The present article makes a brief review about dental management of the patients with cirrhosis. It focus on problems related with infections, haemorrhagic events and treatment with drugs of common use in odontology.
The present article provides a brief review of dental management in patients with cirrhosis. It focusses on problems related to infections, haemorrhagic events, and treatment with commonly used drugs in odontology.
The special characteristics of dental management of cirrhotic patients must be understood to minimize possible complications in the treatment of patients with liver disease. These complications are mainly due to alterations in haemostasis, drug metabolism, and the greater predisposition to infection found in these patients.
Our main objective was to establish evidence-based therapeutic action protocols that can serve as an approach to outpatient dental management of the cirrhotic patient. To that end, we performed a literature review: firstly, to study the main systemic abnormalities related with cirrhosis that might affect dental management of these patients (risk of haemorrhage, infection and altered drug metabolism); and secondly, to review the recommendations and measures reported in the literature to prevent the onset of undesired events during and after outpatient dental treatment in the cirrhotic patient.
Materials, methods and search strategyA literature review of the topic was carried out using the following search strings: “(cirrhosis) AND dental bleeding”, “(liver cirrhosis) AND dental treatment”, “(Hepatic cirrhosis) AND dental infections”,“(liver disease) AND dental infections”, “(Hepatic cirrhosis) AND tooth”, “(Liver disease) AND oral mucosa”, “(Cirrhotic patient) AND prescription”, “(Liver disease) AND endocarditis”, “Analgesics cirrhotic patient”, “(Drug liver injury) AND cirrhosis” and (liver disease) AND antibiotic- induced liver injury”. A total of 2562 articles were found. After reading the title/abstract, a preliminary selection was made of articles related to bleeding, risk of infection and pharmacological risk in the cirrhotic patient. Articles published in English and/or Spanish over the last decade (2004–2013, inclusive) considered of interest for the topic were then selected. As an exception, 1 article in French was included, as it was considered relevant for the review. A total of 19 articles were eventually included.
Literature reviewOral manifestations in the cirrhotic patientThe presence of oral manifestations in patients with cirrhosis, such as the appearance of haemorrhages, petechiae, haematomas, jaundiced mucosa, gingival bleeding, glossitis and sialadenosis, may be concomitant with the appearance of other signs and symptoms of liver dysfunction, and could indicate decompensation of the cirrhosis.1 Furthermore, treatment with diuretics reduces salivary flow (hyposalivation), thus increasing the risk for caries, gingival inflammation and candidiasis.2 Evidence obtained from experiments in animals also suggests that there may be a delay in cicatrisation and in the formation of spongy bone following simple or surgical extractions.3 Differential diagnosis of oral neoplasms should include oral squamous cell carcinoma (OSCC), which has been related to alcoholic cirrhosis,2 and the possibility of oral metastases from a hepatocellular carcinoma, since cirrhosis patients are at high risk for this type of tumour.4
