Extraintestinal manifestations (EIMs) are frequent in patients with inflammatory bowel disease (IBD). Our objective is to characterise and determine the prevalence of MEIs in our cohort of patients with IBD.
Patients and methodsA retrospective study was carried out in adult patients with IBD at the Pablo Tobón Uribe Hospital in Medellín. Colombia. Articular MEIs, primary sclerosing cholangitis (PSC), both ophthalmological and dermatological, were considered. Absolute and relative frequencies were used. The Chi square test of independence was used to compare 2 proportions and the odds ratio (OR) was estimated.
ResultsOur registry has 759 patients with IBD, 544 present UC (71.6%), 200 CD (26.3%) and 15 unclassifiable IBD (1.9%); 177 patients with IBD (23.3%) presented EIMs, 123 of 544 (22.6%) with UC and 53 of 200 (26.5%) with CD (OR: 0.81, 95% CI: 0.55–1.17, p=0.31). Regarding the type of EIMs, the articular ones were the most frequent (13.5%), more in CD than in UC (20.0 vs. 11.3%, OR 1.94, 95% CI: 1.25–3.00, p=0.0037). Patients with IBD and EIMs used more antibodies against tumour necrosis factor (anti-TNFs), compared to those without EIMs (43.5 vs. 18.5%, OR 3.38, 95% CI: 2.31–4.90, p=0.0001).
ConclusionsThe prevalence of EIMs in our cohort is high (23.3%) and the most frequent type is joint. Anti-TNFs are most used when IBD and EIMs coexist. Our study provides valuable information on the association of EIMs and IBD in Latin America.
Las manifestaciones extraintestinales (MEI) son frecuentes en pacientes con enfermedad inflamatoria intestinal (EII). Nuestro objetivo es caracterizar y determinar la prevalencia de MEI en nuestra cohorte de pacientes con EII.
Pacientes y métodosSe realizó un estudio retrospectivo en pacientes adultos con EII del Hospital Pablo Tobón Uribe en Medellín (Colombia). Se consideraron MEI articulares, colangitis esclerosante primaria (CEP), oftalmológicas y dermatológicas. Se emplearon frecuencias absolutas y relativas. Para comparar 2proporciones se utilizó la prueba χ2 de independencia y se estimó el odds ratio (OR).
ResultadosNuestro registro cuenta con 759 pacientes con EII, 544 presentaban CU (71,6%), 200 EC (26,3%) y 15 EII no clasificable (1,9%). Del total, 177 pacientes con EII (23,3%) presentaron MEI, 123 de 544 (22,6%) con CU y 53 de 200 (26,5%) con EC (OR: 0,81; IC 95%: 0,55-1,17; p=0,31). En cuanto al tipo de MEI, las articulares fueron las más frecuentes (13,5%), más en EC que en CU (20,0 vs. 11,3%; OR 1,94; IC 95%: 1,25-3,00; p=0,0037). Los pacientes con EII y MEI utilizaron más anticuerpos contra el factor de necrosis tumoral (anti-TNF) que aquellos sin MEI (43,5 vs. 18,5%; OR 3,38; IC 95%: 2,31-4,90; p=0,0001).
ConclusionesLa prevalencia de MEI en nuestra cohorte es alta (23,3%) y el tipo más frecuente es la articular. Los anti-TNF son más utilizados cuando coexisten EII y MEI. Nuestro estudio aporta información valiosa sobre la asociación de MEI y EII en Latinoamérica.
