To evaluate the cost-effectiveness of a strategy based on direct-acting antivirals (DAAs) following the marketing of simeprevir and sofosbuvir (post-DAA) versus a pre-direct-acting antiviral strategy (pre-DAA) in patients with chronic hepatitis C, from the perspective of the Spanish National Health System.
MethodsA decision tree combined with a Markov model was used to estimate the direct health costs (€, 2016) and health outcomes (quality-adjusted life years, QALYs) throughout the patient's life, with an annual discount rate of 3%. The sustained virological response, percentage of patients treated or not treated in each strategy, clinical characteristics of the patients, annual likelihood of transition, costs of treating and managing the disease, and utilities were obtained from the literature. The cost-effectiveness analysis was expressed as an incremental cost-effectiveness ratio (incremental cost per QALY gained). A deterministic sensitivity analysis and a probabilistic sensitivity analysis were performed.
ResultsThe post-DAA strategy showed higher health costs per patient (€30,944 vs. €23,707) than the pre-DAA strategy. However, it was associated with an increase of QALYs gained (15.79 vs. 12.83), showing an incremental cost-effectiveness ratio of €2439 per QALY. The deterministic sensitivity analysis and the probabilistic sensitivity analysis showed the robustness of the results, with the post-DAA strategy being cost-effective in 99% of cases compared to the pre-DAA strategy.
ConclusionsCompared to the pre-DAA strategy, the post-DAA strategy is efficient for the treatment of chronic hepatitis C in Spain, resulting in a much lower cost per QALY than the efficiency threshold used in Spain (€30,000 per QALY).
Análisis coste-efectividad de una estrategia basada en antivirales de acción directa (AAD) a partir de la comercialización de simeprevir y sofosbuvir (post-AAD) frente a otra previa (pre-AAD), en pacientes con hepatitis C crónica, desde la perspectiva del Sistema Nacional de Salud.
MétodosSe realizó un árbol de decisión combinado con un modelo de Markov para estimar los costes directos sanitarios (€, 2016) y resultados en salud (años de vida ajustados por calidad, AVAC), a lo largo de toda la vida del paciente, con una tasa de descuento anual del 3%. La respuesta virológica sostenida, el porcentaje de pacientes tratados o no en cada estrategia, las características clínicas de los pacientes, las probabilidades anuales de transición, los costes del tratamiento y manejo de la enfermedad, y las utilidades se obtuvieron de la literatura. El análisis coste-efectividad se expresó como relación coste-efectividad incremental (coste incremental por AVAC ganado). Se realizaron análisis de sensibilidad determinísticos y probabilístico.
ResultadosLa estrategia post-AAD mostró mayores costes sanitarios por paciente (30.944€ vs. 23.707€) que la estrategia pre-AAD. Sin embargo, se asoció con un aumento de la ganancia de AVAC (15,79 vs. 12,83), mostrando una relación coste-efectividad incremental de 2439€ por AVAC. Los análisis de sensibilidad mostraron la consistencia de los resultados siendo la estrategia post-AAD, frente a pre-AAD, coste-efectiva en el 99% de los casos.
ConclusionesLa estrategia post-AAD, en comparación con la pre-AAD, es eficiente para el tratamiento de la hepatitis C crónica en España, obteniéndose un coste por AVAC muy inferior al umbral de eficiencia utilizado en España (30.000€ por AVAC).
Infection by the hepatitis C virus (HCV) is characterized by the difficulty of the immune system to eliminate the virus in the acute phase, which leads to the development of chronic hepatitis C (CHC) in more than 70% of patients.1 Among these, depending on various factors, chronic liver damage can lead to the development of liver cirrhosis in up to 25% of patients.2 In most patients, this progression is slow and completely asymptomatic, but once liver cirrhosis has developed, the annual probability of developing clinical decompensations of cirrhosis is 4%, and that of developing hepatocellular carcinoma (HCC) is 1.5%,3 which may eventually lead to the need to receive a liver transplant or the patient's death.
The actual prevalence of CHC in Spain is not well known, but it is estimated that CHC affected 473,000 people in Spain in 2013.4 The magnitude of these figures reveals that CHC is a major socio-sanitary problem in Spain that entails a significant consumption of economic resources for health systems, with regard to both the evident need for treatments to cure the HCV infection and the costs derived from the treatment of the complications of liver disease.5
The sustained virologic response (SVR) for treating the HCV infection prevents disease progression, reduces hepatic mortality and all-cause mortality, and increases patients’ quality of life.6,7 The treatment of the disease has changed radically in recent years due to the availability of direct-acting antivirals (DAAs) that act in combination on different key therapeutic targets for virus replication, allowing SVR rates that exceed 95% in most patients. These treatment regimens, mostly free of interferon, are given orally and have a shorter duration than the alternatives used previously, a better tolerability profile, and greater adherence to treatment.8–11
On the other hand, the use of these new DAA regimes is associated with an increase in the economic impact on short-term health system budgets. This increase is fundamentally related to the significant increase in the number of patients who are eligible to receive treatment because of their excellent safety profile, in addition to the associated cost of the drugs themselves.
For this reason, in Spain and other countries,12–14 health policies such as the National Strategic Plan for CHC (SPCHC)15 have been established. With limited available economic resources and the need of to determine the appropriate treatment strategy giving priority to patients with a higher degree of fibrosis in a first phase (and, therefore, with an increased risk of disease evolution), short- or long-term benefits can be achieved by curing the disease, thereby avoiding clinical complications derived from liver disease or extrahepatic manifestations of HCV.16
These healthcare improvements also affect disease progression and, consequently, future disease-related costs. Therefore, to gain insight into the long-term consequences of the change in management strategy of patients with CHC in our country, it is necessary to provide clinical-economic evidence assessing the likely evolution of the disease with, and without, the use of these new DAAs. The objective of the present pharmacoeconomic analysis is to evaluate the incremental cost-effectiveness ratio of a therapeutic strategy based on the combination of new direct-acting antivirals (post-DAA) during the first year (2015) of application of the SPCHC versus previously available regimens based on double or triple therapy with peginterferon (peg-IFN) plus ribavirin, with telaprevir or boceprevir (pre-DAA), in patients with CHC with different degrees of fibrosis.
