those positive to IgM VCA. A late acute infection was contemplated when both, IgM VCA and IgG VCA, were positive. A past infection was diagnosed if positive IgG VCA was present, considering a late past infection if IgG EBNA was detected. Reactivation of EBV or late acute infection was considered if all three tests were positive.13 The Ethics Committee of the Basque Country accepted the study protocol. All patients signed the informed consent before being included in the study.
Descriptive statistics of sociodemographic and clinical variables were calculated using means and standard deviations (SD) or median with interquartile range (IQR) for quantitative data. Frequencies and percentages were used for categorical variables. We used the Chi-Square and Fisher's exact tests for categorical variables as well as the Wilcoxon and Kruskal–Wallis tests when comparing the main characteristics of the cohort. All effects were deemed statistically significant at p<0.05.
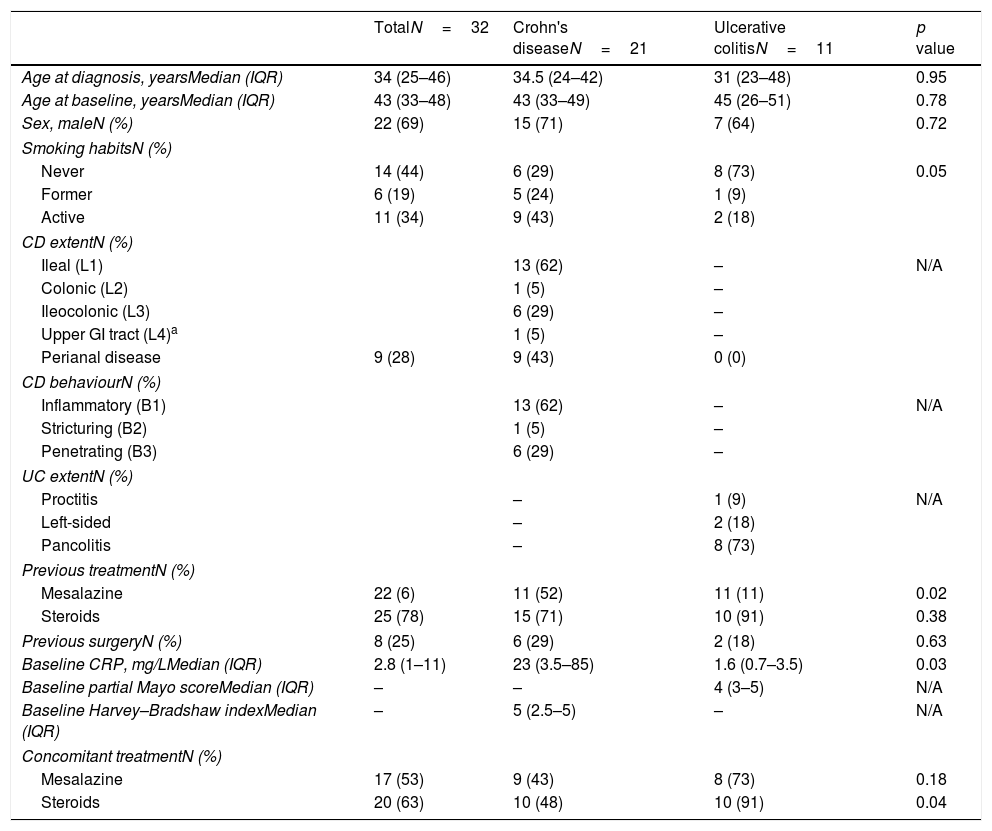
ResultsThirty-two patients were prospectively included in the study. Patient characteristics are summarised in Table 1. The most frequent indications for the immunosuppression were steroid-dependency (41%), moderate to severe disease activity (19%) or fistulising complications (19%). At baseline, 82% and 52% of patients with UC and CD showed clinically active disease, respectively. At this moment, CD patients showed significantly higher CRP levels as compared to UC patients.
