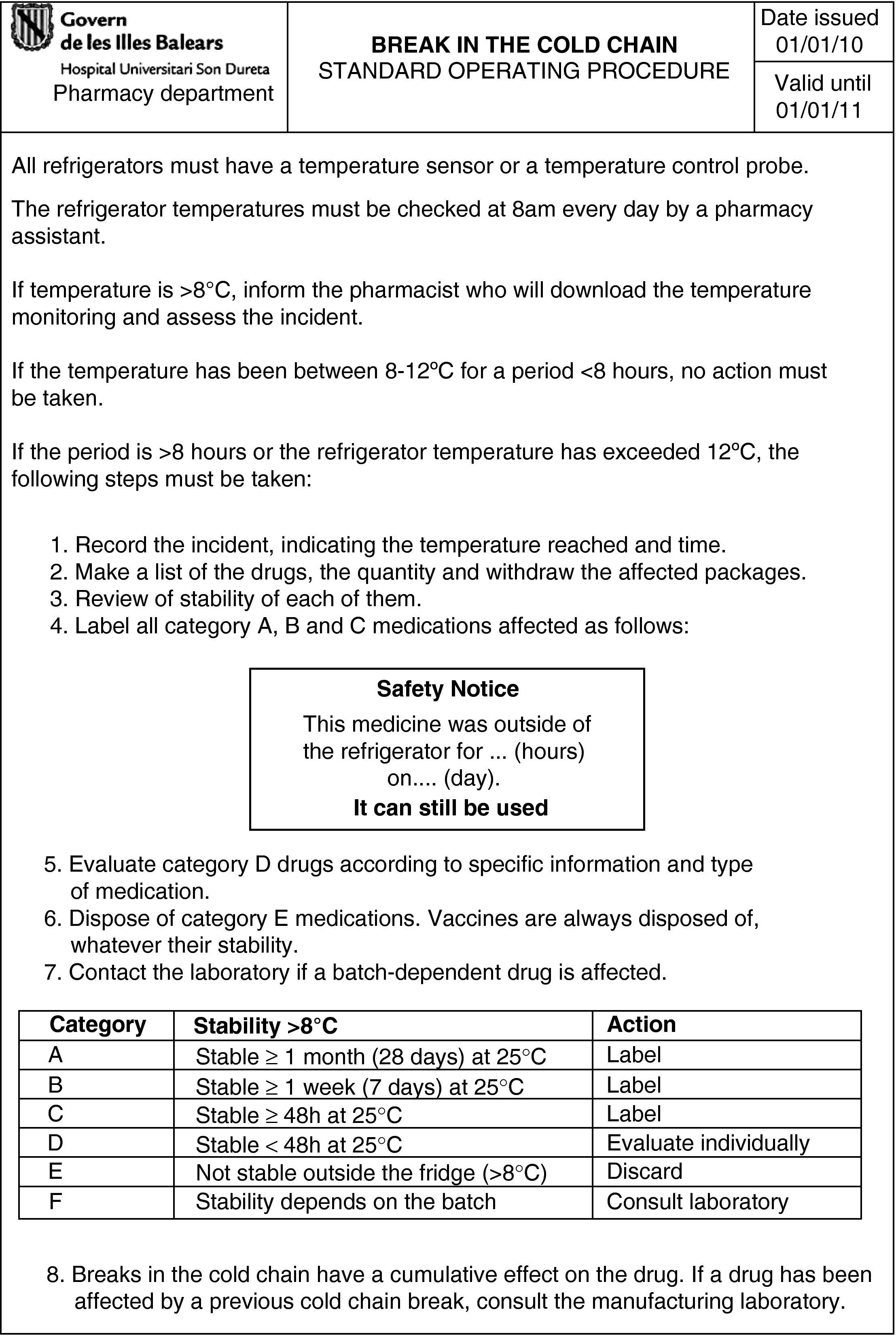
To establish a standard operating procedure in the event of cold chain failure.
MethodWe selected thermolabile drugs included in the hospital's pharmaceutical guide. We performed a review of the available literature, classifying each drug into a given category with an intervention protocol for each one.
ResultsWe reviewed 254 drugs (162 active ingredients). Categories were: A (stable ≥28days at 25°C): 65 drugs; B (≥7days at 25°C): 47 drugs; C (≥48h at 25°C): 30 drugs; D (<48h at 25°C): 47 drugs; E (unstable >8°C): 12 drugs; F (batch-dependent) 22 drugs. Thirty-one drugs were not classified into any category.
The intervention protocol consisted of establishing a system to monitor the products concerned, and discarding or returning them to the laboratory if they were to exceed the time or temperature limit indicated for each category.
DiscussionThe aim of this study is to make intervention quicker in the event of cold chain failure.
Establecer un procedimiento normalizado de trabajo en caso de rotura de cadena de frío.
MétodoSe seleccionaron los medicamentos termolábiles incluidos en la guía farmacoterapéutica del hospital y se revisó la bibliografía disponible, clasificándolos en categorías con un protocolo de actuación en cada caso.
ResultadosSe revisaron 254 medicamentos (162 principios activos). La distribución por categorías fue: A (estable ≥28 días a 25°C): 65 medicamentos; B (≥7días a 25°C): 47; C (≥48h a 25°C): 30; D (<48h a 25°C): 47; E (no estable >8°C): 12; F (depende del lote): 22. No se clasificaron en ninguna categoría 31 medicamentos.
El protocolo de actuación consistió en establecer un sistema de seguimiento de los medicamentos afectados y desechar o devolver al laboratorio en caso de superarse el límite de tiempo o temperatura establecido en cada categoría.
DiscusiónEl trabajo realizado pretende facilitar la rápida actuación en aquellas situaciones de rotura de la cadena de frío.
The cold chain is the set of logistical links that guarantee that a temperature between 2°C and 8°C is maintained during the processes of storage, handling, transport and distribution of drugs. If this is not done, drug properties are liable to change in varying degrees, depending on the temperature reached and the time spent at that temperature.