Inflammatory bowel disease (IBD) comprises two distinct entities: ulcerative colitis (UC) and Crohn's disease (CD). These are uncommon chronic inflammatory diseases of the gastrointestinal tract of multifactorial aetiology that primarily affect the colon and the small intestine.1,2 The clinical course of IBD is characterised by multiple flare-ups, and in recent years, an increased incidence of IBD has been detected worldwide and in Latin America.3,4 Patients with IBD (both CD and UC) may develop extraintestinal manifestations (EIM), which were recently defined at a scientific workshop of the European Crohn's and Colitis Organisation (ECCO) as “an inflammatory pathology in a patient with IBD that is located outside the gut and for which the pathogenesis is either dependent on extension/translocation of immune responses from the intestine, or is an independent inflammatory event perpetuated by IBD or that shares a common environmental or genetic predisposition with IBD”.5 The most common EIMs described involve the joints (peripheral and axial arthropathies), skin (erythema nodosum, pyoderma gangrenosum, aphthous stomatitis), hepato-pancreato-biliary system (primary sclerosing cholangitis, PSC) and eyes (episcleritis, uveitis).6 Reports on the prevalence of EIMs in IBD have been variable and range from 6% to 47%.7–10 The Spanish AQUILES study found an incidence rate of immune-mediated inflammatory diseases at two years of follow-up of 6.5% in 341 patients with IBD (spondyloarthritis, uveitis and psoriasis).11 Certain EIMs, such as pauciarticular peripheral arthritis, mouth ulcers, erythema nodosum and episcleritis, have been reported to be associated with IBD activity. However, ankylosing spondylitis and uveitis have an independent clinical course and other EIMs, such as PSC and pyoderma gangrenosum, may or may not be associated with intestinal inflammatory activity.12 The presence of EIMs in IBD is associated with extensive colitis both in UC and in CD, perianal CD, smoking and a family history of IBD, and increases the likelihood of developing other EIMs.5,8,13 Furthermore, evidence shows that EIMs sometimes precede the diagnosis of IBD.14 Despite the fact that EIMs are often observed in patients with IBD, there are limited data on their prevalence in Latin America. The objective of this study is to determine the prevalence of EIMs in our cohort of patients with IBD and to describe their clinical features.
Patients and methodsData from adult patients with IBD who were seen by the emergency department or who were admitted or seen as outpatients at Hospital Pablo Tobón Uribe in Medellin (Colombia) up until February 2019 were analysed retrospectively to determine the presence of EIMs. The diagnosis of UC and CD was taken from their medical records, with the following codes considered: K50.0 Crohn's disease of small intestine; K50.1 Crohn's disease of large intestine; K50.8 Other types of Crohn's disease; K50.9 Crohn's disease, unspecified; K51.9 Ulcerative colitis, unspecified; and K51.8 Other ulcerative colitis.
Diagnostic criteriaThe recent ECCO consensus guideline for the diagnosis of UC and CD recognises that a ‘gold standard’ for UC and CD diagnosis does not exist and that it must be established by clinical, laboratory, imaging, endoscopic and histopathological findings. The guideline does not recommend the use of genetic or serological testing for diagnostic purposes.15,16 UC activity was defined by the Truelove and Witts’ Severity Index,17 while the extent of UC was defined according to the Montreal classification.18 The disease location and disease behaviour of CD were determined according to the Montreal classification.18 Patients who did not meet the aforementioned criteria for UC and CD, despite clinical, radiological, endoscopic, histological and serological findings, were classified as subjects with IBD unclassified.18,19 Patients whose features were unclear or who did not meet IBD diagnostic criteria were excluded from the study.
Definitions of extraintestinal manifestationsAlthough multiple extraintestinal manifestations are associated with IBD, for this study only those considered true EIMs according to the ECCO definition and consensus guideline5,6 were included. These include manifestations involving: joints, mouth, skin, eyes and PSC. IBD-associated complications were not included.
Joint manifestations were divided into axial and peripheral. Axial joint manifestations include axial sacroiliitis and ankylosing spondylitis, according to the Assessment of SpondyloArthritis international Society (ASAS) classification.20 For peripheral arthropathies, the classification proposed by Orchard in 1998 was used, which includes two types.21 The ASAS classification for peripheral spondyloarthritis was validated in very few patients with IBD,22 which is why we prefer to use Orchard's classification, which was designed purely for IBD patients. Type 1 is defined as joint pain (arthralgia) with evidence of inflammation or effusion (arthritis) affecting fewer than five joints, mainly the large weight-bearing joints of the lower limbs, with symptoms that tend to be acute and self-limiting (resolving in less than 10 weeks), leave no permanent joint damage and mostly occur during IBD flare-ups. Type 2 affects more than five joints, with a symmetrical distribution and predominantly affects the upper limbs. Symptoms persist for months or years and follow an independent course irrespective of IBD activity.21 Patients with arthralgia only but no arthritis were excluded.