Material and methodsThe analysis included 51,900 patients, representing the population of patients who were candidates to receive treatment estimated by the SPCHC, with a mean age of 52 years,17 and fibrosis ≥F2 (METAVIR scale). The analysis compared two characteristic matched subpopulations: the patients treated with the new high-efficacy DAA regimens defined in the SPCHC (post-DAA strategy, Table 1), versus those treated with regimens based on double or triple therapy with peg-IFN plus ribavirin and boceprevir or telaprevir (pre-DAA strategy, Table 1), from the perspective of the National Health System (NHS). A discount rate of 3% was applied to the health costs and outcomes.18
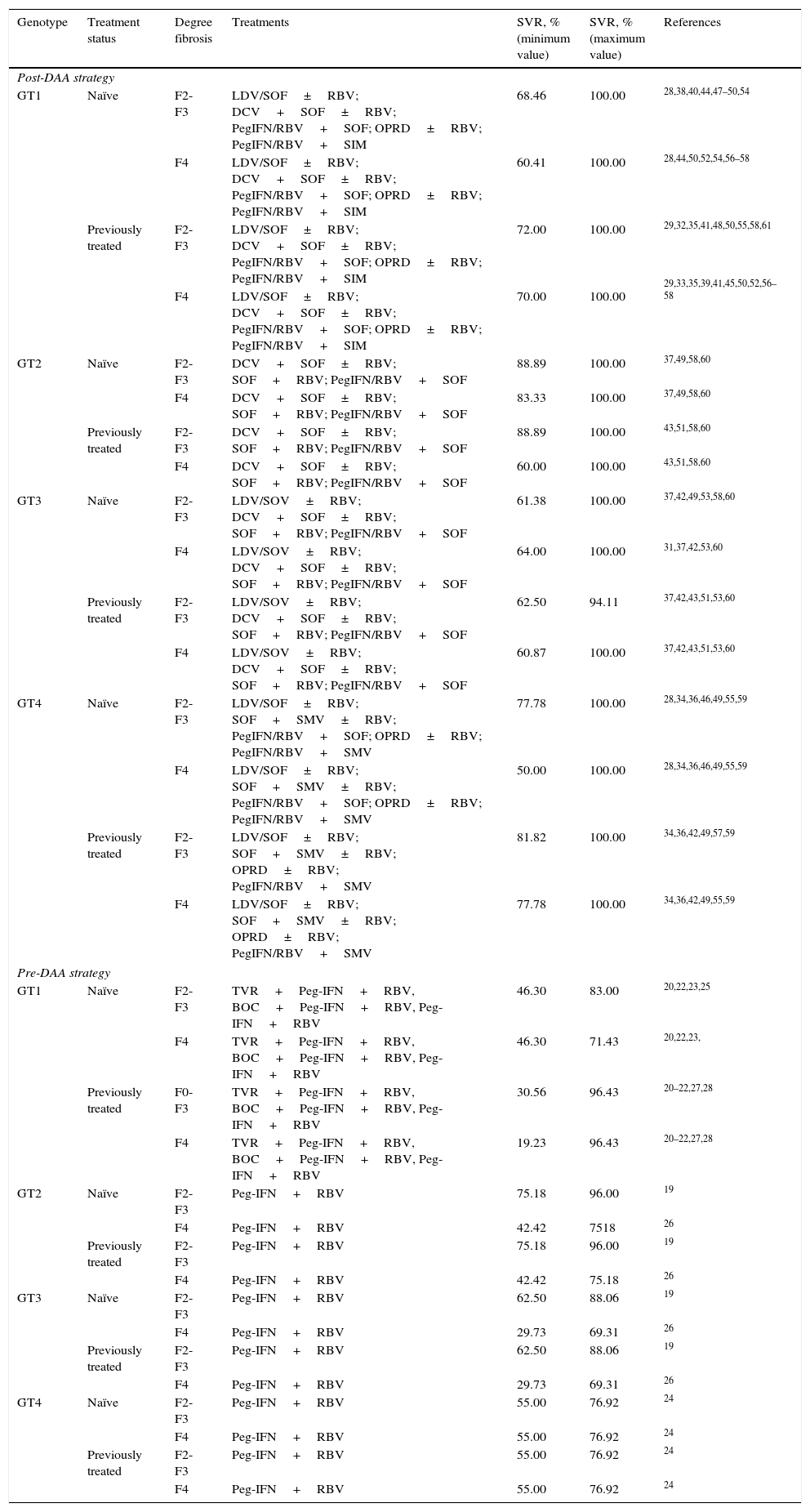
Sustained Virologic Response include in the analysis according to genotype, treatment status and degree fibrosis.
| Genotype | Treatment status | Degree fibrosis | Treatments | SVR, % (minimum value) | SVR, % (maximum value) | References |
|---|---|---|---|---|---|---|
| Post-DAA strategy | ||||||
| GT1 | Naïve | F2-F3 | LDV/SOF±RBV; DCV+SOF±RBV; PegIFN/RBV+SOF; OPRD±RBV; PegIFN/RBV+SIM | 68.46 | 100.00 | 28,38,40,44,47–50,54 |
| F4 | LDV/SOF±RBV; DCV+SOF±RBV; PegIFN/RBV+SOF; OPRD±RBV; PegIFN/RBV+SIM | 60.41 | 100.00 | 28,44,50,52,54,56–58 | ||
| Previously treated | F2-F3 | LDV/SOF±RBV; DCV+SOF±RBV; PegIFN/RBV+SOF; OPRD±RBV; PegIFN/RBV+SIM | 72.00 | 100.00 | 29,32,35,41,48,50,55,58,61 | |
| F4 | LDV/SOF±RBV; DCV+SOF±RBV; PegIFN/RBV+SOF; OPRD±RBV; PegIFN/RBV+SIM | 70.00 | 100.00 | 29,33,35,39,41,45,50,52,56–58 | ||
| GT2 | Naïve | F2-F3 | DCV+SOF±RBV; SOF+RBV; PegIFN/RBV+SOF | 88.89 | 100.00 | 37,49,58,60 |
| F4 | DCV+SOF±RBV; SOF+RBV; PegIFN/RBV+SOF | 83.33 | 100.00 | 37,49,58,60 | ||
| Previously treated | F2-F3 | DCV+SOF±RBV; SOF+RBV; PegIFN/RBV+SOF | 88.89 | 100.00 | 43,51,58,60 | |
| F4 | DCV+SOF±RBV; SOF+RBV; PegIFN/RBV+SOF | 60.00 | 100.00 | 43,51,58,60 | ||
| GT3 | Naïve | F2-F3 | LDV/SOV±RBV; DCV+SOF±RBV; SOF+RBV; PegIFN/RBV+SOF | 61.38 | 100.00 | 37,42,49,53,58,60 |
| F4 | LDV/SOV±RBV; DCV+SOF±RBV; SOF+RBV; PegIFN/RBV+SOF | 64.00 | 100.00 | 31,37,42,53,60 | ||
| Previously treated | F2-F3 | LDV/SOV±RBV; DCV+SOF±RBV; SOF+RBV; PegIFN/RBV+SOF | 62.50 | 94.11 | 37,42,43,51,53,60 | |
| F4 | LDV/SOV±RBV; DCV+SOF±RBV; SOF+RBV; PegIFN/RBV+SOF | 60.87 | 100.00 | 37,42,43,51,53,60 | ||
| GT4 | Naïve | F2-F3 | LDV/SOF±RBV; SOF+SMV±RBV; PegIFN/RBV+SOF; OPRD±RBV; PegIFN/RBV+SMV | 77.78 | 100.00 | 28,34,36,46,49,55,59 |
| F4 | LDV/SOF±RBV; SOF+SMV±RBV; PegIFN/RBV+SOF; OPRD±RBV; PegIFN/RBV+SMV | 50.00 | 100.00 | 28,34,36,46,49,55,59 | ||
| Previously treated | F2-F3 | LDV/SOF±RBV; SOF+SMV±RBV; OPRD±RBV; PegIFN/RBV+SMV | 81.82 | 100.00 | 34,36,42,49,57,59 | |
| F4 | LDV/SOF±RBV; SOF+SMV±RBV; OPRD±RBV; PegIFN/RBV+SMV | 77.78 | 100.00 | 34,36,42,49,55,59 | ||
| Pre-DAA strategy | ||||||
| GT1 | Naïve | F2-F3 | TVR+Peg-IFN+RBV, BOC+Peg-IFN+RBV, Peg-IFN+RBV | 46.30 | 83.00 | 20,22,23,25 |
| F4 | TVR+Peg-IFN+RBV, BOC+Peg-IFN+RBV, Peg-IFN+RBV | 46.30 | 71.43 | 20,22,23, | ||
| Previously treated | F0-F3 | TVR+Peg-IFN+RBV, BOC+Peg-IFN+RBV, Peg-IFN+RBV | 30.56 | 96.43 | 20–22,27,28 | |
| F4 | TVR+Peg-IFN+RBV, BOC+Peg-IFN+RBV, Peg-IFN+RBV | 19.23 | 96.43 | 20–22,27,28 | ||
| GT2 | Naïve | F2-F3 | Peg-IFN+RBV | 75.18 | 96.00 | 19 |
| F4 | Peg-IFN+RBV | 42.42 | 7518 | 26 | ||
| Previously treated | F2-F3 | Peg-IFN+RBV | 75.18 | 96.00 | 19 | |
| F4 | Peg-IFN+RBV | 42.42 | 75.18 | 26 | ||
| GT3 | Naïve | F2-F3 | Peg-IFN+RBV | 62.50 | 88.06 | 19 |
| F4 | Peg-IFN+RBV | 29.73 | 69.31 | 26 | ||
| Previously treated | F2-F3 | Peg-IFN+RBV | 62.50 | 88.06 | 19 | |
| F4 | Peg-IFN+RBV | 29.73 | 69.31 | 26 | ||
| GT4 | Naïve | F2-F3 | Peg-IFN+RBV | 55.00 | 76.92 | 24 |
| F4 | Peg-IFN+RBV | 55.00 | 76.92 | 24 | ||
| Previously treated | F2-F3 | Peg-IFN+RBV | 55.00 | 76.92 | 24 | |
| F4 | Peg-IFN+RBV | 55.00 | 76.92 | 24 | ||
DAA: Direct-Acting Antiviral; SVR: Sustained Virologic Response; GT: Genotype; DCV: Daclatasvir; SOF: Sofosbuvir; RBV: Ribavirin; SMV: Simeprevir; Peg-IFN: peginterferon; OPRD: Ombitasvir/Paritaprevir/Ritonavir/Dasbuvir.
