Immune checkpoint inhibitors (ICIs) are effective agents against several malignancies. However, they are associated with gastrointestinal and liver immune-related adverse events (GI-IrAEs and LI-IrAEs), which can lead to their temporary or permanent discontinuation.
AimThe aim of this study was to evaluate the efficacy and gastrointestinal and liver toxicity of ICIs in oncological treatments in actual clinical practice.
Material and methodsPatients with advanced cancer who received at least 1 ICI dose between May 2015 and September 2018 were retrospectively assessed.
Results132 patients with non-small cell lung cancer (65.15%, n = 86); melanoma (22.7%, n = 30); renal carcinoma (9.09%, n = 12); and other tumours (3%, n = 4) were included. The treatments administered were nivolumab (n = 82), pembrolizumab (n = 28), atezolizumab (n = 13), durvalumab (n = 2), ipilimumab (n = 1) and the antiCTLA-4/PD-1 combination (n = 6). In total, 51 patients (38.6%) developed IrAEs, 17 (12.9%) of which experienced GI-IrAEs. Of these, 8 (47%) needed steroids and 1 patient required surgery due to intestinal perforation. Grade I Li-IrAEs were observed in 4 patients (3.03%): 2 (50%) required corticosteroids and 1 patient had to discontinue treatment. Four patients (66.6%) who received combination therapy experienced GI-IrAEs. IrAE incidence were not associated with age, gender or drug response.
ConclusionsGI-IrAEs are one of the most common adverse events in patients receiving ICIs. A multidisciplinary approach and a greater understanding of these events could help to reduce morbidity and therapy discontinuation.
Los inhibidores del punto de control inmunitario son fármacos eficaces en el tratamiento de diversas neoplasias. Sin embargo, se han relacionado con eventos adversos inmunomediados (EAI) gastrointestinales y hepáticos que pueden desencadenar su interrupción temporal o definitiva.
ObjetivoEvaluar, en condiciones de práctica real, la eficacia y toxicidad gastrointestinal y hepática de los ICIs en tratamientos oncológicos.
Material y métodosEstudio retrospectivo con inclusión de pacientes con diagnóstico de neoplasia avanzada que habían recibido al menos una dosis de ICIs entre mayo de 2015 y septiembre de 2018.
ResultadosSe incluyeron 132 pacientes con neoplasia de pulmón no microcítico (65.15%, n = 86); melanoma (22.7%, n = 30); carcinoma renal (9.09%, n = 12); y otros tumores (3%, n = 4). Los fármacos empleados fueron nivolumab (n = 82), pembrolizumab (n = 28), atezolizumab (n = 13), durvalumab (n = 2), ipilimumab (n = 1) y la combinación antiCTLA-4/PD-1 (n = 6). El 38.6% (n = 51) desarrolló EAI, de tipo gastrointestinal en el 12.9% (n = 17). De ellos, un 47% (n = 8) requirieron esteroides, y en un paciente precisó cirugía por perforación intestinal. En el 3.03% (n = 4) se objetivaron EAI hepáticos grado I: el 50% (n = 2) requirió corticoterapia y en un paciente fue preciso interrumpir el tratamiento. Entre los pacientes con tratamiento combinado, el 66.6% (n = 4) presentó EAI gastrointestinales. La incidencia de EAI no se relacionó con la edad, sexo, ni con la respuesta al fármaco empleado.
ConclusionesLos EAI gastrointestinales son uno de los más frecuentemente observados en pacientes en tratamiento con ICIs. El manejo multidisplicinar y un mayor conocimiento de dichos eventos podría ayudarnos a reducir su morbilidad, así como las interrupciones del tratamiento.
The approach to cancer treatment has changed substantially in recent years with the introduction of new therapeutic targets, helping to improve both patients’ life expectancy and their quality of life.
Checkpoints are molecules on the cell surface which act as endogenous regulators of the immune response by co-inhibiting signalling pathways. They therefore play a crucial role in the prevention of autoimmunity and in the protection of tissues against a pathogenic infection.