There are procedures for receiving, storing and distributing drugs in hospitals to ensure that the cold chain is maintained. Standard operating procedures and facilities ensure that the proper temperature is maintained. The complexity of drug distribution processes in hospitals means that there are cold storage facilities available in a large number of locations in the pharmacy department storage areas, as well as the drug storage areas of inpatient units, day hospitals, operating rooms and outpatient clinics, among others.
The cold chain may be broken in many unexpected ways during daily practice (e.g., due to a power failure, cold room breakdowns, inadequate transportation or an error in storage conditions). These incidents may affect just a few units of a drug in a hospital ward or may affect complete clinical containers due to a refrigerator failure.
Administering a drug which has been inadequately stored can have highly variable potential consequences for the patient. Some medications are affected by a temporary and an isolated break in the cold chain: a number of drugs may lose some efficacy of little clinical relevance, while others may have a total loss of activity or may even become toxic.1
In addition, a break in the cold chain may have a significant economic impact for the hospital if the full activity of a drug cannot be guaranteed and it has to be disposed of, and there were no conditions established for its return to the pharmaceutical company supplying it.
The potential clinical and economic impacts posed by the loss of this group of drugs make it necessary to have a protocol for maintaining the cold chain and establishing actions if it is broken. This includes a drug stability report, including the time and temperature to which the drug has been exposed. It is also important to provide information about any such event, as affected batches will have to be withdrawn, and it is important to know whether they can be used or not.
The aim of this study was to establish a standard procedure for a break in the cold chain, prepare an updated list (to 2010) on the maximum stability of thermolabile active ingredients at room temperature, classify them according to the possibility of re-using them for certain time periods and provide a communication system on the hospital intranet and via the Internet.
MethodThe main active ingredients to be kept at a temperature between 2°C and 8°C or in the freezer were selected from the hospital pharmacotherapeutic guide.
A review of previously published studies in PubMed with the MESH Drug Stability, Drug storage, refrigeration and Cold chain was carried out.2–10 The information available from the summaries of product characteristics (SmPCs) was taken and the pharmaceutical manufacturer was consulted by fax or e-mail if there was any doubt or lack of data.
An Excel table was prepared, which included speciality-specific data, bibliographic references, and the contact details of the pharmaceutical company department, where appropriate.
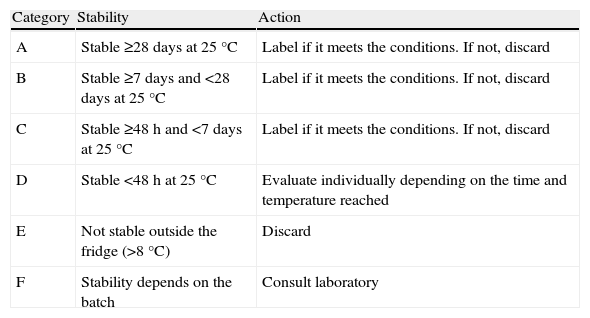
Finally, agreement was reached within the pharmacy department on a standard operating procedure (SOP) for any break in the cold chain, which sorted the drugs into 6 categories based on the time they were stable outside of the refrigerator (Table 1).
Classification of Drugs.
| Category | Stability | Action |
| A | Stable ≥28 days at 25°C | Label if it meets the conditions. If not, discard |
| B | Stable ≥7 days and <28 days at 25°C | Label if it meets the conditions. If not, discard |
| C | Stable ≥48h and <7 days at 25°C | Label if it meets the conditions. If not, discard |
| D | Stable <48h at 25°C | Evaluate individually depending on the time and temperature reached |
| E | Not stable outside the fridge (>8°C) | Discard |
| F | Stability depends on the batch | Consult laboratory |
A total of 254 medicinal products corresponding to 162 active ingredients were reviewed (Table 2).
Recommendations After Breaking the Cold Chain.
| Active Ingredient | Drug Product | Manufacturing Laboratory | Stability at Room Temperature (22–25°C) | Reference | Contact Telephone No. | Category |
| Abciximab | Reopro® vial 50mg/ml | Lilly | 8 days at 28°C (192h) | Consult lab 28/07/09 | 916635000 | B |
| Carglumic acid | Carbaglú® 200mg tablets | Orphan Europe | 1 month at <30°C | SmPCs (2003) | A | |
| Adalimumab | Humira ® 40mg prefilled syringe | Abbott Laboratories | 8h | Cobos Campos et al., 20063 | D | |
| Agalsidase alfa | Replagal® 3.5mg vial | Shire Human Genetic Therapies | 24h | SmPCs (2006) | D | |
| Alemtuzumab | MabCampath® 30mg vial | Bayer Healthcare | 7 days at 25°C | Consult lab 20/2/09 | 934956500 | B |