Risk of infection in the cirrhotic patient. Prophylaxis and treatmentThe likelihood of infections is higher in cirrhotic patients because their immunosuppressed state (which will vary depending on the stage of the disease) increases their susceptibility to systemic infections.5 Dental infections are a port of entry through which bacteria and toxins can enter the blood and increase the state of systemic inflammation. In a healthy body, these small bacteraemias are neutralized by components of the immune system, but in cirrhosis, clearance of circulating endotoxins, bacteria and inflammatory mediators is compromised due to hepatic dysfunction.6
The effect of oral infections on the progression of cirrhosis has been extensively studied in the last decade. The need for dental treatment (apical periodontitis, pockets larger than 6mm, root fragments or large loss of bone support) has been positively correlated in some patients with the more advanced stages of cirrhosis, greater urgency for liver transplant, and alcoholic cirrhosis,6,7 although a cause–effect relationship between the severity of the dental and liver disease has not been demonstrated. Alcohol in turn is a substance that interferes with protein metabolism and tissue healing, both processes related with periodontal disease. Moreover, serum cytokines, also implicated in the process of periodontal inflammation and destruction, are elevated in patients with cirrhosis (especially alcoholic cirrhosis), which could increase the prevalence and severity of periodontitis in these patients.8
Patients with cirrhosis should undergo regular dental checkups to maintain good oral hygiene, thereby preventing oral infections and avoiding invasive treatments.2,6,7
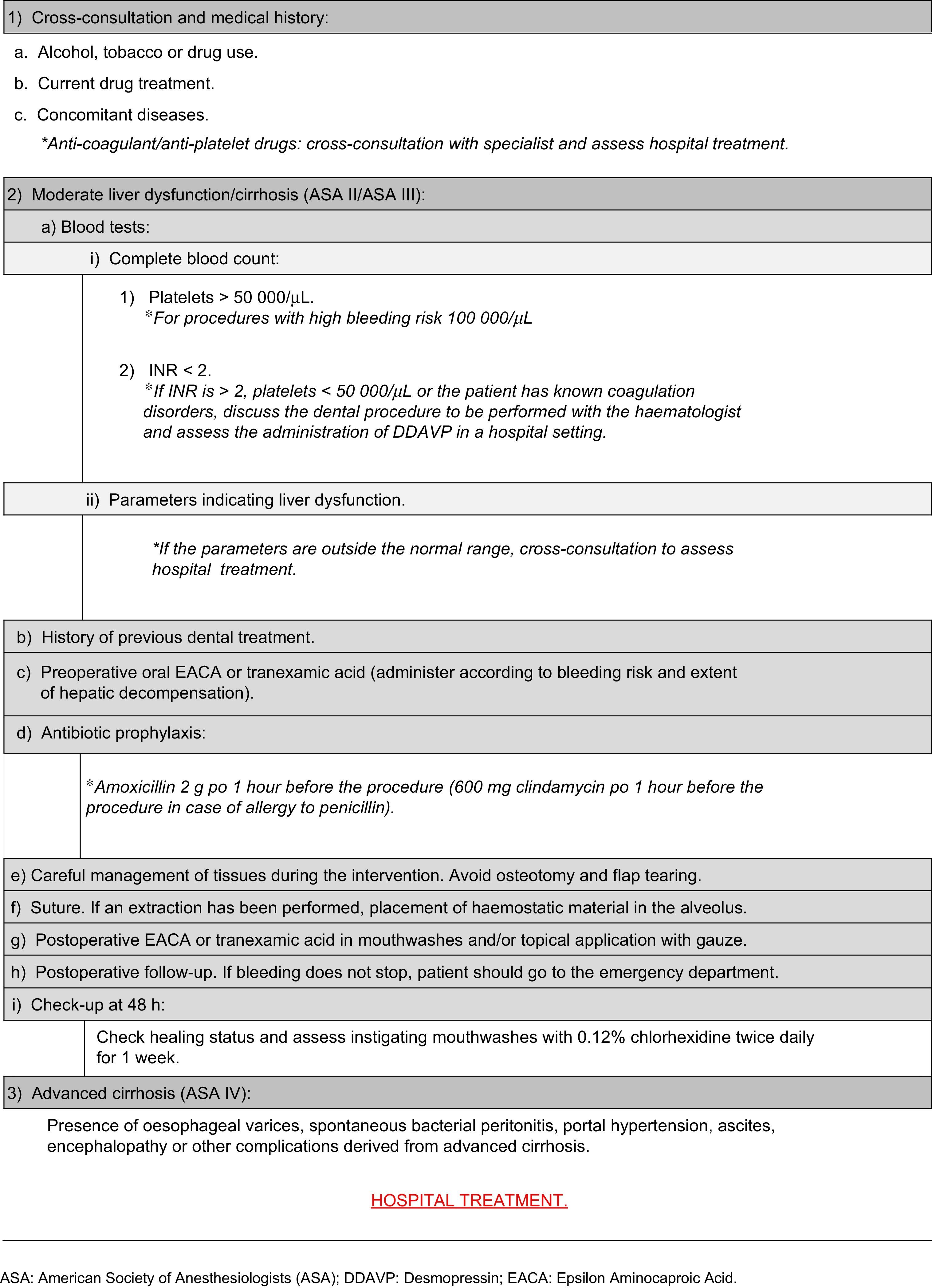
Dental treatment in cirrhotic patients, particularly interventions involving bleeding, should not be undertaken before considering the stage of the disease and the need for antibiotic prophylaxis to reduce the complications derived from the spread of infection, especially in patients with advanced cirrhosis.7 Studies have shown that patients with liver cirrhosis, particularly those with comorbid heart defects, drug abuse and chronic renal failure, are at higher risk for bacterial endocarditis. This justifies the need for antibiotics prior to surgery,5 although there is no clear evidence to recommend administration of 600mg of clindamycin in the case of allergy to penicillin 1h before the procedure.6,9,10
The most widely used antibiotic groups in dentistry include the penicillins-cephalosporins, clindamycin, macrolides and quinolones.