Patient characteristics.
| TotalN=32 | Crohn's diseaseN=21 | Ulcerative colitisN=11 | p value | |
|---|---|---|---|---|
| Age at diagnosis, yearsMedian (IQR) | 34 (25–46) | 34.5 (24–42) | 31 (23–48) | 0.95 |
| Age at baseline, yearsMedian (IQR) | 43 (33–48) | 43 (33–49) | 45 (26–51) | 0.78 |
| Sex, maleN (%) | 22 (69) | 15 (71) | 7 (64) | 0.72 |
| Smoking habitsN (%) | ||||
| Never | 14 (44) | 6 (29) | 8 (73) | 0.05 |
| Former | 6 (19) | 5 (24) | 1 (9) | |
| Active | 11 (34) | 9 (43) | 2 (18) | |
| CD extentN (%) | ||||
| Ileal (L1) | 13 (62) | – | N/A | |
| Colonic (L2) | 1 (5) | – | ||
| Ileocolonic (L3) | 6 (29) | – | ||
| Upper GI tract (L4)a | 1 (5) | – | ||
| Perianal disease | 9 (28) | 9 (43) | 0 (0) | |
| CD behaviourN (%) | ||||
| Inflammatory (B1) | 13 (62) | – | N/A | |
| Stricturing (B2) | 1 (5) | – | ||
| Penetrating (B3) | 6 (29) | – | ||
| UC extentN (%) | ||||
| Proctitis | – | 1 (9) | N/A | |
| Left-sided | – | 2 (18) | ||
| Pancolitis | – | 8 (73) | ||
| Previous treatmentN (%) | ||||
| Mesalazine | 22 (6) | 11 (52) | 11 (11) | 0.02 |
| Steroids | 25 (78) | 15 (71) | 10 (91) | 0.38 |
| Previous surgeryN (%) | 8 (25) | 6 (29) | 2 (18) | 0.63 |
| Baseline CRP, mg/LMedian (IQR) | 2.8 (1–11) | 23 (3.5–85) | 1.6 (0.7–3.5) | 0.03 |
| Baseline partial Mayo scoreMedian (IQR) | – | – | 4 (3–5) | N/A |
| Baseline Harvey–Bradshaw indexMedian (IQR) | – | 5 (2.5–5) | – | N/A |
| Concomitant treatmentN (%) | ||||
| Mesalazine | 17 (53) | 9 (43) | 8 (73) | 0.18 |
| Steroids | 20 (63) | 10 (48) | 10 (91) | 0.04 |
CD: Crohn's disease; GI: gastrointestinal; IQR: interquartile range; N/A: not applicable; UC: ulcerative colitis.
Nine patients started monotherapy, 5 of them with thiopurines and six with anti-TNF agents. Eighteen patients received immunosuppressive therapy with two drugs: 14 with steroids plus thiopurines, 3 with steroids plus anti-TNF agents and 1 with thiopurines plus an anti-TNF agent. Three patients received triple immunosuppression with steroids, thiopurines and anti-TNF.
EBV baseline serological status showed a negative IgM VCA in all subjects, a positive IgG VCA in 31 subjects (97%) and positive IgG EBNA in 31 patients (97%). Both patients with a negative IgG VCA or IgG EBNA had a positive IgG EBNA or IgG VCA, respectively. These findings show that 30 patients (94%) were positive for both tests and thus they are suggestive of a late past infection. There were no patients with detectable EBV viral load at baseline.
The second visit was performed after a median of 19 weeks (IQR, 19–23). At this time, 22% and 24% of patients with UC and CD showed clinically active disease, respectively. We found that CRP declined as compared to baseline levels in the overall cohort (1.7mg/L [IQR, 0.1–4.5]). No patient showed a positive result in IgM VCA. All patients but one (97%) showed a positive IgG VCA. The remaining subject with a negative IgG VCA was previously positive in this test and was positive also for IgG EBNA at both visits. For IgG EBNA there were 30 positive results (94%). One patient (3%) had a negative IgG EBNA, but with a positive IgG VCA, maintaining the same results as in the baseline visit. One patient showed an indeterminate IgG EBNA with a positive result in all previous serologies for IgG VCA and IgG EBNA. In this second visit, all samples for EBV viral load were also considered undetectable.
No patient developed any type of lymphoproliferative disorder or EBV-related disease during the follow-up. During this period, three patients increased the daily dose of azathioprine. There were no further changes in the medical treatment. There were 3 patients (9%) who suffered an adverse events, all of them were considered to be related to azathioprine by their treating physician. Two cases presented hepatotoxicity and one lymphopenia, and all of them resolved after reducing the dose of thiopurines. Three patients were admitted because of a flare and were treated with intravenous steroids. No patient required surgery during the follow-up.