Malignant cells can regulate these checkpoints in order to evade the immune response. In the last few years, immune checkpoint inhibitors (ICIs) have been developed with the aim of enhancing the immune response to cancer cells.1
Ipilimumab, a cytotoxic T lymphocyte antigen 4 (CTLA-4) receptor, was the first ICI approved in 2011 by the US Food and Drug Administration (FDA) for the treatment of advanced melanoma.2 Subsequently, new drugs directed against different therapeutic targets have emerged, such as programmed cell death protein 1 (PD-1, and its ligands PD-L1 and 2), which have increased survival in patients with kidney cancer, non-small cell lung cancer, melanoma and even hepatocellular carcinoma.3–5
As a result of the increasing use of these drugs, a wide variety of secondary adverse effects, called immune-related adverse events (irAEs), have been identified. The pathophysiology is not fully understood, but T cells, antibodies and cytokines are thought to play a role in the development of irAEs.6–9 The characteristics and management of irAEs differ with respect to the toxicity commonly observed with other cancer treatments.10–12 They tend to appear in the first few weeks after the start of treatment, but they have also been observed in the weeks after finishing treatment. Skin toxicity is the earliest and most common manifestation.13 In the case of anti-CTLA-4 drugs, such as ipilimumab, irAEs occur in up to 60–85% of patients, the majority being grade 1−2.12
Figures for gastrointestinal toxicity in previous studies show the incidence of diarrhoea to be 12.1–13.7% in patients treated with an anti-PD-1 and 30.2–35.4% in those treated with an anti-CTLA-4, and that of colitis to be 0.7–1.6% with anti-PD-1, 5.7–9.1% with anti-CTLA-4, and 13.6% with combined therapy.14–16 The incidence of liver toxicity is reported to be in the range of 2–5%, with 1–4% being grades 3 and 4.17,18
Depending on the drug used, irAEs make it necessary to discontinue treatment in up to 25% of patients,19 and gastrointestinal irAEs are one of the main causes.11,16 However, there are no well defined criteria for the possible reintroduction of the drug.20 There are different clinical guidelines for managing the toxicity caused by these drugs.12,21 In the case of mild immune-mediated diarrhoea/colitis (grade 1), observation and symptomatic management of the patient are sufficient. From grade 2 up, the ICI should be withdrawn. Starting low-dose steroids (methylprednisolone 0.5−1 mg/kg/day) or intermediate-to-high doses (1−2 mg/kg/day) should be considered if grade 1 symptoms persist or if there are grade 3 or 4 symptoms. In the case of liver toxicity, the ICI should be temporarily discontinued and stricter analytical monitoring carried out in grades 1 and 2. The ICI should be permanently withdrawn in grade 3 and steroids should be introduced at doses of 0.5−1 or 1−2 mg/kg/day in persistent grade 2 or grade 3, respectively. Biologic therapies, such as infliximab, or immunomodulators, such as mycophenolate mofetil, are indicated in cases of severe immune-mediated colitis or hepatitis with no response to previous treatments.
The aim of our study was to analyse the incidence of gastrointestinal and liver irAEs in cancer patients treated with ICIs at our hospital.
Material and methodsThis was an observational, retrospective, single-centre study based on our experience in the use of ICIs in actual clinical practice from May 2015 to September 2018 at the Miguel Servet University Hospital in Zaragoza, Spain.