The other EIMs taken into consideration were the presence of erythema nodosum, mouth ulcers, pyoderma gangrenosum, psoriasis, uveitis, episcleritis and PSC. All patients with EIMs had been assessed by the respective Rheumatology, Dermatology, Ophthalmology or Hepatology Department.
Data collectionAn Excel database was created and the following data were collected for each patient for analysis purposes: (1) Type of IBD (UC, CD and IBD unclassified); (2) Patient's gender; (3) Overall medical treatment (5-ASA, steroids, immunosuppressants, biological therapy); (4) Presence of EIMs; (5) Number of EIMs; (6) Type of EIM (joint, skin, PSC, eyes); (7) Time to onset of EIMs compared to IBD; and (8) indication for anti-TNF (IBD or EIM).
Statistical analysisAbsolute and relative frequencies were used for qualitative variables and mean and standard deviation or median and interquartile range (P25-P75) were used for quantitative variables, after verification of the assumption of normality using the Kolmogorov-Smirnov test. To compare two proportions, the χ2 test of independence was used and the odds ratio (OR) was estimated with the respective 95% confidence interval.
Ethical considerationsThe project investigators adhered to the international principles of the Declaration of Helsinki (version 2013, Fortaleza, Brazil) and Colombian Ministry of Health resolution 008430 of 1993. According to this resolution, this is a risk-free investigation since the patients’ medical records were reviewed and the confidentiality and privacy of any information collected was guaranteed.
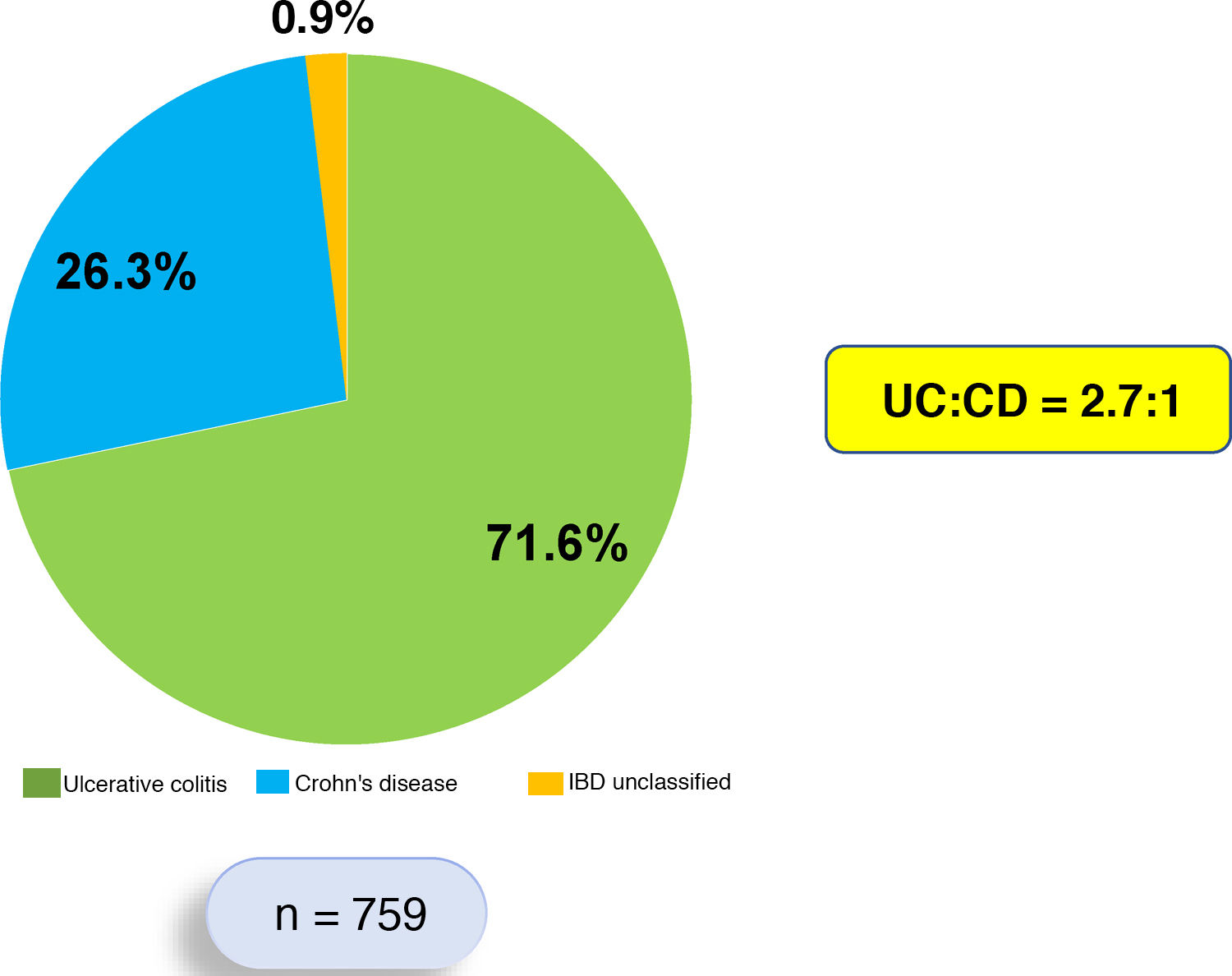
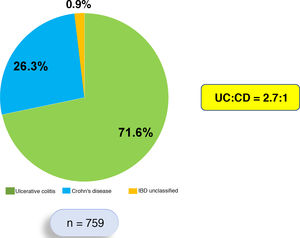
ResultsThis is a retrospective, descriptive and analytical study that systematically included 759 patients who each met diagnostic criteria for IBD. Of these 759 patients, 544 (71.6%) were diagnosed with UC, 200 (26.3%) with CD and 15 (1.9%) with IBD unclassified. The UC:CD ratio was 2.7:1 (Fig. 1). Patients with UC were predominantly female (53.4%), while patients with CD were predominantly male (57.5%).
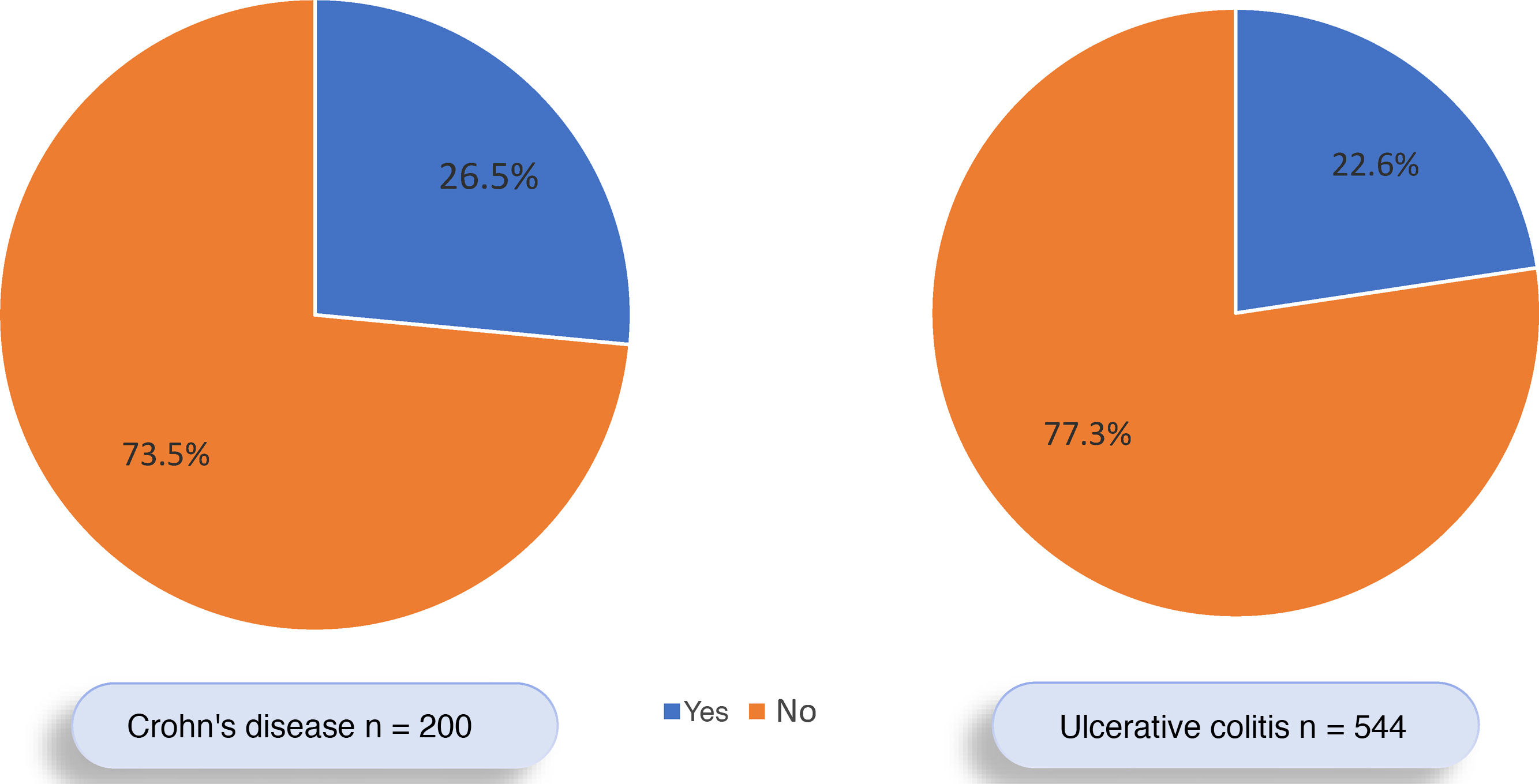
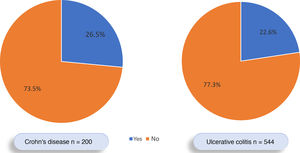
Of all the patients with IBD, 177 (23.3%) had EIMs, 53.1% female and 46.9% male. EIMs were documented in 123 of the 544 (22.6%) patients with UC and in 53 of the 200 (26.5%) with CD. However, this difference was not significant (OR: 0.81; 95% CI: 0.55–1.17; p=0.31) (Fig. 2). Of the 177 patients with EIMs, 145 (81.9%) had one EIM and 32 (18.1%) had two or more EIMs. IBD was diagnosed before the EIMs occurred in 62.5% of the patients, after the EIMs occurred in 16.7% of patients, and at the same time as the EIMs in 20.8%.
