Omeprazole is the first in a series of active ingredients that act by inhibiting the H/K ATPase pump (proton-pump inhibitor [PPI]). Its emergence revolutionised the treatment of peptic ulcers, gastroesophageal reflux and NSAID-induced gastropathy, so much so that it is currently the most prescribed active ingredient.
It is generally a well-tolerated medicine with adverse reactions to it being mild and normally reversible. The most common ones are digestive symptoms that occur in 1.5–3% of cases and effects on the central nervous system. Other adverse reactions that have been reported and are included on the summary of product characteristics are the inhibition of vitamin B12 absorption1 and the appearance of hives.2
We present symptoms of anaphylaxis in a 58-year-old patient after taking an omeprazole tablet and the subsequent cross-reactivity testing with other PPIs.
A 58-year-old male, whose medical history showed a undocumented prior diagnosis of allergy to beta-lactams as a child, and whose surgical history showed a cholesteatoma procedure 18 years ago in his left ear and appendicitis with Meckel's diverticulum 10 years ago. The rest was not of interest.
The symptoms began with heartburn sensations and gastric discomfort 6h after taking a 600mg ibuprofen tablet for knee pain, for which he decides to take a 20mg tablet of omeprazole. Ten minutes after taking it, he began to feel generally unwell, dizzy, sweaty and unsteady which forced him to lie down due to the worsening of his general condition. Upon physical examination, general swelling was observed on his face as well as dysarthria, with feelings of dyspnoea and decreased oxygen saturation measured with the CO-Oximeter, triggering a loss of consciousness. Due to the suspicion of anaphylactic shock, he was cared for at home by the emergency services. He was given 80mg of methylprednisolone and intramuscular adrenaline as well as beclomethasone and salbutamol in a spacer. The oxygen saturation started to progressively improve and the patient regained consciousness after approximately 10min. The patient was taken to the referral hospital where he remained under surveillance for 24h, and he discharged asymptomatic and referred to the allergology department for testing.
The tests performed were as follows:
- -
Skin tests for drugs:
- •
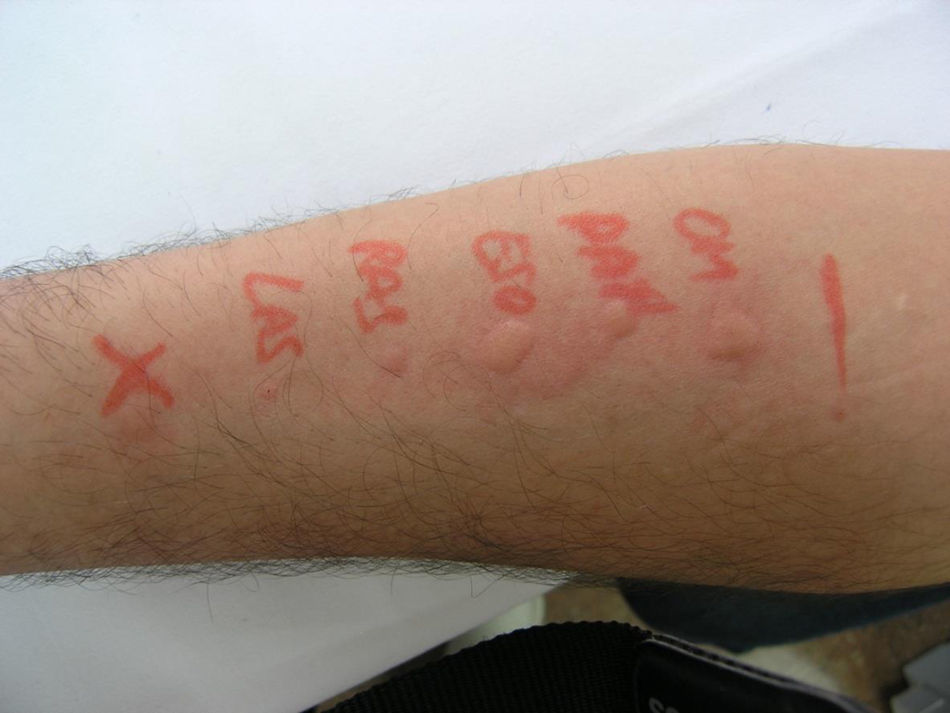
Prick test positive to omeprazole, pantoprazole, esomeprazole, rabeprazole and lansoprazole (Fig. 1).
- •
Prick test negative to arylpropionics (ibuprofen), beta-lactams with re-exposure.
- •
- -
Controlled exposure tests with oral drugs: negative to arylpropionics (ibuprofen) and amoxicillin with re-exposure.
- -
Skin tests for aeroallergens: negative to the common environmental aeroallergens (pollen, dust mites, fungus and dog and cat dander).
- -
Spirometry: FVC=92% theoretical, FEV1=98% theoretical. FVC/FEV1=97, bronchodilator test negative.
After the examination of the described signs and symptoms and the tests performed in the allergology department, the patient was diagnosed with anaphylaxis due to hypersensitivity to omeprazole and PPIs.
The impact of hypersensitivity reactions to PPIs is increasing due to the growing use of these drugs, mainly for 2 reasons: their efficacy and the frequent use of non-prescription drugs.3
The skin tests conducted showed a type of immediate allergic reaction to the 5 PPIs that are marketed in Spain. The negative control results also exclude the possibility of an irritant reaction.
This is not surprising given that, upon carrying out a detailed analysis of the clinical cases reported in the literature, different papers are observed where the different reactivity patterns amongst the PPIs are shown.4,5
In order to develop a hypersensitivity symptom, the patient should have previously been in contact with that substance. However, it was consciously the first time that he took an omeprazole tablet and so his history was studied in detail where it was actually affirmed that during his last surgical procedure 10 years ago due to acute appendicular symptoms, this drug was used intravenously.
Facing the presence of anaphylaxis symptoms, the possibility of the patient having previously taken the drug must be taken into account, even if it is not reflected in his medical record. It must also be taken into account that not all anaphylaxis symptoms are caused by NSAIDs.6
For this and other reasons, we believe that it would be highly advisable for the patient's medical record to include all of the information related to his pharmacotherapeutic profile and the possible adverse reactions that they may have had throughout their history by the different health services.6 Equally, we must go as far as possible to make the public aware that they should avoid self-medication, even with medicines that are popularly considered to be “safe drugs”, and that we should not let our guard down in the event of adverse effects because, as we have seen in this case, they can be very serious.
Conflicts of interestThere is no financial support for this project nor conflicts of interests by any of those that took part in it.
Please cite this article as: González-Rubio F, Esteban-Jiménez O, Garcés-Sotillos MM, Colás-Sanz C. Shock anafiláctico por omeprazol. Gastroenterol Hepatol. 2017;40:20–21.
Case reported to the Spanish Agency of Medicines via the Aragon Pharmacovigilance Network.