| Alfacalcidol | Etalpha® ampoules 1mcg | Leo Pharma | 1 year at 25°C | Bovaira García et al., 20044 | 932213366 | A |
| Alfacalcidol | Etalpha® ampoules 2mcg | Leo Pharma | 1 year at 25°C | Bovaira García et al., 20044 | 932213366 | A |
| Alfacalcidol | Etalpha® drops | Leo Pharma | 1 year | Consult lab 16/02/09, Bovaira García et al., 20044 | 932213366 | A |
| Alprostadil | Alprostadil® 500mcg/ml | Pfizer | 4 months | Cuervas-Mons et al., 2004,6 García Vázquez et al., 19977 | A | |
| Alteplase | Actilyse® 20mg | Boehringer Ingelheim Spain | 3 years at <25°C | Bovaira García et al., 20044 | A | |
| Alteplase | Actilyse® 50mg | Boehringer Ingelheim Spain | 3 years at <25°C | Bovaira García et al., 2004,4 Cuervas-Mons et al., 20046 | A | |
| Amphotericin B | Abelcet® 50mg vial | Cephalon Pharma | 1 day outside fridge=9 days in fridge (change expiry date) | Cobos Campos et al., 20063 | Specific recommendation | |
| Cuervas-Mons et al., 20046 | ||||||
| Amphotericin B | Fungizona® 50mg vial | Bristol Myers Squibb | 2 weeks-1 month | Cobos Campos et al., 2006,3 Cuervas-Mons et al., 20046 | B | |
| Digoxin antidote | Digitalis Antidot® 80mg vial | Roche Farma Farma | 20 days up to 40°C | Silgado et al., 20062 | B | |
| Asparaginase | Erwinase® (ME) 10,000IU vial | Opi | 2 years | García Vázquez et al., 19977 | A | |
| Asparaginase | Kidrolase® 10,000IU vial | Opi | 48h (1), 7 days (2) | Cuervas-Mons et al., 20046 (1) | ||
| C | García Vázquez et al., 19977 (2) | |||||
| Atosiban | Tractocile® 37.5 | Ferring | 24h at 25°C | Cobos Campos et al., 2006,3 Cuervas-Mons et al., 20046 | D | |
| Atosiban | Tractocile® 6.75 | Ferring | 24h at 25°C | Cobos Campos et al., 2006,3 Cuervas-Mons et al., 20046 | D | |
| Atracurium | Atracurio® 25mg amp. | Inibsa | 1 month 30°C (loss of 8%) | Cobos Campos et al., 2006,3 Cuervas-Mons et al., 20046 | A | |
| Basiliximab | Simulect® 20mg vial | Novartis Farmaceutica | 4 days at <25°C | Cuervas-Mons et al., 20046 | C | |
| Bevacizumab | Avastin® 100mg vial | Roche Farma | 9h at 30°C; 5 days at 15°C | Consult lab 20/2/09 | 913248100 | D |
| Avastin® 400mg vial | Roche Farma | 9h at 30°C; 5 days at 15°C | Consult lab 20/2/09 | 913248100 | D | |
| BH4 | Tetrahydrobiopterin® 10mg | Schircks Laboratories | ≤−20°C: 36 months//≤−10°C: 18 months//≤+5°C: 4 months//>6 months at 25°C: maintains >99.5% activity (although may turn yellow or break more easily)//20–30°C: expires in 2 months//Can be re-frozen | SmPCs (review year not available) | A | |
| BH4 | Tetrahydrobiopterin® 50mg | Schircks Laboratories | ≤−20°C: 36 months//≤−10°C: 18 months//≤+5°C: 4 months//>6 months at 25°C: maintains >99.5% activity (although may turn yellow or break more easily)//20–30°C: expires in 2 months//Can be re-frozen | SmPCs (review year not available) | A | |
| Bleomycin | Bleomicina® 15IU vial | Almirall | 28 days | Cobos Campos et al., 2006,3 Sala et al., 2004,5 Cuervas-Mons et al., 2004,6 Silgado et al., 20062 | A | |
| Busulphan | Busilvex® 60mg vial | Pierre Fabre Iberica | Note batch no., expiry, temperature, exposure time and contact laboratory | Consult lab 20/02/09 | 934833000 | F |
| Calcitonin | Calcitonina Hubber® inject. 100IU | Valeant Pharmaceuticals Iberica | 1 month | Cobos Campos et al., 20063 | A | |
| Calcitonin | Calcitonina Hubber® nasal 200IU | Valeant Pharmaceuticals Iberica | 1 month | Cobos Campos et al., 20063 | A | |
| Carboprost | Hemabate® 250 | Zlb Behring | Note batch no., expiry, temperature, exposure time and contact laboratory | Consult lab 20/02/09 | 933068117 | F |
| Carmustine | BiCNU® (ME) 100mg vial | Bms | The appearance of droplets indicates that the product is not fit to be used | Cobos Campos et al., 20063 | F | |
| Caspofungin | Cancidas® 50mg | Merck Sharp Dohme | 48h | Cobos Campos et al., 2006,3 Sala et al., 2004,5 Cuervas-Mons et al., 2004,6 Silgado et al., 20062 | C | |
| Caspofungin | Cancidas® 70mg | Merck Sharp Dohme | 48h | Cobos Campos et al., 2006,3 Sala et al., 2004,5 Cuervas-Mons et al., 2004,6 Silgado et al., 20062 | C | |
| Cefonizid | Monocyd® 1g IM/IV | Rottapharm | 18 months | Silgado et al., 2006,2 Cuervas-Mons et al., 20046 | A | |
| Cetuximab | Erbitux® 100mg vial | Merck Sharp Dohme | 20h | Bovaira García et al., 20044 | D | |
| Cisatracurium | Nimbex® 10mcg/ml | Glaxo SmithKline | 1 month at 25°C: 5% degradation 1 day outside fridge=10 days in fridge | Cobos Campos et al., 2006,3 Sala et al., 20045 | Specific recommendation | |
| Cisatracurium | Nimbex® 2mcg/ml | Glaxo SmithKline | 1 month at 25°C: 5% degradation 1 day outside fridge=10 days in fridge | Cobos Campos et al., 2006,3 Sala et al., 20045 | Specific recommendation | |
| Liposomal cytarabine | Depocyte® 50mg vial | Mundi Pharma Pharmaceuticals | 72h at 25°C | Consult lab 20/02/09 | 913821870 | C |