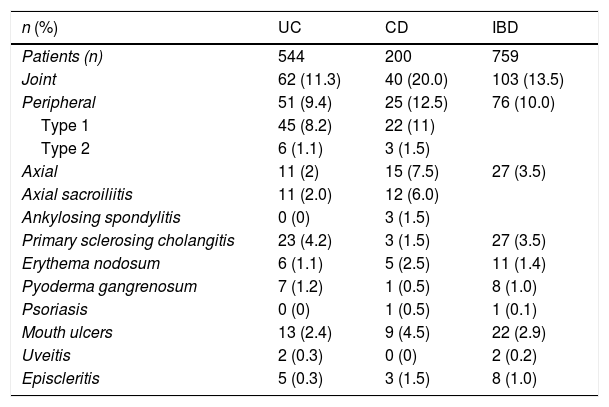
With regard to the type of EIM, EIMs involving the joints were the most common. Such EIMs were documented in 13.5% of the patients with IBD, in 10.0% with peripheral arthropathies and in 3.5% with axial involvement. Joint manifestations were more common in CD (20.0%) than in UC (11.3%) and this difference was significant (OR 1.94; 95% CI: 1.25–3.00; p=0.0037). In total, 3.0% of patients with IBD had sacroiliitis and only 0.4% had ankylosing spondylitis (1.5% in CD). It should be noted that no patients with UC had ankylosing spondylitis. With regard to peripheral arthropathies, the most common was type 1, which had a similar distribution for both UC and CD (8.2% and 11.0%), as did type 2 (1.1% and 1.5%), respectively.
PSC was identified in 3.5% of the subjects with IBD. It was more common in UC (4.2%) than in CD (1.5%) but this difference was not significant (OR 2.89; 95% CI: 0.86–9.76; p=0.11). Other EIMs were uncommon in our IBD patients: erythema nodosum (1.4%), pyoderma gangrenosum (1.0%), psoriasis (0.1%), mouth ulcers (2.9%), uveitis (0.2%) and episcleritis (1.0%). Table 1 shows the incidence rates of EIM per type of IBD.