A decision tree combined with a Markov model was used for the estimation of long-term costs and health outcomes with each of the strategies evaluated.
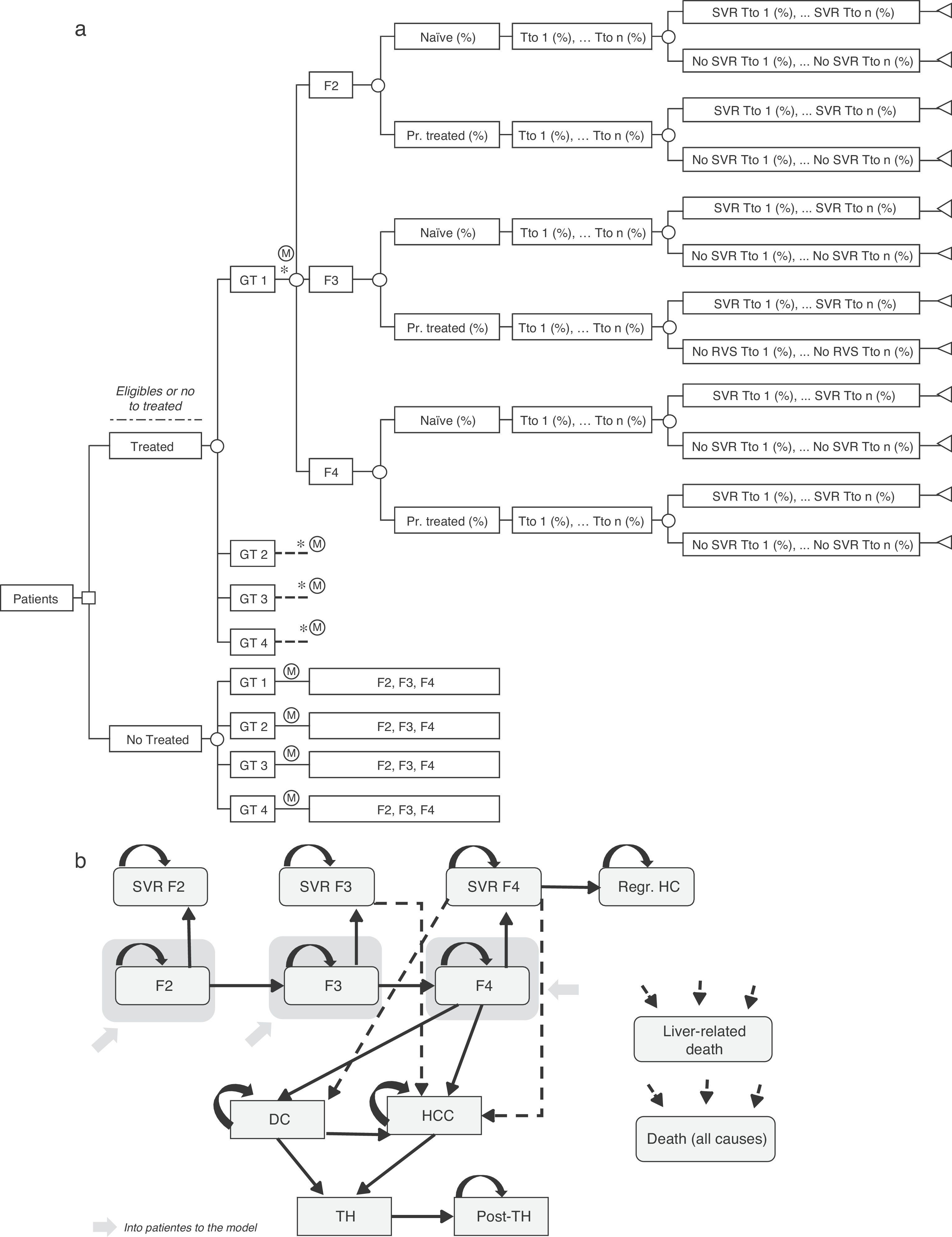
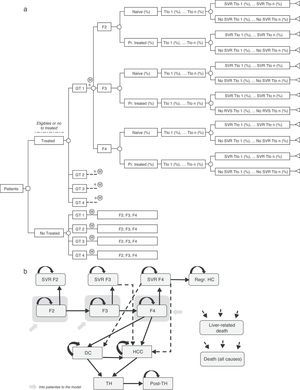
Decision treeA decision tree was developed to estimate the overall SVR rates of the two strategies (pre-DAA and post-DAA) included in the analysis. These responses were the product of the proportion of patients treated or not treated, genotype, fibrosis level, treatment status (naïve or previously treated), percentage of use of each treatment, and the SVR rates associated with each type of patient (Fig. 1a).19–61
(a) Decision analysis tree of treated or untreated patients based on their Clinical characteristics, treatment options and SVR rates. * Similar branch for GT2, GT3 y GT4 including the different treatments associated to each genotype (Table 2) and percentage of use of each them depending on the characteristic of the patient. GT: Genotype; Pr. Treated: Previusly treated; Tto: Treatment; SVR: Sustained Virologic Response. (b) Diagram of Markov model of CHC. SVR: Sustained Virologic Response; PSA: Probabilistic Sensitivity Analysis; DC: Decompensated Cirrhosis; Regr. HC: Regression of hepatic cirrhosis; HCC: Hepatocellular carcinoma; LT: Liver transplant; Post-LT: Post-Liver transplant.