Penicillins and their derivatives very rarely cause liver damage, and it is usually asymptomatic. Penicillin G, penicillin V, ampicillin and amoxicillin are drugs in which very few cases of hepatotoxicity have been described.11 For this reason, the risk/benefit ratio of prophylactic administration of 2g of amoxicillin alone or combined with 500mg metronidazole 1h before the procedure6,9,10 could be favourable in patients with advanced cirrhosis and a risk of sepsis, which may explain its use in the majority of invasive dental procedures. However, cases of hepatotoxicity due to amoxicillin–clavulanic acid have been widely reported,12 and it is considered responsible for between 13% and 23% of cases of drug-induced hepatotoxicity,11 especially in patients aged over 65 years, in women, and following repeated courses of treatment, generally lasting longer than 2 weeks.11 Clavulanic acid appears to be responsible for the marked increase in hepatotoxicity cases. The liver damage caused by this component can appear up to 2 weeks after completing treatment11; it is therefore advisable to avoid the use of beta-lactamase inhibitors due to their risk of causing liver damage.
Cephalosporins have been occasionally implicated in cases of cholestatic hepatitis, assuming a hypersensitivity mechanism similar to that of the penicillins.11,12
Macrolides have also been implicated in liver damage. Cases of hepatotoxicity with erythromycin have been described,11 sometimes occurring up to 2 weeks after completing treatment. Azithromycin has been considered responsible for some cases of cholestasis,11 as has clarithromycin, especially in high doses.12 Clarithromycin, erythromycin and especially telithromycin have been associated with cases of acute liver failure and death. For these reasons, the use of macrolides should be avoided in patients with advanced cirrhosis.13
Liver damage secondary to clindamycin is exceptional.18 Nonetheless, as with other macrolides, it should either be avoided or used at lower doses in patients with advanced cirrhosis,13 especially if more effective treatment alternatives are available. Despite this, clindamycin is the antibiotic of choice in the prophylaxis of cirrhotic patients who are allergic to penicillin (single dose of 600mg of clindamycin in case of allergy to penicillin, 1h before the procedure6,9,10).
Few cases of hepatotoxicity have been caused by quinolones,12 although ciprofloxacin has been associated with isolated cases of high serum transaminases with hepatocellular damage, cholestasis and acute liver failure resulting in death.11,12 Despite this, quinolones are commonly used in cirrhotic patients, and are a relatively safe option. Furthermore, dose adjustment for cirrhosis is not usually required,13 although it should be adjusted in cases of renal failure.