DiscussionIn our study we explore for the first time the short-term influence of immunosuppression on EBV kinetics in naïve patients with IBD. Interestingly, we have observed that all patients included in our cohort showed signs of previous exposure to EBV. In this context, no patient showed signs of reactivation of the virus, measured by the EBV viral load at baseline or after starting immunosuppression. However, our results can only be extrapolated to those patients with a previous exposure to EBV.
There is an increasing interest in the influence of EBV in patients with IBD under immunosuppressive therapy. The data from the French cohort demonstrated that most cases of lymphoproliferative disorders reported in patients with IBD receiving thiopurines were post-transplant lymphoproliferative disorder-like B-cell disorders and they were associated with EBV.14 These type of complications arise when there is an impaired function of T-lymphocytes or natural killer cells in the surveillance of a latent EBV infection.15 Two patients in this cohort died from an early fatal postmononucleosis lymphoproliferative disorder in young seronegative men receiving thiopurines. Although these complications are rarely observed (1–3 cases per 1000 patient-years) some authors have advocated to avoid the use of thiopurines in young males seronegative for EBV.16 More recently, there is data indicating that this infection may be related to some complications like the EBV-positive mucocutaneous ulcer.17,18 This lesion can arise in the oropharynx, skin and the gastrointestinal tract. Immunosuppression may play an important role in this complication, as it has been suggested by its resolution in most of the cases after the withdrawal of the immunosuppressive therapy.
Previous studies have evaluated the possible role of EBV in patients already receiving immunosuppressive therapy for their underlying disease.19,20 The first study in this field showed no differences in the EBV viral load in patients with CD being hospitalized or undergoing endoscopic examinations, as compared to seropositive controls without IBD.20 The authors found that 16% and 22% of the samples had detectable EBV viral load in patients receiving thiopurines or infliximab, respectively. Furthermore, they did not find a significant influence on the EBV viral load after the infusion of infliximab. The absence of influence of anti-TNF biologics on EBV viral load has been confirmed in later studies in patients with IBD and rheumatologic diseases.21–23 Nevertheless, current evidence supports that anti-TNF drugs may predispose to the activation of the lytic phase of the virus, at least in the IBD population.24 This may explain the results of the Portuguese cohort, where patients receiving anti-TNF agents showed a higher rate of EBV DNA positivity.19 However, these authors found that it was the group of patients under mesalazine and thiopurines who had the higher EBV viral load (defined as >500copies/mL) as compared to controls. In our cohort we have not observed detectable levels of EBV DNA, but our results are in line with one previous report in our country where undetectable levels were found in a cohort of 30 patients with IBD.25
Our study has some limitations that should be addressed. Although it was performed in four IBD Units, the number of patients included in each cohort is very limited. Moreover, our determination of EBV viral load was performed only after a short period of time after starting immunosuppressive therapy, so we are not able to evaluate the long-term consequences of these drugs. As this is not a routine examination in our centres, we did not evaluate the possible association between different metabolites of thiopurine metabolism or the blood trough levels of the anti-TNF agents and the EBV viral load.
We have evaluated for the first time the influence on EBV viral load in naïve patients with IBD starting immunosuppressive therapy. In this prospective study we did not find an influence on EBV viral load in the short-term. Nevertheless, more prospective studies including a higher number of patients, with multiple determinations of EBV viral load and a longer follow-up should be carried out in order to obtain definite conclusions in this field.
FundingNone.
Author contributionsIR-L conception and design of the study, analysis and interpretation of data and drafted the manuscript. IR-L, OM, LZ, JOZ and NM acquisition of data. MJLG, MA and GC performed the microbiological test. JLC critically reviewed the manuscript for important intellectual content.
Conflicts of interestIR-L: financial support for educational activities from MSD, Pfizer, Abbvie, Takeda, Janssen, Tillotts Pharma, Shire Pharmaceuticals, Ferring, Dr. Falk Pharma and Otsuka.
The remaining authors declare no conflicts of interest related to this manuscript.
Conference presentations: The results of this study were presented in the 2016 Spanish Gastroenterology Annual Meeting and the 2016 European Crohn's and Colitis Organisation Congress.