In the base case analysis, the allotted treatment proportions were measured to be 73.4% (38,067)62 of the patients on the post-DAA strategy compared to 18.9% (9800)4 of the patients on the pre-DAA strategy (Table 2).
Characteristics of the patients cohort with CHC.
| Parameter | Base case | Minimum value | Maximum value | Distribution (for PSA) | Reference |
|---|---|---|---|---|---|
| Cohort age, years | 52 | 17 | |||
| Proportion genotype (GT) | |||||
| GT1, % | 69.32 | 62.39 | 76.25 | Dirichlet | 15, Estimation (±10%) |
| GT2, % | 2.27 | ----- | ----- | Dirichlet | 15 |
| GT3, % | 15.91 | ----- | ----- | Dirichlet | 15 |
| GT4, % | 12.50 | ----- | ----- | Dirichlet | 15 |
| Stage of fibrosis distribution (F) | |||||
| F2, % | 31.48 | ----- | ----- | Dirichlet | 15 |
| F3, % | 27.78 | ----- | ----- | Dirichlet | 15 |
| F4, % | 40.74 | Dirichlet | 15, Estimation (±10%) | ||
| Treatment status | |||||
| Treatment-naïve, % | 41.42 | 37.28 | 45.56 | Beta | 62, Estimation (±10%) |
| Treatment-experienced, % | 58.58 | ----- | ----- | Beta | 62 |
| Percent of patients treated or not treated (post-DAA strategy) | |||||
| Treated, % | 73.35 | 66.01 | 100.00 | Beta | 62, Estimation (±10%) |
| No treated, % | 26.65 | ----- | ----- | Beta | 62, Estimation |
| Percent of patients treated or not treated (pre-DAA strategy) | |||||
| Treated, % | 18.88 | 16.99 | 100.00 | Beta | 62, Estimation (±10%) |
| No Treated, % | 81.12 | ----- | ----- | Beta | 62 |
PSA: Probabilistic Sensitivity Analysis; SPCHC: Strategic Plan for CHC.
The clinical characteristics for the distribution of patients according to genotype, degree of fibrosis, and treatment status (naïve or previously treated) were the same in both strategies and were obtained from studies based on the Spanish population (Table 2).
To establish the drugs included in one strategy or another, the date of commercialization of simeprevir and sofosbuvir was considered. Thus, the therapies included in the pre-DAA strategy are based on telaprevir and boceprevir in triple therapy with peg-IFN and peg-IFN plus ribavirin for genotype 1 and peg-IFN plus ribavirin for the remaining genotypes. The therapies included in the post-DAA strategy, based on the recommendations of the current clinical guidelines, were founded on treatment with the new DAAs, with or without ribavirin.11,15,63 The use of each treatment according to the clinical characteristics of the patients was estimated from market research data.64 These parameters are shown in Table 2.
The SVR rates of the treatments included in the study in each of the strategies for the different genotypes, degrees of fibrosis, naïve or previously treated patients were obtained from the most relevant clinical studies for each.19–61 In addition to the overall SVR rate, the decision tree made it possible to calculate the minimum and maximum SVR values used in the sensitivity analysis (Table 1).
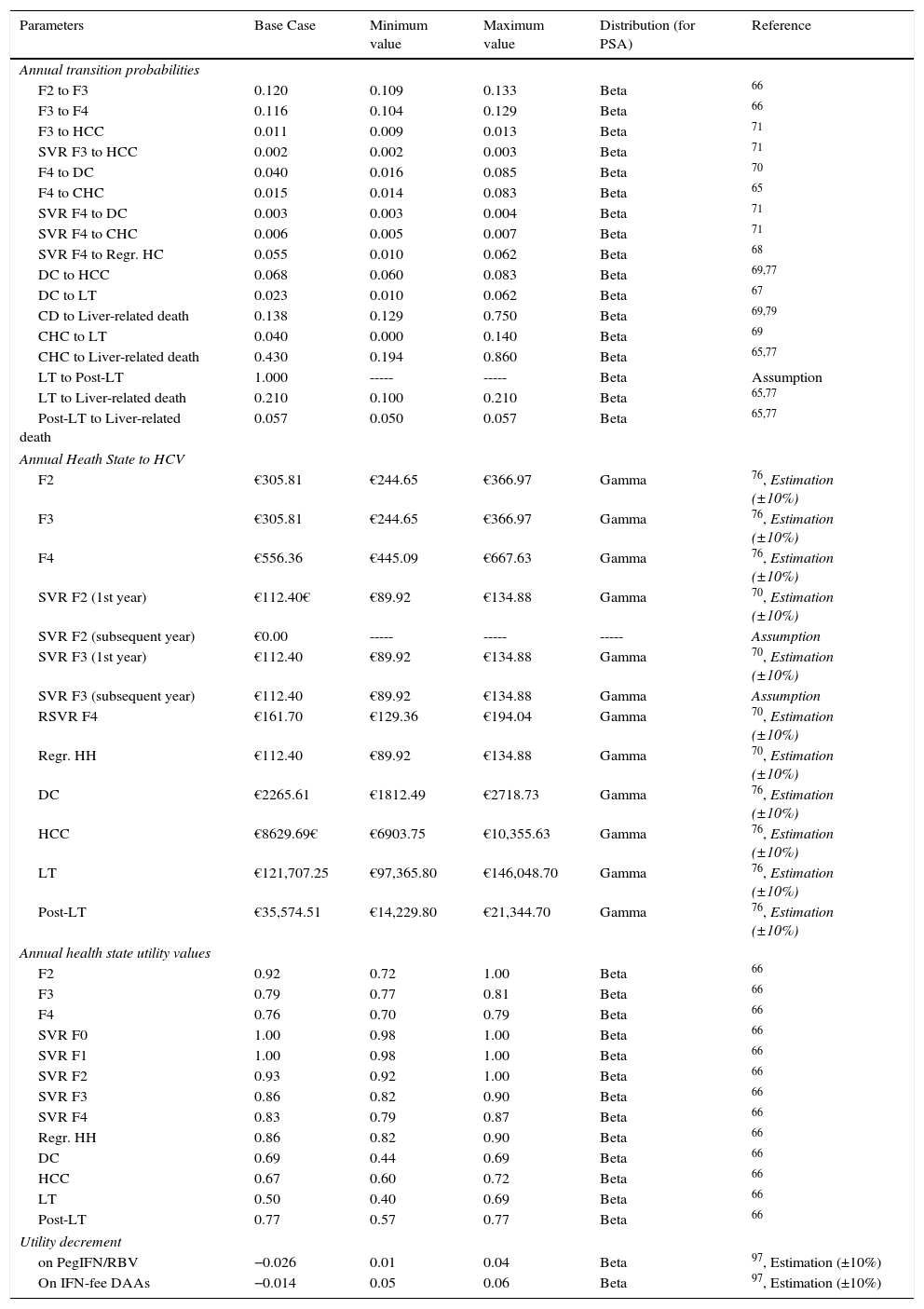
Markov modelA novel Markov model was designed to project the progression of the disease over a lifetime for the two cohorts of patients, depending on the two treatment strategies described previously (Fig. 1b). The simulation was performed in annual cycles, and the transition probabilities for each health state were obtained from the literature65–71 (Table 3). Patients entered the model at varying stages of liver fibrosis differentiating into treated or untreated patients. Treated patients transitioned to SVR states based on the global response rates previously calculated in the decision tree.