Type of inflammatory bowel disease and frequency of extraintestinal manifestations.
| n (%) | UC | CD | IBD |
|---|---|---|---|
| Patients (n) | 544 | 200 | 759 |
| Joint | 62 (11.3) | 40 (20.0) | 103 (13.5) |
| Peripheral | 51 (9.4) | 25 (12.5) | 76 (10.0) |
| Type 1 | 45 (8.2) | 22 (11) | |
| Type 2 | 6 (1.1) | 3 (1.5) | |
| Axial | 11 (2) | 15 (7.5) | 27 (3.5) |
| Axial sacroiliitis | 11 (2.0) | 12 (6.0) | |
| Ankylosing spondylitis | 0 (0) | 3 (1.5) | |
| Primary sclerosing cholangitis | 23 (4.2) | 3 (1.5) | 27 (3.5) |
| Erythema nodosum | 6 (1.1) | 5 (2.5) | 11 (1.4) |
| Pyoderma gangrenosum | 7 (1.2) | 1 (0.5) | 8 (1.0) |
| Psoriasis | 0 (0) | 1 (0.5) | 1 (0.1) |
| Mouth ulcers | 13 (2.4) | 9 (4.5) | 22 (2.9) |
| Uveitis | 2 (0.3) | 0 (0) | 2 (0.2) |
| Episcleritis | 5 (0.3) | 3 (1.5) | 8 (1.0) |
No significant differences were observed between the extent of UC, disease location and disease behaviour of CD with respect to the presence or absence of EIMs.
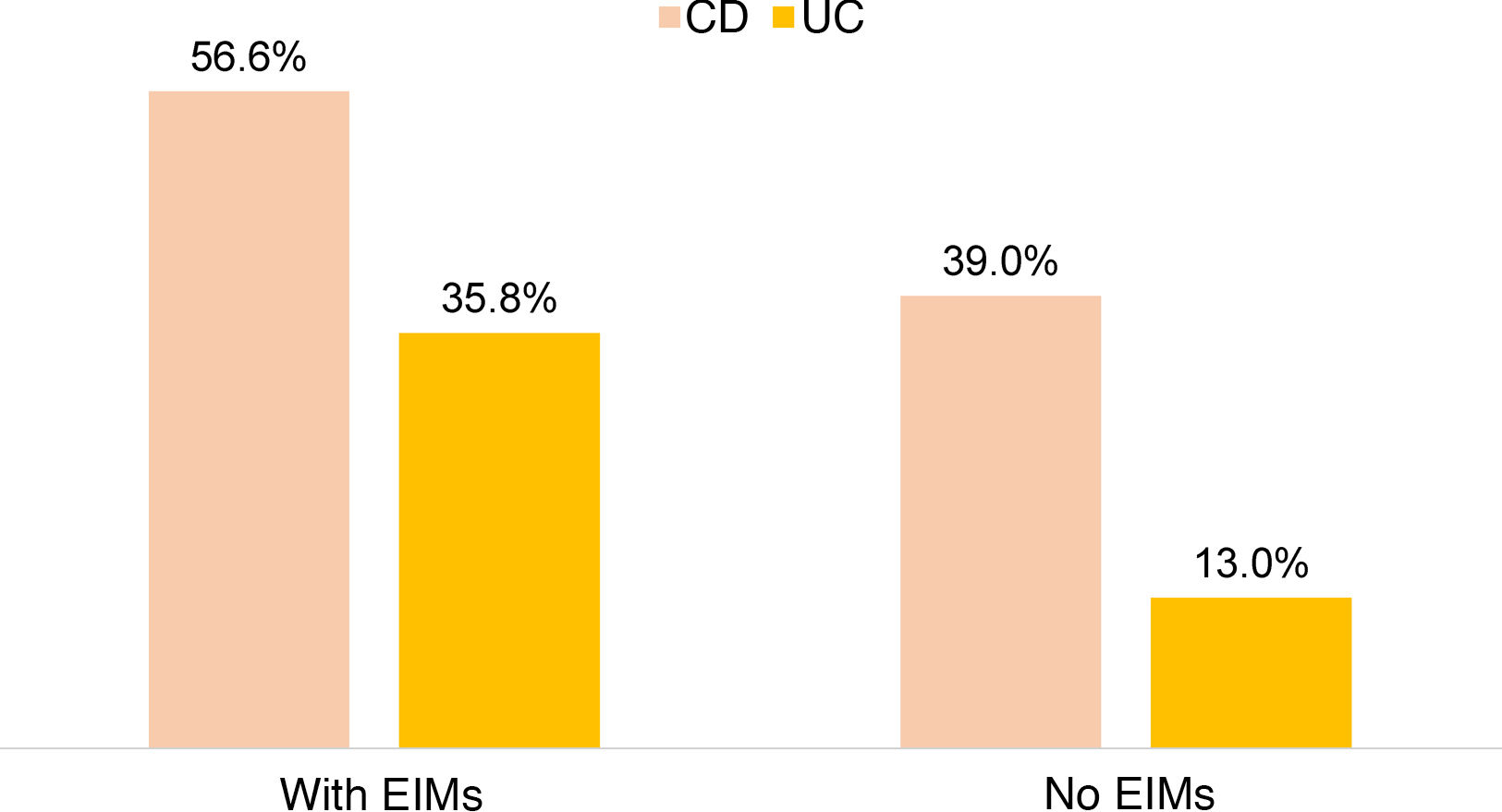
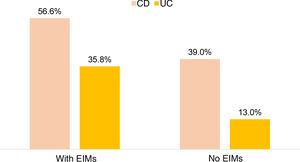
There was no significant difference between the use of steroids in IBD and the presence of EIMs, but greater use of anti-TNF was observed in patients with IBD and EIMs compared to those with no EIMs (43.5% vs. 18.5%; OR: 3.38; 95% CI: 2.31–4.90; p=0.0001). This was demonstrated separately for both UC (35.8% vs. 13.0%; OR 3.89; 95% CI: 2.38–6.36; p=0.0001) and CD (56.6% vs. 39.9%; OR 3.35; 95% CI: 1.74–6.46; p=0.0001) (Fig. 3). In 17.1% of subjects receiving biological therapy, the indication for anti-TNF usage was the presence of EIMs.
DiscussionOur study showed a prevalence of EIMs in our cohort of IBD patients of 23.3%. EIMs were more common in CD (26.5%) than in UC (22.6%) and in 18.1% of subjects, more than one EIM was documented. In addition, we found that joint manifestations were the most common and these occurred more in CD than in UC (20% vs. 11.3%; p=0.0037). In a Canadian population-based study conducted in the province of