Management of haemostatic alterations in the cirrhotic patientPreoperative management involves analysis of the patient's medical record and evaluation of the dental treatment to be performed; a history of bleeding in other previous procedures must also be assessed, as this is an important indicator of coagulation disorders.9 In case of doubt, cross-consultation with a specialist is advisable, in order to determine the degree of severity of the disease.14
Cirrhosis can result in low plasma levels of coagulation factors due to poor absorption and utilization of vitamin K, which affects the synthesis of factors II, VII, IX and X.14 As a result, it is advisable to evaluate the patient's haemostatic function before any dental procedure that involves bleeding. This should include a complete blood count with platelet count, prothrombin time, activated partial thromboplastin time, international normalized ratio (INR) and liver function test (transaminases, bilirubin and albumin levels).1,9,15
The INR alone does not always accurately predict the risk of postoperative bleeding, nor do the aforementioned laboratory tests. However, it is associated with the degree and progression of liver dysfunction.
Thrombocytopenia or platelet dysfunction suggests an increased risk of postoperative bleeding, more so if found together with elevated INR. A platelet count of ≥100,000μl is recommended for surgical dental procedures with a higher risk of bleeding, such as bone surgery, large flaps or multiple extractions, and a minimum level of 50,000–55,000μl for lower risk surgical procedures, such as simple extractions or small flaps.9,14
Anti-fibrinolytic agents, such as epsilon aminocaproic acid (EACA) or tranexamic acid, inhibit fibrinolysis. Therefore, the use of these drugs to control bleeding following a simple extraction, as well as intranasal administration of desmopressin (1-desamino-8D-arginine vasopressin, DDAVP), which promotes factor VIII synthesis and stimulates the release of Von Willebrand factor, could be a good alternative in patients with platelet counts between 30,000–50 000μl and INR values between 2 and 3,10 in whom platelet transfusion is not suitable.14
Depending on the type of surgery and bleeding risk, anti-fibrinolytics can be used preoperatively in oral form or postoperatively in mouthwashes.2 Nevertheless, good post-extraction bleeding control has been described in liver pretransplant patients by simple compression of the surgical wound with gauze, providing that the INR is less than or equal to 2.5 and the platelet count is above 30,000μl, and there are no other coagulation disorders, conditions or drug use that may affect the haemostasis.9
With respect to laboratory test parameters, low haematocrit values (below 25%) have been correlated with increased bleeding time, even in patients with normal platelet counts.9,15 Similarly, the determination of blood fibrinogen values and their replacement when levels fall below 120mg/dL is a an important parameter.14
It is important to bear in mind that the higher bleeding risk in the cirrhotic patient is also due to the presence of ectasias and varicosities in the gastro-oesophageal mucosa, pharynx and oral mucosa,16 and that certain invasive procedures on these areas can cause profuse bleeding, especially if the patient's haemostatic parameters are still significantly compromised. For this reason, it is important to minimize soft tissue trauma.1
American Society of Anesthesiologists (ASA) III compensated cirrhotic patients can usually be seen on an outpatient basis, providing that the foregoing criteria are met and haemostatic parameters are within the above-mentioned ranges, and that the dental procedures can be performed in these conditions. However, dental treatment in a hospital setting is advisable in ASA III or ASA IV cirrhotic patients with severe haemostatic alterations that coexist with other conditions, or with a high risk of complications derived from a complex dental treatment.1 Cross-consultation with a haematology or digestive medicine specialist is advisable, particularly when the patient may require blood transfusion or administration of non-conventional haemostatic agents, such as intravenous DDAVP.
Drugs for dental use in the cirrhotic patientAlthough there is no reliable liver function parameter for adjusting drug doses,17,18 recommendations are usually based on the Child–Pugh classification and model for end-stage liver disease (MELD) scores, which correlate the cirrhosis-related degree of dysfunction and survival.13,18 In patients with compensated liver disease, drug metabolism is similar to that of the general population.17 In cirrhotic patients with high MELD and Child–Pugh scores, however, lower, less frequent doses should be given over short periods of time17,18 to avoid accumulation and potential adverse effects.