Parameters used in the model: transition probabilities rates, utilities and unit cost.
| Parameters | Base Case | Minimum value | Maximum value | Distribution (for PSA) | Reference |
|---|---|---|---|---|---|
| Annual transition probabilities | |||||
| F2 to F3 | 0.120 | 0.109 | 0.133 | Beta | 66 |
| F3 to F4 | 0.116 | 0.104 | 0.129 | Beta | 66 |
| F3 to HCC | 0.011 | 0.009 | 0.013 | Beta | 71 |
| SVR F3 to HCC | 0.002 | 0.002 | 0.003 | Beta | 71 |
| F4 to DC | 0.040 | 0.016 | 0.085 | Beta | 70 |
| F4 to CHC | 0.015 | 0.014 | 0.083 | Beta | 65 |
| SVR F4 to DC | 0.003 | 0.003 | 0.004 | Beta | 71 |
| SVR F4 to CHC | 0.006 | 0.005 | 0.007 | Beta | 71 |
| SVR F4 to Regr. HC | 0.055 | 0.010 | 0.062 | Beta | 68 |
| DC to HCC | 0.068 | 0.060 | 0.083 | Beta | 69,77 |
| DC to LT | 0.023 | 0.010 | 0.062 | Beta | 67 |
| CD to Liver-related death | 0.138 | 0.129 | 0.750 | Beta | 69,79 |
| CHC to LT | 0.040 | 0.000 | 0.140 | Beta | 69 |
| CHC to Liver-related death | 0.430 | 0.194 | 0.860 | Beta | 65,77 |
| LT to Post-LT | 1.000 | ----- | ----- | Beta | Assumption |
| LT to Liver-related death | 0.210 | 0.100 | 0.210 | Beta | 65,77 |
| Post-LT to Liver-related death | 0.057 | 0.050 | 0.057 | Beta | 65,77 |
| Annual Heath State to HCV | |||||
| F2 | €305.81 | €244.65 | €366.97 | Gamma | 76, Estimation (±10%) |
| F3 | €305.81 | €244.65 | €366.97 | Gamma | 76, Estimation (±10%) |
| F4 | €556.36 | €445.09 | €667.63 | Gamma | 76, Estimation (±10%) |
| SVR F2 (1st year) | €112.40€ | €89.92 | €134.88 | Gamma | 70, Estimation (±10%) |
| SVR F2 (subsequent year) | €0.00 | ----- | ----- | ----- | Assumption |
| SVR F3 (1st year) | €112.40 | €89.92 | €134.88 | Gamma | 70, Estimation (±10%) |
| SVR F3 (subsequent year) | €112.40 | €89.92 | €134.88 | Gamma | Assumption |
| RSVR F4 | €161.70 | €129.36 | €194.04 | Gamma | 70, Estimation (±10%) |
| Regr. HH | €112.40 | €89.92 | €134.88 | Gamma | 70, Estimation (±10%) |
| DC | €2265.61 | €1812.49 | €2718.73 | Gamma | 76, Estimation (±10%) |
| HCC | €8629.69€ | €6903.75 | €10,355.63 | Gamma | 76, Estimation (±10%) |
| LT | €121,707.25 | €97,365.80 | €146,048.70 | Gamma | 76, Estimation (±10%) |
| Post-LT | €35,574.51 | €14,229.80 | €21,344.70 | Gamma | 76, Estimation (±10%) |
| Annual health state utility values | |||||
| F2 | 0.92 | 0.72 | 1.00 | Beta | 66 |
| F3 | 0.79 | 0.77 | 0.81 | Beta | 66 |
| F4 | 0.76 | 0.70 | 0.79 | Beta | 66 |
| SVR F0 | 1.00 | 0.98 | 1.00 | Beta | 66 |
| SVR F1 | 1.00 | 0.98 | 1.00 | Beta | 66 |
| SVR F2 | 0.93 | 0.92 | 1.00 | Beta | 66 |
| SVR F3 | 0.86 | 0.82 | 0.90 | Beta | 66 |
| SVR F4 | 0.83 | 0.79 | 0.87 | Beta | 66 |
| Regr. HH | 0.86 | 0.82 | 0.90 | Beta | 66 |
| DC | 0.69 | 0.44 | 0.69 | Beta | 66 |
| HCC | 0.67 | 0.60 | 0.72 | Beta | 66 |
| LT | 0.50 | 0.40 | 0.69 | Beta | 66 |
| Post-LT | 0.77 | 0.57 | 0.77 | Beta | 66 |
| Utility decrement | |||||
| on PegIFN/RBV | −0.026 | 0.01 | 0.04 | Beta | 97, Estimation (±10%) |
| On IFN-fee DAAs | −0.014 | 0.05 | 0.06 | Beta | 97, Estimation (±10%) |
SVR: Sustained Virologic Response; PSA: Probabilistic Sensitivity Analysis; DC: Decompensated Cirrhosis; Regr. HC: Regression of hepatic cirrhosis; HCC: Hepatocellular carcinoma; LT: Liver transplant; Post-LT: Post-Liver transplant; peg-IFN/RBV: peginterferon/ribavirin.
The patients in SVR F2 were considered cured patients and remained in that condition until death from non-liver related causes. The patients in SVR F3, although having attained an improvement of the disease, maintain the risk of developing HCC. The patients in SVR F4 could experience a stabilization or cirrhosis regression (Regr. CH) or, on the contrary, could develop complications related to the cirrhosis such as decompensated cirrhosis (DC) or HCC. The patients who did not attain SVR could remain in their current fibrosis state, or transition to other states according to the course of the disease similar to untreated patients. Patients in DC and HCC states were eligible to receive a liver transplant (LT). The patients receiving an LT remained in this state for only one cycle and transitioned to post-LT, in which they remained until death (Fig. 1b).
Three types of mortality were considered in the analysis, according to each health state. Mortality for patients in SVR, Regr. CH and the fibrosis states regardless of their degree, was assumed to be equal to the all-cause mortality by age.72 The patients in more advanced stages of the disease, such as DC, HCC, LT, and post-LT, were associated with the liver-related mortality and the non-hepatic mortality. The latter was calculated from the all-causes mortality rate less the liver-related mortality,73 all by age range.
CostsAll of the costs included in the model are direct health costs and are expressed in euros (€) for the year 2016.
The drug cost per patient for the post-DAA strategy (€28,738) was calculated from the total cost of all patients with CHC treated with the new therapies in 201574 divided by the number of patients treated with the new therapies in the same year.62 In the pre-DAA strategy, the average drug cost per patient (€15,003) was calculated from the drug cost for the National Health System (NHS)75 used in this strategy, adjusted according to different deductions. As premises, it was assumed that the cost of ribavirin (RBV) is €0, that patients are treated only once, and that all complete the therapy, with no discontinuation of treatment for any cause.
The monitoring costs were calculated as an average of the costs associated with the administration of the treatment, based on previously published data,76 according to the different durations, considering 8–24 weeks for the post-DAA strategy (€1257.52) and 24–48 weeks for the pre-DAA strategy (€2371.39).