We included all patients diagnosed with any type of advanced cancer who had received at least one dose of ICI therapy. Gastrointestinal and liver toxicity were classified using the Common Terminology Criteria for Adverse Events v4.0 (CTCAE 4.0),22 from grade 1 to grade 4, according to severity. In the case of gastrointestinal toxicity: grade 1, asymptomatic or mild clinical or diagnostic alteration which does not require treatment; grade 2, moderate symptoms requiring medical treatment; grade 3, severe clinical condition requiring medical treatment; and grade 4, life-threatening. In the case of liver toxicity: grade 1, AST or ALT 1−2.5 × upper limit of normal (ULN) and/or total bilirubin (TotB) 1–1.5 × ULN; grade 2, AST or ALT 2.5−5 × ULN and/or TotB 1.5−3 × ULN; grade 3, AST or ALT > 5 × ULN and/or TotB >3 × ULN, and grade 4, AST or ALT > 8 × ULN. The pattern of liver toxicity was defined according to the ratio (R) (alanine-aminotransferase [ALT] × ULN/alkaline phosphatase [AP] × ULN) in hepatocellular (R ≥ 5), cholestatic (R ≤ 2) and mixed (R 2–5).23,24
Response to treatment was defined based on the iRECIST criteria: complete response, in the event of complete disappearance of malignant lesions and absence of pathological nodes (<10 mm); partial response, if reduction of the tumour burden of the main lesion is >30%, or the main lesion disappears with persistence of secondary lesions; stable disease, in the absence of tumour progression, without meeting criteria for partial or complete response; and disease progression, with an increase in the size of the main lesion ≥20% (at least ≥5 mm) or a significant increase in secondary lesions.25
The quantitative variables are described with the mean and standard deviation, and the qualitative variables by the frequency distribution and the percentages of each category. The chi-squared test with continuity correction was used to compare proportions of the categorical variables. Student's t test was used for continuous variables. The level of significance was taken as p < 0.05. All statistical calculations were carried out using the statistical software R version 3.1.3 (www.https://www.r-project.org/).
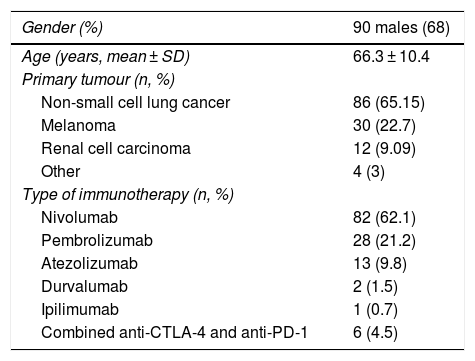
ResultsWe included a total of 132 patients: 32% females (n = 42) and 68% males (n = 90), with a mean age of 66.3 (SD: 10.4). The primary cancers treated were: non-small cell lung cancer (86 patients, 65.15%); melanoma (30 patients, 22.7%); urothelial carcinomas (12 patients, 9.09%); and other tumours (4 patients, 3%). The drugs used were: nivolumab (82 patients, 62.1%); pembrolizumab (28 patients, 21.2%); atezolizumab (13 patients, 9.8%); durvalumab (2 patients, 1.5%); and ipilimumab (1 patient, 0.7%). Six patients (4.5%) received combined treatment with anti-CTLA-4 and anti-PD-1 (Table 1).
Baseline characteristics of the patients.