| Chlorambucil | Leukeran® 2mg tablets | Glaxo SmithKline | Note batch no., expiry, temperature, exposure time and contact laboratory | Consult lab 22/12/09 | 918070301 | F |
| Chloramphenicol | Colircusi cloranfenicol® | Alcon Cusi | Storage: 15 days at 25°COnce open: 1 month | Bovaira García et al., 2004,4 Silgado et al., 20062 | B | |
| Chloramphenicol | Colircusi de Icol® | Alcon Cusi | Storage: 15 days at 25°COnce open: 1 month | Bovaira García et al., 20044 | B | |
| Anti-inhibitor coagulant complex | Feiba® 4 1000UF | Baxter | 6 months | Sala et al., 20045 | A | |
| Daclizumab | Zenapax® 5mg/ml | Roche Farma | 14 days at 25°C, 7 days at 30°C | Bovaira García et al., 2004,4 Silgado et al., 20062 | B | |
| Daptomycin | Cubicin® 500mg vial | Novartis Farmaceutica | 60h at 25°C | Consult lab 16/02/09 | 933064200 | C |
| Denileukin diftitox | Ontak® 300mg/2ml | Ferrer Farma | No information available | Consult lab 20/12/09 | 936003700 | F |
| Desmopressin | Minurin® drops | Ferring | 1 month | Cobos Campos et al., 2006,3 Sala et al., 2004,5 Cuervas-Mons et al., 20046 | A | |
| Desmopressin | Minurin® inject | Ferring | 24h | Cobos Campos et al., 2006,3 Sala et al., 2004,5 Cuervas-Mons et al., 20046 | D | |
| Dinoprostone | Prepidil® 0.5 gel | Pfizer | 1 month | Cobos Campos et al., 2006,3 Cuervas-Mons et al., 20046 | A | |
| Dinoprostone | Propess® 10mg | Ferring SAU | <24h at 2–8°C: re-freeze. If thawed completely: 4 weeks at 2–8°C. Room temperature: 24h | Consult lab 03/08/09 | D | |
| Dinoprostone | Prostaglandina E2® 10mg/ml | Pfizer | 1 month at 25°C | Cobos Campos et al., 20063 | A | |
| Dopamine hydrochloride | Duodopa gel intestinal cassettes 100ml | Solvay Pharma | 4 days at 25°C | Consult lab 05/03/09 | 934954500 | C |
| Dornase alpha | Pulmozyme® 2.5mg amp. | Roche Farma | 24h at 30°C (1)2.5 days at 30°C (2) | Cuevas (1), Sala et al., 20045 (1), Silgado et al., 20062 (2), Bovaira García et al., 20044 (2) | D | |
| Doxycycline | Vibravenosa® 100mg | Pfizer | 1 month | Cobos Campos et al., 20063 | A | |
| Doxorubicin (=adriamycin) | Doxorrubicina® 50mg vial | Ferrer Farma | Note batch no., expiry, temperature, exposure time and contact laboratory | Consult lab 20/02/09 | 936003700 | F |
| Liposomal doxorubicin | Myocet® 50mg vial | Cephalon Pharma | <1 month | Cobos Campos et al., 2006,3 Silgado et al., 20062 | B | |
| Pegylated liposomal doxorubicin | Caelyx® 20mg vial | Schering-Plough | Note batch no., expiry, temperature, exposure time and contact laboratory | Consult lab, Sala et al., 20045 | F | |
| Drotrecogin | Xigris® 20mg | Lilly | 72h up to 28°C | Silgado et al., 20062 | C | |
| Drotrecogin | Xigris® 5mg | Lilly | 72h up to 28°C | Silgado et al., 2006,2 Cuervas-Mons et al., 2004,6 Bovaira García et al., 2004,4 Sala et al., 20045 | C | |
| Eculizumab | Soliris® 300mg vial | Alexion Pharma Spain | 6–7 days | Consult lab 20/02/09 | 932723017 | C |
| Epirubicin | Farmorubicina® 200mg vial | Pfizer | Note batch no., expiry, temperature, exposure time and contact laboratory | Consult lab 15/02/09 | F | |
| Eptifibatide | Integrilin® 20mg vial | Glaxo SmithKline | 2 months | Cuervas-Mons et al., 20046 | A | |
| Erythropoietin alpha | Eprex® 40,000IU prefilled syringe | Janssen-Cilag | 1h 6h | Cobos Campos et al., 2006,3 Cuervas-Mons et al., 2004,6 Silgado et al., 2006,2 Bovaira García et al., 20044 | D | |
| Erythropoietin beta | Neorecormon® prefilled syringe | Roche Farma | 3 days | Cuervas-Mons et al., 2004,6 Cobos Campos et al., 2006,3 Silgaro, Sala et al., 20045 | C | |
| Streptokinase | Streptase® 750,000IU | CSL Behring | Keep at temperature ≤25°C | Cuervas-Mons et al., 2004,6 Bovaira García et al., 20044 | D | |
| Streptozocin | Zanosar® (ME) 1g vial | Pfizer | Note batch no., expiry, temperature and exposure time and contact laboratory | Cobos Campos et al., 2006,3 Silgado et al., 20062 | F | |
| Etanercept | Enbrel® 25mg prefilled syringe | Wyeth Farma | 24h at ≤25°C; >25°C contact laboratory | Consult lab 21/12/09 | 913346400 | D |
| Etanercept | Enbrel® 25ng/ml paediatric vial | Wyeth Farma | 24h at ≤25°C; >25°C contact laboratory | Consult lab 21/12/09 | 913346400 | D |
| Etanercept | Enbrel® 50mg prefilled syringe | Wyeth Farma | 24h at ≤25°C; >25°C contact laboratory | Consult lab 21/12/09 | 913346400 | D |
| Factor IX | Factor® IX 600IU | Behring | 3 months at 25°C (do not re-refrigerate) | SmPCs (2010) | Specific recommendation | |
| Factor VIII+Von Willebrand factor | Fanhdi® | Grifols | 2 years at 25°C (1)months at 40°C (2) | Cobos Campos et al., 20063 (1), Cuervas-Mons et al., 20046 (2) | A | |
| Recombinant Factor VIII | Advate® 1000IU | Baxter | 6 months at 25°C (do not re-refrigerate) | SmPCs (review year not available) | Specific recommendation | |