The healthcare costs associated with each health state were obtained from different published studies and adjusted to 2016 prices70,76 (Table 3). Patients in SVR F2 and Regr. CH states were assumed as cured after the first year so that no consumption of resources will be generated and, therefore, no health status cost will be considered in the second and subsequent years. Conversely, it is considered that patients in states F3 and F4 with SVR and who remain there for the subsequent years of the simulation have a cost due to the risk of disease progression (Table 3).
Utilities: Cost-effectiveness analysisThe utility value is referred to the quality perceived by patients in the different health states of a disease. The utilities associated in this analysis for each health state of the Markov model were obtained from the literature66 (Table 3). The impact of drug toxicity, differentiating treatments with or without peg-IFN, was considered as a decrease in the quality of life or disutility during treatment (Table 3).
Health outcomes were measured in terms of the mean survival per patient, measured as life-years gained (LYGs), at the end of the simulation of the disease progression, throughout the life of the patients. Subsequently, this value was adjusted with the corresponding utility values, and expressed as quality adjusted life years (QALYs) gained per patient with each of the two treatment strategies evaluated.
The efficiency or relationship between health costs and outcomes is expressed as an incremental cost-effectiveness ratio, therefore, the mean incremental cost per patient of the post-DAA strategy versus the pre-DAA strategy divided by the mean incremental QALYs per patient, obtained with the post-DAA strategy versus the pre-DAA strategy.
Sensitivity analysesA one-way deterministic sensitivity analysis (DSA) and a probabilistic sensitivity analysis (PSA) were performed to evaluate the robustness of the outcomes by varying the most relevant parameters in the analysis.
In the DSA, the variables evaluated were: SVR rates in a range between the maximum and minimum values calculated previously, the proportion of patients with genotype 1 (GT1), the proportion of patients in F4 treated or not treated, the transition probabilities from SVR F3 or SVR F4 to HCC, the probability from SVR F4 to DC, the probabilities from DC or HCC to LT, and the utilities associated with SVR states (Table 2, Table 3).
In the PSA, 10,000 Monte Carlo simulations were performed. Parametric distributions were applied to the parameters model, a beta distribution was assigned the proportion of treated and untreated patients, patient characteristics (genotypes, fibrosis states, and treatment states), transition probabilities and utilities, and a gamma distribution for costs. The ranges of the values were estimated from the minimum and maximum values calculated and from the literature77 (Table 2, Table 3).
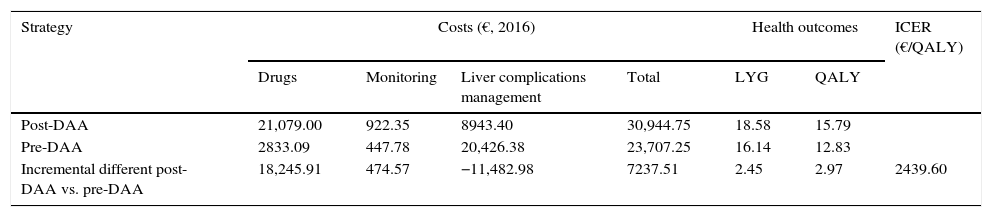
ResultsBase caseIn the 50-year simulation of the natural history of CHC, the post-DAA strategy was more effective than the pre-DAA strategy, achieving an increase of 2.45 LYGs and 2.97 QALYs per patient (18.58 versus 16.14 LYGs and 15.79 versus 12.83 QALYs, respectively). The average total cost to achieve these gains in health outcomes was €30,944 and €23,707 per patient with the post-DAA and pre-DAA strategies, respectively. The cost items with the greatest impact on total cost were the drug cost (€21,079, 68% of the total cost) in the post-DAA strategy and the cost of managing the liver complications associated with disease progression (€20,426, 86% of the total cost) in the pre-DAA strategy (Table 4).