| Gender (%) | 90 males (68) |
|---|---|
| Age (years, mean ± SD) | 66.3 ± 10.4 |
| Primary tumour (n, %) | |
| Non-small cell lung cancer | 86 (65.15) |
| Melanoma | 30 (22.7) |
| Renal cell carcinoma | 12 (9.09) |
| Other | 4 (3) |
| Type of immunotherapy (n, %) | |
| Nivolumab | 82 (62.1) |
| Pembrolizumab | 28 (21.2) |
| Atezolizumab | 13 (9.8) |
| Durvalumab | 2 (1.5) |
| Ipilimumab | 1 (0.7) |
| Combined anti-CTLA-4 and anti-PD-1 | 6 (4.5) |
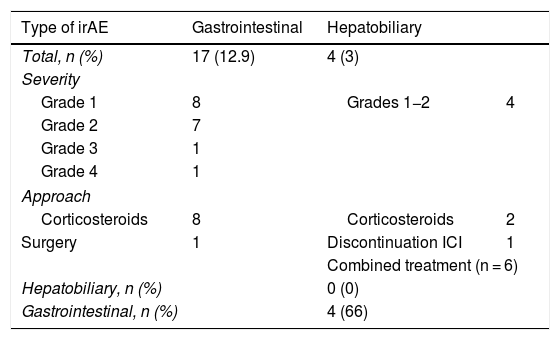
None of the patients had a complete response to treatment, and 30 patients died before the response was assessed. Of the remaining patients, 19.6% (n = 20) obtained a partial response, 27.5% (n = 28) showed criteria of stable disease, and in 52.9% (n = 54), the disease progressed despite treatment. In 38.6% (n = 51), some type of irAE occurred, with liver involvement in 3.03% and colitis-type gastrointestinal involvement in 12.9% (n = 17): eight grade 1; seven grade 2; one grade 3; and one grade 4. Seven of these patients went to Accident and Emergency, with a mean of 1.71 visits (1–3 visits), and six of them required hospitalisation, with a mean stay of 18.8 days (range 1–70 days). Colonoscopy was performed in two patients who met criteria for grade 2 colitis, with histologically confirmed findings of moderate proctitis and mild pancolitis. Oral steroids were required in 47% (n = 8) of cases (1 mg/kg/day) with a good response, except in one patient who required surgery for intestinal perforation, without further treatment. Liver irAEs occurred in 3.03% (n = 4) of patients, consisting of mild hepatitis (grade 1 and 2) with a hepatocellular pattern in three patients (mean ratio 11.03, range 7.48–18) and cholestasis in one (ratio 0.47), resolved with the withdrawal of the drug in the two grade 1 cases, but not in the two cases with grade 2, who required oral corticosteroid therapy (1 mg/kg/day), as their abnormal lab test results persisted. Of the six patients with combined treatment, four (66.6%) had gastrointestinal adverse effects, but none had any reported liver involvement (Table 2). Other types of irAEs were also observed, which were cutaneous in 9.09% (n = 12), endocrine in 9.09% (n = 12), pulmonary in 6% (n = 8) and renal in 2.27% (n = 3). The incidence of irAEs was not related to age, gender or response to the drug used. There were no deaths as a consequence of gastrointestinal or liver adverse effects.
Description of gastrointestinal and hepatic immune-related adverse events.
| Type of irAE | Gastrointestinal | Hepatobiliary | |
|---|---|---|---|
| Total, n (%) | 17 (12.9) | 4 (3) | |
| Severity | |||
| Grade 1 | 8 | Grades 1−2 | 4 |
| Grade 2 | 7 | ||
| Grade 3 | 1 | ||
| Grade 4 | 1 | ||
| Approach | |||
| Corticosteroids | 8 | Corticosteroids | 2 |
| Surgery | 1 | Discontinuation ICI | 1 |
| Combined treatment (n = 6) | |||
| Hepatobiliary, n (%) | 0 (0) | ||
| Gastrointestinal, n (%) | 4 (66) | ||
irAE: immune-related adverse event.