Manitoba, 6.2% of patients with IBD had EIMs, 5.5% with CD and 7% with UC, and only 0.3% of patients had multiple EIMs. However, patients with peripheral arthropathy were excluded.7 In a Hungarian study involving 873 patients with IBD, 21.3% had EIMs. These were more common in CD (36.6%) than in UC (15.0%) and more joint manifestations were documented in CD than in UC (22.4% vs. 10.2%; p<0.01), which is similar to our results.8 A study conducted by the Mayo Clinic in Minnesota found that 40% of the 243 consecutive IBD patients experienced EIMs, 36% with UC and 43% with CD.9 More recently, in a Swiss cohort10 of 950 patients with IBD, 38.1% experienced EIMs, 43% with CD and 31% with UC. The most common EIMs involved the joints (28.6% in CD and 21.3% in UC) and 12.3% of the IBD patients experienced more than one EIM. This finding is similar to the results reported with our cohort. In a Spanish study involving 173 patients with CD only, 35.5% of patients experienced EIMs and 9.8% had two or more EIMs. The most common were joint manifestations, while female gender, colonic involvement and higher steroid dependency were associated with a higher risk of developing EIMs, which was not shown in our study.23
A systematic review and meta-analysis of 71 studies of joint manifestations in IBD found prevalences for peripheral arthritis of 13%, for sacroiliitis of 10% and for ankylosing spondylitis of 3%.24 A more recent study by the IBSEN Study Group in Norway identified axial spondyloarthritis (7.7%) and ankylosing spondylitis (4.5%) in 470 patients with IBD at 20 years of follow-up.25 These percentages are higher than those recorded in our study, which can probably be explained by the longer patient follow-up time.
With regard to PSC, there were more cases among subjects with UC than with CD (4.2% vs. 1.5%). The studies conducted in Hungary8 and Switzerland,10 as mentioned above, had similar results, with more cases of PSC in UC than in CD (1.6% vs. 0.8% and 4% vs. 1%, respectively).