| Recombinant Factor VIII | Advate® 500IU | Baxter | 6 months at 25°C (do not re-refrigerate) | SmPCs (review year not available) | Specific recommendation | |
| Factor IX plasma | Inmunine® Stim plus 600 | Baxter | 3 months at 25°C | Sala et al., 20045 | A | |
| Recombinant Factor IX | Benefix® 500IU | Baxter | 1 month at 25°C | Cuervas-Mons et al., 2004,6 Silgado et al., 2006,2 Sala et al., 20045 | A | |
| Recombinant Factor VIII | Refacto AF® 1000IU vial 4ml | Wyeth Farma | 3 months at 25°C. After this time, do not re-refrigerate | SmPCs (review year not available) | A | |
| Recombinant Factor VIII | Refacto AF® 250IU vial 4ml | Wyeth Farma | 3 months at 25°C. After this time, do not re-refrigerate | SmPCs (review year not available) | A | |
| Recombinant Factor VIII | Refacto AF® 500IU vial 4ml | Wyeth Farma | 3 months at 25°C. After this time, do not re-refrigerate | SmPCs (review year not available) | A | |
| Factor VIIa | Novoseven® 1.2 | Novo Nordisk Pharma | 24h at 25°C | Silgado et al., 2006,2 Bovaira García et al., 2004,4 Sala et al., 2004,5 Consult lab 18/02/09 | 913349800 | D |
| Factor VIIa | Novoseven® 2.4 | Novo Nordisk Pharma | 24h at 25°C | Silgado et al., 2006,2 Bovaira García et al., 2004,4 Sala et al., 2004,5 Consult lab 18/02/09 | 913349800 | D |
| Factor VIIa | Novoseven® 4.8 | Novo Nordisk Pharma | 24h at 25°C | Silgado et al., 2006,2 Bovaira García et al., 2004,4 Sala et al., 2004,5 Consult lab 18/02/09 | 913349800 | D |
| Phenylephrine | Colircusi fenilefrina® | Alcon Cusi | 1 month (1)15 days (2) | SmPCs (1), Cobos Campos et al., 20063 (2), Silgado et al., 20062 (2) | B | |
| Phentolamine | Regitine® | Novartis Farmaceutica | 7 days at 25°C or 2 days at 30°C | Consult lab 21/07/09 | 933064200 | B |
| Fibrin | Tissucol® 2ml | Baxter | 48h. Do not re-freeze | Cuervas-Mons et al., 2004,6 Bovaira García et al., 2004,4 Sala et al., 20045 | D | |
| Filgrastim | Neupogen® 300MU | Amgen | 7 days, 9–30°C | Cobos Campos et al., 2006,3 Cuervas-Mons et al., 2004,6 Bovaira García et al., 2004,4 Silgado et al., 2006,2 Sala et al., 20045 | B | |
| Filgrastim | Neupogen® 480MU | Amgen | 7 days, 9–30°C | Cobos Campos et al., 2006,3 Cuervas-Mons et al., 2004,6 Bovaira García et al., 2004,4 Silgado et al., 2006,2 Sala et al., 20045 | B | |
| Filgrastim | Neupogen® vials | Amgen | 7 days, 9–30°C | Cuervas-Mons et al., 2004,6 Bovaira García et al., 2004,4 Silgado et al., 2006,2 Sala et al., 20045 | B | |
| Fluorescein+oxybuprocaine | Colircusi fluotest® | Alcon Cusi | 15 days | Cobos Campos et al., 2006,3 Silgado et al., 2006,2 Bovaira García et al., 20044 | B | |
| Folinic acid | Folinato calcico® 10mg/ml | Ferrer Farma | 7–14 days at 25°C | Consult lab 22/12/09 | 936003700 | B |
| Galsulfase | Naglazyme® 5mg vial | Biomarin Europe Ltd | Note batch no., expiry, temperature, exposure time and contact laboratory | Consult lab 22/12/09 | 620988250 | F |
| Antiophidic immunoglobulin | Viperfav® | Sanofi Pasteur MSD | 7 days | Cuervas-Mons et al., 2004,6 Sala et al., 20045 | B | |
| Anti D immunoglobulin 3 | Rhopylac® 300mcg syr 2ml | CSL Behring | 24h at 25°C | Consult lab 25/08/09 | 933671870 | D |
| Specific immunoglobulin | Gamma Anti D® 1500IU | Grifols | 3 months at 25°C, although discarding is recommended | Cobos Campos et al., 20063 | E | |
| Specific immunoglobulin | Gamma Antihepatitis B 200IU/ml | Grifols | 3 months at 25°C, 2 months at 35°C, although discarding is recommended as it is a vaccine | Cobos Campos et al., 20063 | E | |
| Specific immunoglobulin | Gamma Antitetanos® 500IU | Grifols | 12 months at 25°C, 3 months at 35°C, although discarding is recommended as it is a vaccine | Cobos Campos et al., 20063 | E | |
| Specific immunoglobulin | Imogam® 150IU/ml | Sanofi Pasteur MSD | Note batch no., expiry, temperature, exposure time and contact laboratory | Consult lab 21/12/09 | 913717800 | F |
| Non specific human immunoglobulin | Kiovig® 10g | Baxter | 9 months (do not re-refrigerate) | SmPCs (2006) | Specific recommendation | |
| Non specific human immunoglobulin | Kiovig® 2.5g | Baxter | 9 months (do not re-refrigerate) | SmPCs (2006) | Specific recommendation | |
| Non specific human immunoglobulin | Kiovig® 5g | Baxter | 9 months (do not re-refrigerate) | SmPCs (2006) | Specific recommendation | |
| Non specific human immunoglobulin | Vivaglobin® 160 | CSL Behring | 3 months or until expiry date if earlier (do not re-refrigerate) | SmPCs (2008) | Specific recommendation | |
| Gemtuzumab | Mylotarg® 5mg vial | Wyeth Farma | 90 days | Sala et al., 20045 | A | |
| Glatiramer | Copaxone® 20mg prefilled syringe | Sanofi-Aventis | 7 days, 15–25°C | Cobos Campos et al., 2006,3 Cuervas-Mons et al., 2004,6 Bovaira García et al., 2004,4 Silgado et al., 2006,2 Sala et al., 20045 | B | |