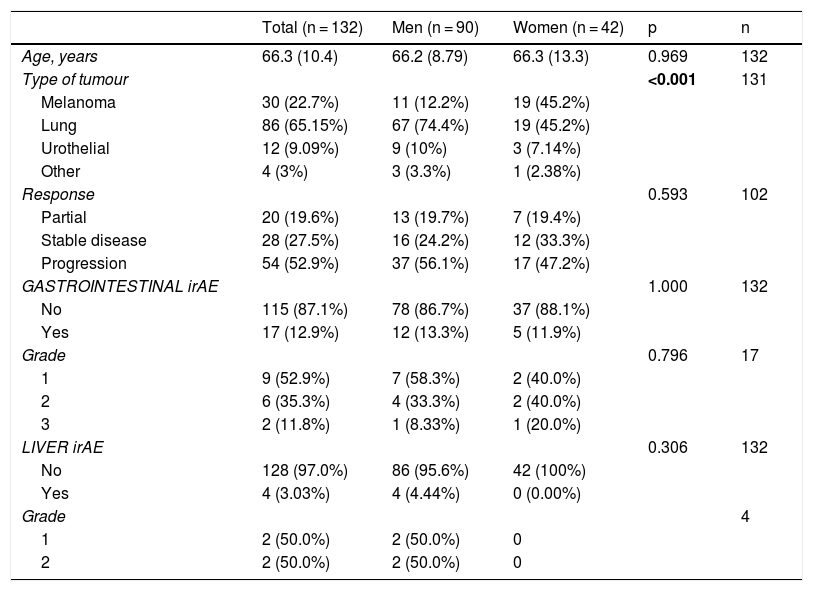
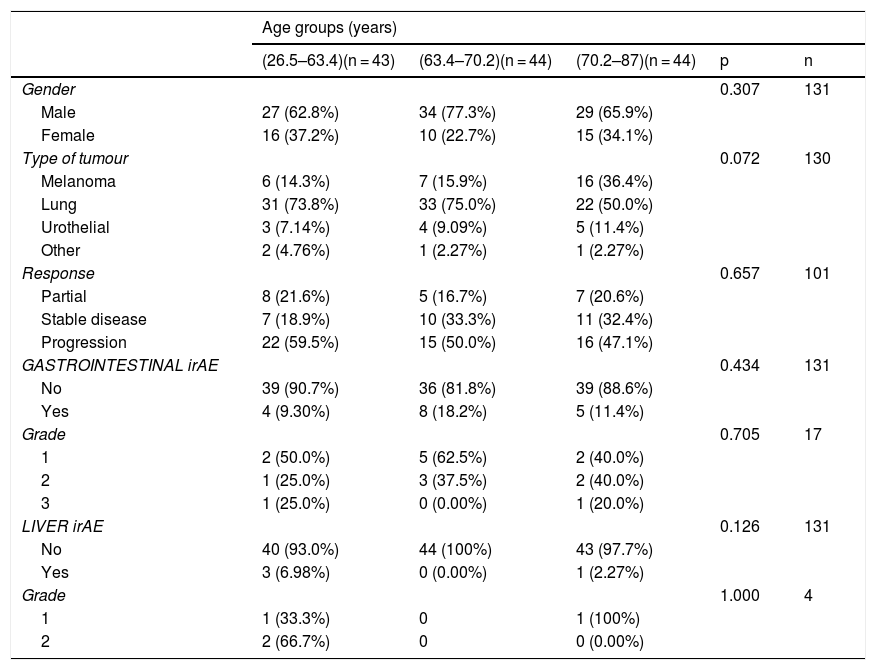
The gender-stratified analysis identified statistically significant results in terms of cancer type and gender, with lung cancer being the most prevalent among males; among females, lung cancer and melanoma were equally prevalent. There were no differences between males and females in terms of age, type of response to immunotherapy, presence or absence of toxicity and grade of toxicity (Table 3). In the age group-stratified analysis, no statistically significant differences were found in relation to gender, response to immunotherapy, or the incidence and grade of irAE (Table 4).
Analysis of patients stratified by gender.