Another interesting finding of this study is that, in 16.7% of patients, the EIM was diagnosed prior to IBD. In the Swiss cohort, the EIM preceded the IBD diagnosis in 25.8% of patients.14 A more recent study found that 7% of patients with IBD exhibited joint EIMs prior to being diagnosed with IBD.26
To explain this association between EIMs and IBD found in this and other earlier studies, experts have suggested two theories. The first considers that EIMs arise from an extension of antigen-specific immune responses from the intestine to non-intestinal sites, and the second proposes that EIMs are independent inflammatory events initiated or perpetuated by the presence of IBD or by shared genetic or environmental risk factors in the host.5 Various potential mechanisms have also been proposed by which gut microbiota may drive the pathogenesis of EIMs in IBD patients: similarity between gut microbiota epitopes and non-microbial epitopes present at the extraintestinal site; microbial translocation from the gut to the extraintestinal site; bacterial lipopolysaccharides released into the bloodstream, promoting inflammation at extraintestinal sites; disruption of the gut barrier; and production of microbiota-derived metabolites, which could alter the immune response.5
Finally, we found that patients with IBD and EIMs require significantly more anti-TNF therapy than those with no EIMs (43.5% vs. 18.5%). This was demonstrated both in CD and in UC. In addition, in 17.1% of patients, the indication for anti-TNF usage was the presence of EIMs. One recent systematic review and meta-analysis showed the effectiveness of anti-TNF in EIMs, especially in EIMs involving the joints and skin.27 This can be explained by TNFα-dependent mechanisms common in the pathophysiology of IBD and some EIMs.5 One Swiss study found that patients with IBD and EIMs received more anti-TNF therapy than those with no EIMs (58.2% vs. 21.0%) and these percentages are similar to those found in our study. Furthermore, in 43.2% of patients, anti-TNF drugs were started to treat EIMs.28
With regard to the limitations of this study, it should be noted that, since this is a retrospective study based on data collected from the patients’ medical records, it may suffer from selection biases. Moreover, our hospital is a highly complex institution and a referral centre for IBD patients from all over the country. As such, we probably included more serious and complicated patients in our study than other sites around the country and we know that this type of patient is associated with an increased presence of EIMs. However, due to being a retrospective record review study, we were unable to reliably determine the activity of either CD or UC at the time of the EIMs in order to establish any link. With regard to the lower rate of axial joint involvement in our study, this may be due to under-diagnosis and we, as gastroenterologists, should be more aware of the co-existence of IBD with this type of manifestation, remember to consider the diagnostic criteria and take a detailed history of any joint symptoms.
ConclusionsTo summarise, EIMs in patients with IBD are common in Colombia, more so in CD than in UC. Joint manifestations are the most common EIMs and the association between IBD and EIMs requires greater use of biological therapy. All of these findings are similar to those reported before in other studies. It is therefore important to form multidisciplinary teams at institutions, including gastroenterologists, rheumatologists, dermatologists, ophthalmologists and hepatologists, among others, to improve the diagnosis of both IBD and EIMs, reduce the delay in diagnosing these diseases and offer appropriate and timely treatment to these complex patients, as international consensuses have tried to do.29 Our study provides valuable information on the association between EIMs and IBD in our country, despite the limited information available on this association in Latin America.
FundingNo funding was received from any entity to conduct this study.
Authors/contributorsF. Juliao-Baños: Study design, patient recruitment and drafting of the manuscript
M. Arrubla: Data collection
L. Osorio: Data collection
J. Camargo: Data collection
J. Londoño: Data collection
C. Cáceres: Data collection
J. Carvajal: Patient recruitment
G. Mosquera: Patient recruitment
J. Donado: Statistical analysis
Conflicts of interestNone of the study authors has conflicts of interest to report.
Please cite this article as: Juliao-Baños F, Arrubla M, Osorio L, Camargo J, Londoño J, Cáceres C, et al. Caracterización y prevalencia de manifestaciones extraintestinales en una cohorte de pacientes con enfermedad inflamatoria intestinal en Medellín (Colombia). Gastroenterol Hepatol. 2021;44:398–404.