| Gonadotrophin | Gonal F® 300IU 22mcg/0.5ml pref. pen | Serono Spain | 3 months | Consult lab 21/12/09 | 917454400 (Merck) | A |
| Gonadotrophin | Gonal F® 450IU 33mcg/0.75ml pref. pen | Serono Spain | 3 months | Consult lab 21/12/09 | 917454400 (Merck) | A |
| Gonadotrophin | Ovitrelle® 250mcg prefilled syringe | Serono Spain | 30 days | Consult lab 21/12/09 | 917454400 | A |
| Gonadotrophin | Puregon® 300IU cassette 0.36ml | Organon Spain | 3 months at <25°C, once open 1 month | Consult lab 05/03/09 | 915673000 | A |
| Gonadotrophin | Puregon® 600IU cassette 0.72ml | Organon Spain | 3 months at <25°C, once open 1 month | Consult lab 05/03/09 | 915673000 | A |
| Gonadotrophin | Puregon® 900IU cassette 1.08ml | Organon Spain | 3 months at <25°C, once open 1 month | Consult lab 05/03/09 | 915673000 | A |
| Hyaluronate | Healon® “5” 23mg/ml prefilled syringe | Panalab | 30 days 25°C | Cobos Campos et al., 20063 | A | |
| Hemin | Normosang® 250mg vial | Orphan Europe | 1 week up to 26°C | Silgado et al., 2006,2 Bovaira García et al., 2004,4 Sala et al., 20045 | B | |
| Infliximab | Remicade® 100 vial | Schering Plough | 12 months | Cobos Campos et al., 20063 | A | |
| C1 esterase inhibitor | Berinert® 500IU vial | Csl Behring | Stable, can be stored at temps up to 25°C | SmPCs (review year not available) | A | |
| Insulin | Insuman infusat® 100U | Sanofi-Aventis | Storage: no information available Once in use: 4 weeks | Consult lab 21/12/09 | 934859400 | Specific recommendation |
| Insulin glargine | Lantus® 100U/ml solostar | Sanofi-Aventis | 4 weeks at <30°C (including usage time) | Bovaira García et al., 20044 | A | |
| Insulin aspart | Novorapid flexpen® 100U/ml pens | Novo Nordisk Pharma | Storage: 24h at 25°C. Once in use: 4 weeks | Bovaira García et al., 20044 | D | |
| Insulin aspart-protamine | Novomix 30 flexpen® 100U/ml pref. pens | Novo Nordisk Pharma | Storage: 24h at 25°C. Once in use: 4 weeks | Bovaira García et al., 20044 | D | |
| Insulin detemir | Levemir® 100U/ml | Novo Nordisk Pharma | Once in use: 6 weeks (do not re-refrigerate once open or if carried as a spare) | SmPCs (review year not available) | Specific recommendation | |
| Insulin glulisine | Apidra® 100U/ml solostar | Sanofi-Aventis | 4 weeks (including administration time) | SmPCs (review year not available) | Specific recommendation | |
| Insulin regular | Actrapid® 100IU/ml vial | Novo Nordisk Pharma | Storage: 24h at 9–25°C Once in use: 6 weeks | Cobos Campos et al., 2006,3 Consult lab 25/08/09 | 913349800 | D |
| Insulin regular | Actrapid innolet® 100IU/ml pref. pen | Novo Nordisk Pharma | Storage: 24h at 9–25°C Once in use: 4 weeks | Cobos Campos et al., 2006,3 Consult lab 25/08/09 | 913349800 | D |
| Insulin protamine | Insulatard® 100IU/ml vial | Novo Nordisk Pharma | Storage: 24h at 9–25°C. Once in use: 4 weeks | Cobos Campos et al., 20063 | D | |
| Insulin protamine | Insulatard flexpen® 100IU/ml pref. pen | Novo Nordisk Pharma | D | |||
| Insulin protamine | Insulatard innolet® 100IU/ml | Novo Nordisk Pharma | D | |||
| Insulin regular-isophane | Humulina® 20:80 pen 100U/ml | Lilly | Storage: 4 days (20°C), 48h (25°C), 24h (30°C), 12h (35°C). Once in use: 28 days (25°C) | Bovaira García et al., 20044 | C | |
| Insulin regular-isophane | Humulina® 30:70 pen 100U/ml | Lilly | Storage: 4 days (20°C), 48h (25°C), 24h (30°C), 12h (35°C). Once in use: 28 days (25°C) | Bovaira García et al., 20044 | C | |
| Insulin regular-protamine | Mixtard 20 innolet® 100IU/ml | Novo Nordisk Pharma | Storage: 24h at 9–25°C. Once in use: 4 weeks | Cobos Campos et al., 20063 | D | |
| Insulin regular-protamine | Mixtard 30 innolet® 100IU/ml | Novo Nordisk Pharma | Storage: 24h at 9–25°C. Once in use: 4 weeks | Cobos Campos et al., 20063 | D | |
| Insulin lispro | Humalog® 100IU/ml vial | Lilly | Storage: 7 days (20°C), 48h (25°C), 24h (30°C), 12h (35°C). Once in use: 28 days (25°C) | Cobos Campos et al., 2006,3 SmPCs (review year not available) | C | |
| Insulin lispro | Humalog humaject® 100U/ml pref. pen | Lilly | Storage: 7 days (20°C), 48h (25°C), 24h (30°C), 12h (35°C). Once in use: 28 days (25°C) | Cobos Campos et al., 2006,3 SmPCs (review year not available) | C | |
| Insulin lispro | Humalog pen® 100U/ml pref. pen | Lilly | Storage: 7 days (20°C), 48h (25°C), 24h (30°C), 12h (35°C). Once in use: 28 days (25°C) | Cobos Campos et al., 2006,3 SmPCs (review year not available) | C | |
| Insulin lispro-protamine | Humalog MIX25 pen® 100IU/ml pref. pen | Lilly | Storage: 7 days (20°C), 48h (25°C), 24h (30°C), 12h (35°C). Once in use: 28 days (25°C) | Cobos Campos et al., 2006,3 SmPCs (review year not available) | C | |