| Total (n = 132) | Men (n = 90) | Women (n = 42) | p | n | |
|---|---|---|---|---|---|
| Age, years | 66.3 (10.4) | 66.2 (8.79) | 66.3 (13.3) | 0.969 | 132 |
| Type of tumour | <0.001 | 131 | |||
| Melanoma | 30 (22.7%) | 11 (12.2%) | 19 (45.2%) | ||
| Lung | 86 (65.15%) | 67 (74.4%) | 19 (45.2%) | ||
| Urothelial | 12 (9.09%) | 9 (10%) | 3 (7.14%) | ||
| Other | 4 (3%) | 3 (3.3%) | 1 (2.38%) | ||
| Response | 0.593 | 102 | |||
| Partial | 20 (19.6%) | 13 (19.7%) | 7 (19.4%) | ||
| Stable disease | 28 (27.5%) | 16 (24.2%) | 12 (33.3%) | ||
| Progression | 54 (52.9%) | 37 (56.1%) | 17 (47.2%) | ||
| GASTROINTESTINAL irAE | 1.000 | 132 | |||
| No | 115 (87.1%) | 78 (86.7%) | 37 (88.1%) | ||
| Yes | 17 (12.9%) | 12 (13.3%) | 5 (11.9%) | ||
| Grade | 0.796 | 17 | |||
| 1 | 9 (52.9%) | 7 (58.3%) | 2 (40.0%) | ||
| 2 | 6 (35.3%) | 4 (33.3%) | 2 (40.0%) | ||
| 3 | 2 (11.8%) | 1 (8.33%) | 1 (20.0%) | ||
| LIVER irAE | 0.306 | 132 | |||
| No | 128 (97.0%) | 86 (95.6%) | 42 (100%) | ||
| Yes | 4 (3.03%) | 4 (4.44%) | 0 (0.00%) | ||
| Grade | 4 | ||||
| 1 | 2 (50.0%) | 2 (50.0%) | 0 | ||
| 2 | 2 (50.0%) | 2 (50.0%) | 0 |
irAE: immune-related adverse event.
Analysis of patients stratified by age groups.
| Age groups (years) | |||||
|---|---|---|---|---|---|
| (26.5–63.4)(n = 43) | (63.4–70.2)(n = 44) | (70.2–87)(n = 44) | p | n | |
| Gender | 0.307 | 131 | |||
| Male | 27 (62.8%) | 34 (77.3%) | 29 (65.9%) | ||
| Female | 16 (37.2%) | 10 (22.7%) | 15 (34.1%) | ||
| Type of tumour | 0.072 | 130 | |||
| Melanoma | 6 (14.3%) | 7 (15.9%) | 16 (36.4%) | ||
| Lung | 31 (73.8%) | 33 (75.0%) | 22 (50.0%) | ||
| Urothelial | 3 (7.14%) | 4 (9.09%) | 5 (11.4%) | ||
| Other | 2 (4.76%) | 1 (2.27%) | 1 (2.27%) | ||
| Response | 0.657 | 101 | |||
| Partial | 8 (21.6%) | 5 (16.7%) | 7 (20.6%) | ||
| Stable disease | 7 (18.9%) | 10 (33.3%) | 11 (32.4%) | ||
| Progression | 22 (59.5%) | 15 (50.0%) | 16 (47.1%) | ||
| GASTROINTESTINAL irAE | 0.434 | 131 | |||
| No | 39 (90.7%) | 36 (81.8%) | 39 (88.6%) | ||
| Yes | 4 (9.30%) | 8 (18.2%) | 5 (11.4%) | ||
| Grade | 0.705 | 17 | |||
| 1 | 2 (50.0%) | 5 (62.5%) | 2 (40.0%) | ||
| 2 | 1 (25.0%) | 3 (37.5%) | 2 (40.0%) | ||
| 3 | 1 (25.0%) | 0 (0.00%) | 1 (20.0%) | ||
| LIVER irAE | 0.126 | 131 | |||
| No | 40 (93.0%) | 44 (100%) | 43 (97.7%) | ||
| Yes | 3 (6.98%) | 0 (0.00%) | 1 (2.27%) | ||
| Grade | 1.000 | 4 | |||
| 1 | 1 (33.3%) | 0 | 1 (100%) | ||
| 2 | 2 (66.7%) | 0 | 0 (0.00%) | ||
irAE: immune-related adverse event.