Complications following administration of drugs in cirrhotic patients include: acute liver failure, hepatic encephalopathy, acute renal failure and gastrointestinal bleeding.17
Local anaesthetics should be administered with caution, as amides are metabolized in the liver and can cause toxic reactions at lower than expected doses.1 Some local anaesthetics, such as articaine, are extensively metabolized in the plasma, while prilocaine is partially metabolized in the lungs. Since they are less dependent on liver metabolism, these drugs should cause fewer toxic effects.1
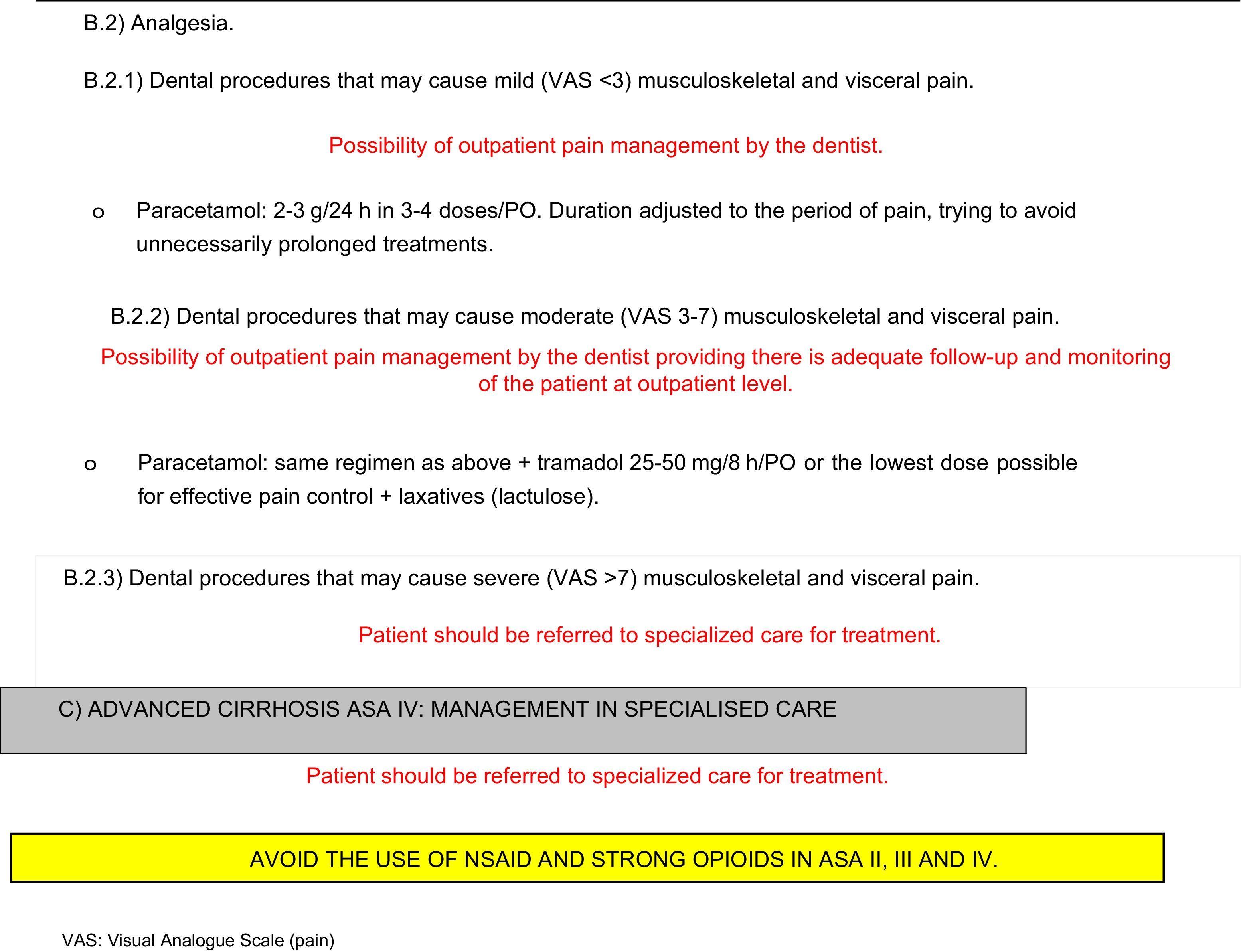
With respect to analgesic drugs, most dental procedures require drugs recommended for the first and second steps of the World Health Organization analgesic ladder, i.e. non-steroidal anti-inflammatory drugs (NSAIDs), paracetamol and weak opioids. The use of strong opioids (third step on the WHO analgesic ladder) or coadjuvants is not usually required. If they are needed, they should not be used before consulting with a specialist.