| Insulin zinc | Monotard® 100IU/ml vial | Novo Nordisk Pharma | Storage: 24h at 25°C. Once in use: 6 weeks | Cobos Campos et al., 20063 | D | |
| Interferon 2A | Intron A® 10MIU vial solution | Schering-Plough | 7 days | Cobos Campos et al., 2006,3 Bovaira García et al., 2004,4 Cuervas-Mons et al., 2004,6 Sala et al., 20045 | B | |
| Interferon 2A | Intron A® 18MIU pen | Schering-Plough | 48h at 25°C | Bovaira García et al., 2004,4 Cuervas-Mons et al., 2004,6 Sala et al., 20045 | C | |
| Interferon 2A | Intron A® 60MIU pen | Schering-Plough | 48h at 25°C | Bovaira García et al., 2004,4 Cuervas-Mons et al., 2004,6 Sala et al., 20045 | C | |
| Interferon B-1A | Rebif® 22mcg prefilled syringe | Serono Spain | Storage: 6 days 25°C. Once in use: 1 month | Silgado et al., 2006,2 Cobos Campos et al., 2006,3 Bovaira García et al., 2004,4 Cuervas-Mons et al., 2004,6 Sala et al., 20045 | C | |
| Interferon B-1A | Rebif® 44mcg prefilled syringe | Serono Spain | Storage: 6 days 25°C. Once in use: 1 month | Silgado et al., 2006,2 Cobos Campos et al., 2006,3 Bovaira García et al., 2004,4 Cuervas-Mons et al., 2004,6 Sala et al., 20045 | C | |
| Isoprenaline | Aleudrina® 0.2mg vial | Reig Jofre | 6 months at 25°C (at this temperature, reduce expiry 3 months) | Cobos Campos et al., 2006,3 Silgado et al., 2006,2 Bovaira García et al., 20044 | Specific recommendation | |
| Lactobacilli | Casenfilus® | Casen Fleet | 24h | Silgado et al., 20062 | D | |
| Laronidase | Aldurazyme® 500IU vial | Genzyme | 6 months at 25°C | Consult lab 26/02/09 | 916591670 | A |
| Latanoprost | Xalatan® eye drops | Pfizer | 1 month at 25°C | SmPCs, Bovaira García et al., 20044 | A | |
| Latanoprost+timolol | Xalocom® eye drops | Pfizer | 1 month at 25°C | SmPCs, Bovaira García et al., 20044 | A | |
| Leuprolide | Eligard® | Astellas Pharma | 14 days at room temperature before use | Consult lab 21/12/09 | 914952700 | B |
| Levosimendan | Simdax® 2.5mg/ml | Orion Corporation | Storage time (contact laboratory if exceeded): >25°C: not acceptable15–25°C: 2–3 days8–15°C: 1 week0–2°C: 1 week<0°C: 1 week | Consult lab 21/12/09 | 915030252 | F |
| LHRH | LHRH® | Ferring | 15 days at 25°C | Consult lab 16/02/09 | 917994780 | B |
| Organ preservation fluid | Viaspan® | Bristol Myers | Use not recommended 12–24h after breaking chain | Consult lab 18/02/09 | 914565300 | D |
| Lopinavir+ritonavir | Kaletra® oral solution 60ml | Abbott Laboratories | 42 days | SmPCs, Silgado et al., 2006,2 Cobos Campos et al., 20063 | A | |
| Tetryzoline+medroxyprogesterone+chloramphenicol | Colircusi medrivas® | Alcon Cusi | Storage: 15 days 25°C. Once in use: 1 month | Consult lab 20/2/09, Bovaira García et al., 20044 | 934977000 | B |
| Melphalan | Melfalan® 2mg tablets | Lilly | Note batch no., expiry, temperature, exposure time and contact laboratory | Consult lab 21/12/09 | 916635000 | F |
| Methylergometrine | Methergin® 0.2mg/ml | Novartis Farmaceutica | 2 weeks | Cobos Campos et al., 2006,3 Silgado et al., 20062 | B | |
| Methoxy peg epoetin beta | Mircera® 100mcg/0.3ml prefilled syringe | Roche Farma | 1 month at ≤30°C. Do not re-refrigerate | Consult lab 21/12/09 | 913248100 | Specific recommendation |
| Methoxy peg epoetin beta | Mircera® 150mcg/0.3ml prefilled syringe | Roche Farma | 1 month at ≤30°C. Do not re-refrigerate | Consult lab 21/12/09 | 913248100 | Specific recommendation |
| Methoxy peg epoetin beta | Mircera® 200mcg/0.3ml prefilled syringe | Roche Farma | 1 month at ≤30°C. Do not re-refrigerate | Consult lab 21/12/09 | 913248100 | Specific recommendation |
| Methoxy peg epoetin beta | Mircera® 250mcg/0.3ml prefilled syringe | Roche Farma | 1 month at ≤30°C. Do not re-refrigerate | Consult lab 21/12/09 | 913248100 | Specific recommendation |
| Methoxy peg epoetin beta | Mircera® 50mcg/0.3ml prefilled syringe | Roche Farma | 1 month at ≤30°C. Do not re-refrigerate | Consult lab 21/12/09 | 913248100 | Specific recommendation |
| Methoxy peg epoetin beta | Mircera® 75mcg/0.3ml prefilled syringe | Roche Farma | 1 month at ≤30°C. Do not re-refrigerate | Consult lab 21/12/09 | 913248100 | Specific recommendation |
| Muromonab | Orthodone® OKT3 | CILAG Gmbh | 1 week at 30°C if at least 6 months of expiry period is left. 48h at 25°C if 3 months of expiry period is left | Cuervas-Mons et al., 20046 | C | |
| Natalizumab | Tysabri® 300mg 1 vial perfusion | Elan Farma | No information available | Consult lab 06/03/09 | 935677880 | F |
| Nonacog alfa | Benefix® 500IU/1000IU | Baxter | 1 month at ≤25°C | Cobos Campos et al., 2006,3 Silgado et al., 2006,2 Cuervas-Mons et al., 2004,6 Sala et al., 20045 | A | |