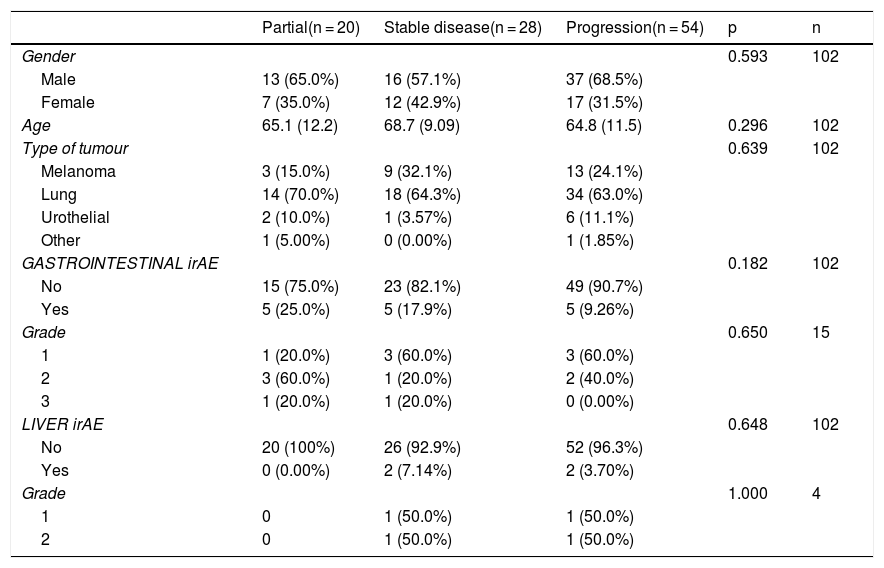
According to type of response to treatment, gastrointestinal toxicity occurred in 25% (n = 5) of those who obtained a partial response, 17.9% (n = 5) of those with stable disease and 9.26% (n = 5) of those with disease progression. None of the patients who had a partial response developed liver toxicity. However, liver toxicity did occur in 7.14% of the patients who had stable disease and in 3.7% (n = 2) of those with disease progression. These results were not statistically significant (Table 5).
Analysis of patients stratified by type of response.
| Partial(n = 20) | Stable disease(n = 28) | Progression(n = 54) | p | n | |
|---|---|---|---|---|---|
| Gender | 0.593 | 102 | |||
| Male | 13 (65.0%) | 16 (57.1%) | 37 (68.5%) | ||
| Female | 7 (35.0%) | 12 (42.9%) | 17 (31.5%) | ||
| Age | 65.1 (12.2) | 68.7 (9.09) | 64.8 (11.5) | 0.296 | 102 |
| Type of tumour | 0.639 | 102 | |||
| Melanoma | 3 (15.0%) | 9 (32.1%) | 13 (24.1%) | ||
| Lung | 14 (70.0%) | 18 (64.3%) | 34 (63.0%) | ||
| Urothelial | 2 (10.0%) | 1 (3.57%) | 6 (11.1%) | ||
| Other | 1 (5.00%) | 0 (0.00%) | 1 (1.85%) | ||
| GASTROINTESTINAL irAE | 0.182 | 102 | |||
| No | 15 (75.0%) | 23 (82.1%) | 49 (90.7%) | ||
| Yes | 5 (25.0%) | 5 (17.9%) | 5 (9.26%) | ||
| Grade | 0.650 | 15 | |||
| 1 | 1 (20.0%) | 3 (60.0%) | 3 (60.0%) | ||
| 2 | 3 (60.0%) | 1 (20.0%) | 2 (40.0%) | ||
| 3 | 1 (20.0%) | 1 (20.0%) | 0 (0.00%) | ||
| LIVER irAE | 0.648 | 102 | |||
| No | 20 (100%) | 26 (92.9%) | 52 (96.3%) | ||
| Yes | 0 (0.00%) | 2 (7.14%) | 2 (3.70%) | ||
| Grade | 1.000 | 4 | |||
| 1 | 0 | 1 (50.0%) | 1 (50.0%) | ||
| 2 | 0 | 1 (50.0%) | 1 (50.0%) |
irAE: immune-related adverse event.