Paracetamol can be safely prescribed in cirrhotic patients in small doses of 2 or 3g per day, even for long periods, due to the absence of sedation and nephrotoxicity.17–19 It is recommended as first-line treatment for pain, even in patients with alcoholic cirrhosis in whom this drug has a higher risk of toxicity.13,17,18 Good tolerance of short courses of up to 4g of paracetamol per day has been described, even in patients with cirrhosis and alcohol consumption, even though dosage should ideally not exceed 2g/day in these cases.17
Although they can be tolerated in patients with moderate liver disease,17 NSAIDs should be avoided or used with extreme caution in the cirrhotic patient,13 as the combination of their antiplatelet action with the haemostatic alterations and thrombocytopenia frequently found in these patients increases the risk of gastrointestinal bleeding. This risk is even further aggravated in patients with gastric-oesophageal varices and ectasias or portal hypertension.17 NSAIDS should be also be avoided because they have been associated with hepatotoxicity with acute hepatic decompensation, with the subsequent risk of renal failure.13,17–19 Many NSAIDS also depend on protein-mediated transport, so that serum levels of free drug are increased in cirrhotic patients with low serum protein; for this reason, if they are necessary, they should be prescribed in lower doses than usual. There is still little experience in the use of coxibs in these patients. They seem to be effective in pain control, and could be better tolerated, as they do not interfere in platelet and renal metabolism, although they should also be used with caution.17,18
Weak opioids should also be used with caution, since their toxicity increases in cases of hypoalbuminaemia associated with cirrhosis. They should also be administered concomitantly with laxatives to prevent constipation and the development of encephalopathy.13,18 Due to the marked bioaccumulation of these drugs, low doses should be used in patients with cirrhosis, following cross-consultation with a specialist.13,18
Erratic analgesic power has also been described in codeine and tramadol,13,17,18 since the biotransformation of these drugs to active metabolites is affected by the first-pass effect. Nevertheless, low-dose tramadol can be considered a second-line analgesic in the management of pain in the cirrhotic patient when paracetamol is insufficient to control the pain.13 However, caution is recommended when administered in patients with epilepsy due to the risk of causing convulsions, and the dose should be adjusted when renal dysfunction is suspected in the cirrhotic patient.13,17,18
ConclusionsOutpatient dental treatment of the cirrhotic patient requires extraordinary measures to be taken in order to avoid the onset of complications as a result of chronic hepatic dysfunction. Haemostasis, the risk of infection, and drug metabolism must be considered and studied individually in patients with cirrhosis, given the difficulty of achieving correct homeostasis in these situations. Therefore, we conclude that:
- –
There is a higher risk of systemic infection and bacterial endocarditis derived from oral infectious foci in the cirrhotic patient. As a result, it is advisable to administer antibiotic prophylaxis, although there is no protocol to recommend this at present.
- –
Haemostatic alterations can lead to excessive bleeding in dental surgery, so a detailed case study should be made, following an appropriate action protocol (Appendix 1).
- –
Drug metabolism may be affected, according to the degree of hepatic dysfunction. To prevent the appearance of undesirable effects, the safest drugs can be prescribed. In advanced stages, and when recommended by a specialist, lower, less frequent doses should be given. The protocols include the most common clinical situations and the drugs recommended for each of these in the outpatient dental treatment of the cirrhotic patient (Appendix 2).
The authors declare that they have no conflict of interests.
Please cite this article as: Rodríguez Martínez S, Talaván Sernab J, Silvestre FJ. Manejo odontológico en el paciente cirrótico. Gastroenterol Hepatol. 2016;39:224–232.