Result of the cost-effectiveness analysis per patient (Base Case).
| Strategy | Costs (€, 2016) | Health outcomes | ICER (€/QALY) | ||||
|---|---|---|---|---|---|---|---|
| Drugs | Monitoring | Liver complications management | Total | LYG | QALY | ||
| Post-DAA | 21,079.00 | 922.35 | 8943.40 | 30,944.75 | 18.58 | 15.79 | |
| Pre-DAA | 2833.09 | 447.78 | 20,426.38 | 23,707.25 | 16.14 | 12.83 | |
| Incremental different post-DAA vs. pre-DAA | 18,245.91 | 474.57 | −11,482.98 | 7237.51 | 2.45 | 2.97 | 2439.60 |
DAA: Direct-Acting Antiviral; LYG: Life Year Gained; QALY: Quality adjusted life-years; ICER: Incremental cost-effectiveness ratio (incremental cost of the post-DAA strategy vs. pre-DAA strategy divide by incremental QALY of post-DAA vs. pre-DAA).
The incremental cost-effectiveness ratio (ICER) of the post-DAA strategy versus the pre-DAA strategy was €2439 per additional QALY (Table 4).
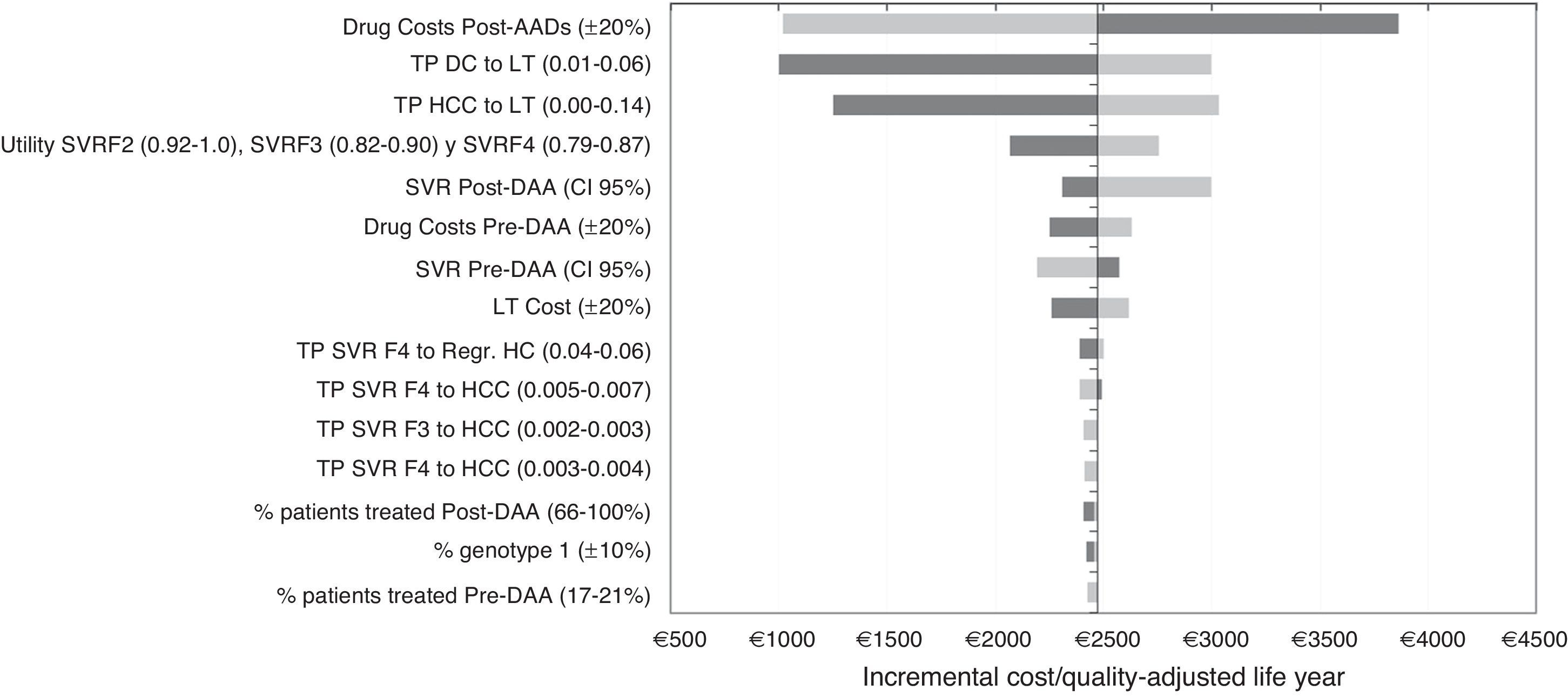
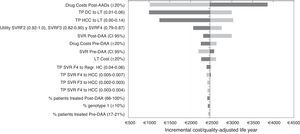
Sensitivity analysisIn the DSA, the ICER values ranged between €1002 and €3860 for each additional QALY gained with the post-DAA strategy versus the pre-DAA. The representation of this analysis was performed using a tornado diagram, in which the parameters that produce greater variation in the results of the base case analysis are situated at the top and those of less influence at the bottom (Fig. 2). The most relevant parameters in the analysis were the drug cost and the percentage of patients treated or not treated in the post-DAA strategy. A decrease in the pharmacological cost of treatment in the post-DAA strategy caused a reduction in the ICER to €1019; conversely, an increase caused an increment of the ICER to values of €3860. Variations in the transition probabilities from DC or HCC to LT made the results sensitive to this parameter, causing a variation of €3032 to €1250 in the ICER, showing that it is a parameter that influences the results of the analysis. When the SVR rates were varied with the minimum or maximum values, the ICER ranged from €2997 to €2193. When 100% of patients were considered to be treated in the post-DAA strategy, the ICER did not suffer much variation because the increase in the incremental difference in total costs from the base case (from €7237 to €10,237) was compensated by the increase in the incremental difference in the QALYs (2.97 to 4.25 QALY).
One-way deterministic sensitivity analyses (DSA). Tornado Diagram. DAA: Directly acting agents; SVR: Sustained Virologic Response; DC: Decompensated Cirrhosis; Regr. HC: Regression of hepatic cirrhosis; HCC: Hepatocellular carcinoma; LT: Liver transplant; TP: Transition probabilities. The dark grey colour represents a variation of the ICER with the maximum values of each parameters and the light grey colour represents the variations of the ICER with the minimum values.
Finally, variations in the other parameters included in the DSA did not influence the results.
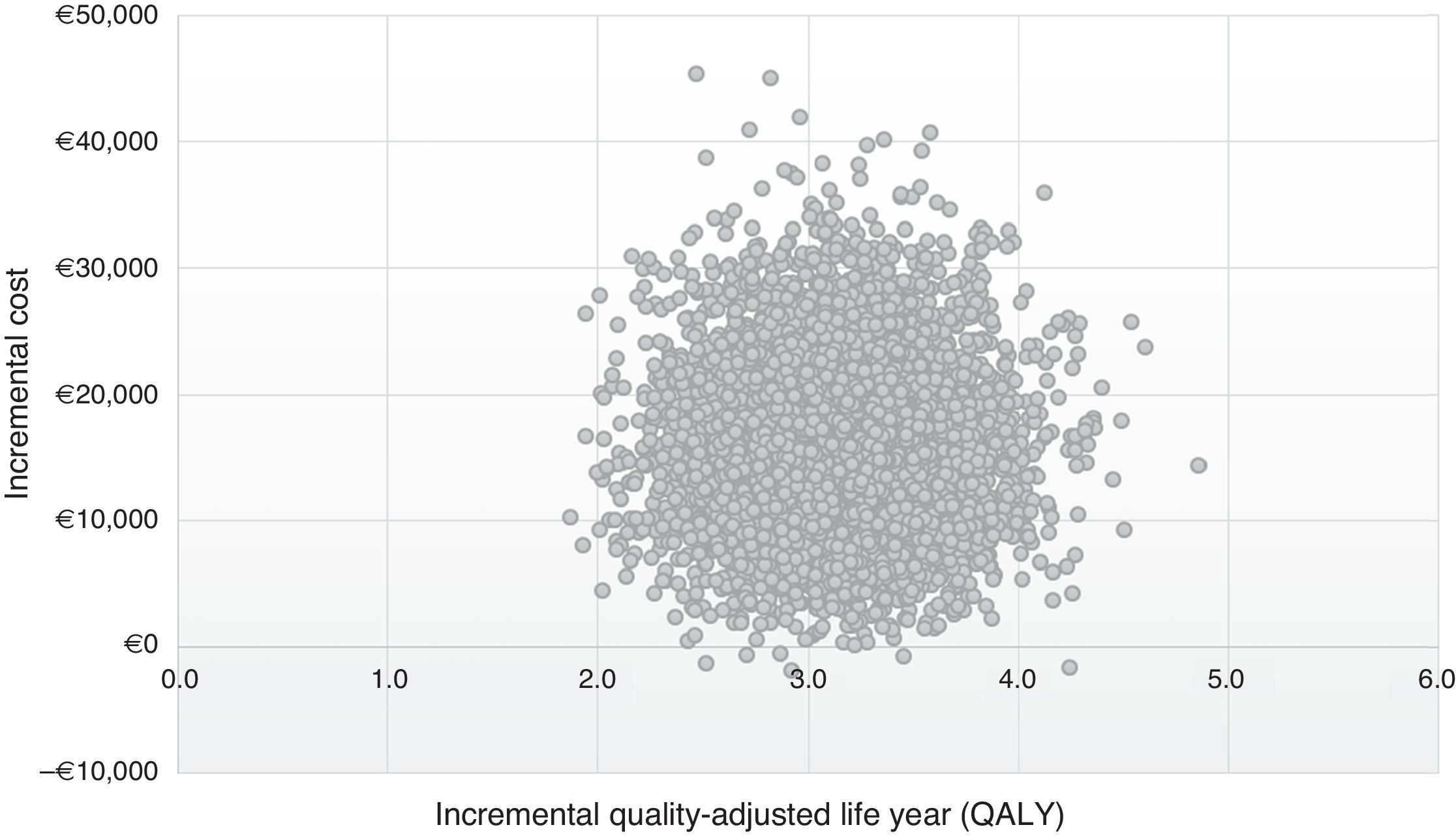
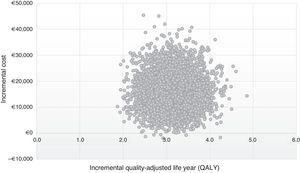
The results of 10,000 interactions performed on PSA are shown at the cost-effectiveness plane (Fig. 3). In 99% of the simulations, the values are in quadrant I, which showed that the post-DAA strategy is cost-effective, more effective, although with a higher average cost per patient than the pre-DAA strategy; while 1% was situated in quadrant II, in which the post-DAA strategy is cost-effective and also supposed a lower cost than the pre-DAA strategy.