The possibility of toxicity should be considered in all patients on ICI treatment who develop gastrointestinal symptoms. The symptoms and the radiological or endoscopic findings can mimic other conditions, and this requires multidisciplinary management and differential diagnosis with inflammatory bowel disease, infections (clostridioides, cytomegalovirus, etc.)26 and even tumour progression.27
In our study, gastrointestinal and skin toxicity were the most common, with an irAE incidence similar to those reported in other series: 12.9% for gastrointestinal toxicity and 3% for liver toxicity.14–18 No cases of severe liver failure were recorded. There were no deaths associated with the use of the ICI and only one case of grade 4 gastrointestinal toxicity was identified in a patient on combined treatment. In other series, pulmonary and cardiac toxicity have been described as the main causes of death.28,29
According to the current European Society for Medical Oncology (ESMO) recommendations,12 in patients with mild diarrhoea (grade 1), symptomatic management is sufficient, and in most cases the treatment can continue. In moderate-to-severe cases (grades 2–4), the ICI will need to be postponed or discontinued and the use of corticosteroids or immunosuppressants such as anti-TNFs considered if the severity persists despite the withdrawal of the drug.30 The use of vedolizumab in these patients has also been reported, but the available data are still limited.31
In cases of liver toxicity, the recommendation is to monitor transaminase levels and start corticosteroids or even immunosuppressants in patients with severe hepatitis, assessing the need for a liver biopsy and referring the patient to a specialist, if necessary.12 It has to be taken into account that starting immunosuppressive treatment in these patients means an additional risk of complications which will need to be closely monitored.32
In our study, irAEs were managed according to current clinical practice guidelines,12,33 although some exceptions were made regarding the use of steroids: three of the nine patients with grade 1 colitis were given low-dose corticosteroids due to persistence of symptoms despite symptomatic treatment. In all cases, it was possible to withdraw the steroid without subsequent deterioration in the patient’s clinical or analytical parameters. In two of the six patients with grade 2 gastrointestinal toxicity, remission was obtained with conservative management, without requiring the use of corticosteroids.
At present, we do not have predictive factors of toxicity, nor has any clear relationship been established between the dose of ICI used and its severity.34,35 Research has been underway over the last few years to find markers that will allow us to detect or predict the risk of these adverse effects. Faecal calprotectin has high sensitivity and specificity in the diagnosis of inflammatory diarrhoea. However, it is not specific for the detection of immune-mediated colitis, and although its elevation reflects intestinal inflammation, it does not predict the development of immune-related toxicity.36 Other markers such as C-reactive protein and serum albumin can be helpful, but their low specificity has to be taken into account, as their values can be affected by other systemic inflammatory processes. The utility of other biomarkers such as serum interleukin-17 and eosinophil blood count have been studied in these patients; an increase in these parameters was identified in cases of toxicity, but they were not clear predictors of the condition.37,38
Inflammatory or autoimmune diseases and solid organ or haematopoietic stem cell transplantation are some of the other situations reported as possible risk factors for the development of irAEs, although the data published to date are contradictory, and our study did not identify a clear risk profile.39,40 Similarly, the degree of immune-mediated toxicity does not seem to be a predictor of the efficacy of these therapies, and our findings were consistent with that idea.41 However, previous studies have described a greater oncological response with some combined treatments, as well as a higher incidence of certain irAEs with certain ICIs,15,42–44 even going as far as relating the development of irAEs to the patient's intestinal microbiota.45,46
ConclusionsGastrointestinal irAEs are among the most common AEs in patients receiving ICI therapy. The development of irAEs was not related to treatment response in our study. Multidisciplinary management and a greater understanding of these events could help us reduce the associated morbidity and mortality rates and interruptions in treatment.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Sanz-Segura P, García-Cámara P, Fernández-Bonilla E, Arbonés-Mainar JM, Bernal Monterde V. Efectos adversos inmunomediados gastrointestinales y hepáticos inducidos por los inhibidores del punto de control inmunitario: estudio descriptivo observacional. Gastroenterol Hepatol. 2021;44:261–268.