DiscussionThe present cost-effectiveness analysis shows that the new therapeutic regimens based on DAAs manage to efficiently improve the results in health and the quality of life of patients with CHC. In other words, the additional investment made to guarantee the patients’ access to these new drugs, analyzed in the long-term, is well below the willingness to pay or efficiency threshold of the NHS in Spain, compared to previous strategies according to double or triple therapy with peg-IFN, ribavirin, telaprevir, or boceprevir.
The inclusion of new treatments in the therapeutic arsenal improves the health outcomes for patients, but is generally accompanied by an increase in pharmaceutical expenditure directly associated with treatment. In the present analysis, the post-DAA strategy, compared to the treatments used previously, entails an increase in the overall cost associated with the management of CHC derived from a higher drug cost, as well as a significant increase in the number of patients who have had access to treatment as a result of the introduction of the new therapeutic strategies with greater effectiveness and tolerability (19% of the patients treated with the pre-DAA strategy versus 73% with the post-DAA strategy).4,62 However, any economic evaluation of the treatment of a disease based only on the drug costs is incomplete and short-term. Untreated patients also generate direct healthcare costs associated with the consumption of health resources that are necessary for the treatment of complications resulting from the progression of liver disease.78 The average cost of an untreated patient in our study during the entire simulation of the evolution of the CHC is approximately €22,600.
The incremental cost-effectiveness ratio of the DAAs used in the treatment of CHC has been evaluated in numerous recent studies, and most of them conclude that the new DAAs are cost-effective treatments.79–84 However, there are few published studies that analyze the incorporation of the new DAAs from the perspective of economic and health policy/public health impact,85–87 and to date, there are no published studies that have been conducted in the Spanish context. This analysis is novel with respect to the cost-effectiveness analysis of DAAs previously published in our country because it evaluates the economic and clinical impact of the first year of implementation of the strategy included in the SPCHC for the treatment of patients with CHC (≥F2) and its viability or long-term projection. Although we cannot directly compare our study with other previously published analyses, because of the differences in methodology and particularly because the studies have been conducted in other countries where both drug and healthcare costs are different and not comparable, we can establish some similarities in relation to the impact of the new DAAs in reference to the burden of the disease and healthcare expenditure (average cost per patient). In this sense, the results of our study agree with the rest of the analyses, reflecting the efficiency of the DAAs versus the old therapies.
The new DAA regimens are characterized by their high effectiveness, with SVR rates above 95% in most patients, and an excellent safety profile that, in practice, allows any patient to be a candidate to receive antiviral treatment to eliminate HCV infection.88 It is evident that the questions regarding which patients should have access to the new DAAs and the timing of appropriate treatment for patients with CHC are controversial. The SPCHC prioritizes the administration of treatment in patients with significant fibrosis (≥F2), but it provides access to patients with mild fibrosis (F0 or F1) in various situations. Several studies that evaluate the effectiveness of the new drugs administered in the early stages of fibrosis versus advanced fibrosis conclude that early treatment is more cost effective and has greater health benefits compared to treating more advanced states of fibrosis.66,83,85,86,89–91 Ensuring access to the new DAAs for a greater number of patients would allow a faster reduction in the prevalence of HCV infection in our population, with the additional benefit of reducing the incidence of new cases of HCV infection due to a decline transmission of infection of the disease at the population level, as demonstrated in a recent study.92,93
Our study has some limitations. First, the parameters used in the modelling have been extracted from different sources. However, all of the variables are based on official sources published by MSSSI or in publications with a high level of clinical evidence.
Second, due to a lack of data availability, it was considered that all of the treated patients completed their treatment, and the possibility that the patients who failed could eventually receive a retreatment was not included. In real-world practice, some of these patients may not complete the therapy due to the application of stopping-rules based on a poor virological response (pre-DAA scenario) or the appearance of adverse effects. Both situations are more common in the pre-DAA scenario, in which an overall 13–21%21,23,28,94,95 of the patients did not complete the treatment, compared to 0–2% with current combinations.29,30,38,39,47,56 If this had been taken in account, drug costs may have decreased but being patients with worse health, the cost of managing the disease could have increased.96 From the perspective of the conclusions of the study, this conservative approach favours the pre-DAA strategy and, to a certain extent, underestimates measuring the ICER favourable to the post-DAA strategy. Equally, the adverse events of the different therapeutic strategies evaluated have not been directly considered in the analysis, although disutilities have been included in the model97 to analyze the changes in the health state of the patients due to having more or fewer adverse effects.
Third, the study has been conducted from the perspective of the NHS and not from the social perspective; therefore, the loss of productivity in patients with CHC and future cost unrelated of disease has not been considered. These patients are missing many employment hours due to the clinical management of the disease. Several studies have demonstrated that the absenteeism and presentism of a patient with CHC in Spain would be a significant total annual cost98 of €2395. The greater effectiveness and safety of the new DAAs represent an improvement in labour productivity in these patients, thereby reducing the costs related to lost productivity from the perspective of Spanish society.98,99 The inclusion of the social perspective in the model would predictably increase the average cost per patient of the pre-DAA strategy, although a study specifically designed from the social perspective would be necessary to quantify the differences between the two treatment strategies.
Fourth, due to the lack of efficacy data in real-world cohorts disaggregated for all of the treatments and for each genotype and fibrosis state, the SVR rates associated with the analysis come from clinical trials. Nevertheless, the results of global SVR rates obtained in the decision tree for patients with fibrosis ≥F2 are similar to the results of published systematic reviews or meta-analyses that evaluate the response according to different genotypes and degrees of fibrosis.8,88,100,101
Fifth, in our analysis, two public sources with estimated data of the global pharmacological cost generated by DAAs in the year 2015 and the number of patients treated in the same year were considered for the calculation of the cost of treatment. To evaluate the sensitivity of the analysis to this parameter, a DSA was performed, increasing and decreasing the drug cost of both strategies. The results showed that, if the price of the drugs were lower, the ICER would be reduced, which would mean a reduction in the overall cost generated by the management of the disease.
Despite the limitations described, the results of the sensitivity analysis confirm that the uncertainty associated with the parameters used in the modelling did not represent a significant deviation from the results obtained in the base case, with the post-DAA strategy being a cost-effective option in all simulations, having ratios below the efficiency threshold of €20,000–30,000102,103 used in Spain, which supports the conclusion that the strategy based on treatment with DAAs (post-DAA) is a very efficient option compared to the pre-DAA strategy in Spain.
Previous studies have shown that curing CHC in early stages is more cost-effective, and has greater health benefits at the population level, compared to strategies based on treatment only at more advanced stages. Together with the results from this analysis, the evidence suggests that universal access to treatment, regardless of the degree of fibrosis, could be a more efficient strategy than the current practice. Additional studies should be performed to confirm this theory.
Conflicts of interestJuan Turnes has received unconditional funding from Gilead Sciences for the development of the analysis.
Raquel Domínguez-Hernández and Miguel Ángel Casado are employees of Pharmacoeconomics & Outcomes Research Iberia, a consultancy firm specializing in the economic evaluation of healthcare interventions, which has received unconditional funding from Gilead Sciences for the development of the analysis.
Please cite this article as: Turnes J, Domínguez-Hernández R, Casado MÁ. Análisis coste-efectividad de dos estrategias de tratamiento para la hepatitis C crónica: antes y después del acceso a los agentes antivirales de acción directa en España. Gastroenterol Hepatol. 2017;40:433–446.