| Octreotide | Sandostatin® 0.05mg/ml | Novartis Farmaceutica | 14 days at <30°C | Cobos Campos et al., 2006,3 Silgado et al., 2006,2 Cuervas-Mons et al., 2004,6 Bovaira García et al., 20044 | B | |
| Octreotide | Sandostatin® 0.1mg/ml | Novartis Farmaceutica | 14 days at <30°C | Silgado et al., 2006,2 Cuervas-Mons et al., 20046 | B | |
| Omalizumab | Xolair® 150mg vial | Novartis Farmaceutica | Note batch no., expiry, temperature, exposure time and contact laboratory | Consult lab 18/02/09 | 933064200 | F |
| Oxytocin | Syntocinón® 10IU/ml | Defiante Farmaceutica Lda | 3 months at <30°C | Cobos Campos et al., 20063 | A | |
| Oxytocin | Syntocinon® 10IU/ml | Defiante Farmaceutica Lda | 3 months at <30°C | Cobos Campos et al., 20063 | A | |
| Palivizumab | Synagis® 100 | Abbott Laboratories | 2 weeks at <25°C | Silgado et al., 2006,2 Sala et al., 20045 | B | |
| Palivizumab | Synagis® 50 | Abbott Laboratories | 2 weeks | Sala et al., 20045 | B | |
| Panitumumab | Vectibix® 100mg vial | Amgen | 24h at 25°C | Consult lab 20/2/09 | 936001900 | D |
| Panitumumab | Vectibix® 400mg vial | Amgen | 24h at 25°C | Consult lab 16/02/09 | 936001900 | D |
| Pegaptanib sodium | Macugen® 0.3 prefilled syringe | Pfizer | Note batch no., expiry, temperature, exposure time and contact laboratory | Consult lab 04/03/09 | 932213366 | F |
| Pegfilgrastim | Neulasta® 6mg prefilled syringe | Amgen | 72h at <30°C | Cobos Campos et al., 2006,3 Cuervas-Mons et al., 2004,6 Bovaira García et al., 2004,4 Sala et al., 20045 | C | |
| Peginterferon alpha 2a | Pegasys® 135mcg prefilled syringe | Roche Farma | 7 days | Cuervas-Mons et al., 2004,6 Bovaira García et al., 20044 | B | |
| Peginterferon alpha 2a | Pegasys® 180mcg prefilled syringe | Roche Farma | 7 days | Cuervas-Mons et al., 2004,6 Cobos Campos et al., 2006,3 Silgado et al., 2006,2 Bovaira García et al., 20044 | B | |
| Peginterferon alpha 2b | Pegintron® 100mcg pref. pen | Schering-Plough | 18 months at <25°C | Cuervas-Mons et al., 2004,6 Bovaira García et al., 2004,4 Sala et al., 20045 | A | |
| Peginterferon alpha 2b | Pegintron® 120mcg pref. pen | Schering-Plough | 18 months at <25°C | Cuervas-Mons et al., 2004,6 Bovaira García et al., 2004,4 Sala et al., 20045 | A | |
| Peginterferon alpha 2b | Pegintron® 150mcg pref. pen | Schering-Plough | 18 months at <5°C | Cuervas-Mons et al., 2004,6 Bovaira García et al., 2004,4 Sala et al., 20045 | A | |
| Peginterferon alpha 2b | Pegintron® 50mcg pref. pen | Schering-Plough | 18 months at <5°C | Cuervas-Mons et al., 2004,6 Bovaira García et al., 2004,4 Sala et al., 20045 | A | |
| Peginterferon alpha 2b | Pegintron® 80mcg pref. pen | Schering-Plough | 18 months at <25°C | Cuervas-Mons et al., 2004,6 Bovaira García et al., 2004,4 Sala et al., 20045 | A | |
| Protamine | Protamina® Rovi 50mg vial | Rovi | 48h at 25°C – 1 week | Cobos Campos et al., 2006,3 Sala et al., 20045 | C | |
| Prothrombin | Prothromplex Inmuno® Tim4 600IU | Baxter | 6 months | Sala et al., 20045 | A | |
| Ranibizumab | Lucentis® 10mg vial | Novartis Farmaceutica | Note batch no., expiry, temperature, exposure time and contact laboratory | Consult lab 16/02/09 | 933064200 | F |
| Rasburicase | Fasturtec® 1.5mg vial | Sanofi-Aventis | 15 days at 25°C | Cobos Campos et al., 2006,3 Silgado et al., 2006,2 Cuervas-Mons et al., 2004,6 Bovaira García et al., 2004,4 Sala et al., 20045 | B | |
| Risperidone | Risperdal® 25mg | Janssen-Cilag | 7 days | Silgado et al., 2006,2 Cuervas-Mons et al., 2004,6 Sala et al., 20045 | B | |
| Risperidone | Risperdal® 37.5mg | Janssen-Cilag | 7 days | Silgado et al., 2006,2 Cuervas-Mons et al., 2004,6 Sala et al., 20045 | B | |
| Risperidone | Risperdal® 50mg | Janssen-Cilag | 7 days | Silgado et al., 2006,2 Cuervas-Mons et al., 2004,6 Sala et al., 20045 | B | |
| Ritonavir | Norvir® 100mg soft capsules | Abbott Laboratories | 1 month at <25°C | Cobos Campos et al., 2006,3 Silgado et al., 2006,2 Cuervas-Mons et al., 2004,6 Sala et al., 2004,5 Bovaira García et al., 20044 | A | |
| Rituximab | Mabthera® 100mg vial | Roche Farma | 18 days at <30°C | Cobos Campos et al., 2006,3 Silgado et al., 2006,2 Cuervas-Mons et al., 2004,6 Bovaira García et al., 20044 | B | |
| Rituximab | Mabthera® 500mg vial | Roche Farma | 18 days at <30°C | Cobos Campos et al., 2006,3 Silgado et al., 2006,2 Cuervas-Mons et al., 2004,6 Bovaira García et al., 20044 | B | |
| Rocuronium | Esmeron® 100mg vial | Organon Spain | 12 weeks at <30°C | Cobos Campos et al., 2006,3 Silgado et al., 2006,2 Cuervas-Mons et al., 2004,6 Sala et al., 2004,5 Bovaira García et al., 20044 | A | |
| Rotigotine | Neupro® patches | Ucb Pharma | 7 days at 25°C | Consult lab 25/08/09 | 915703444 | B |
| Measles-Mumps-Rubella | Vacuna® MSD triple | Sanofi Pasteur MSD | 7 days at 25°C | Cobos Campos et al., 20063 | B | |
| Secretin | Secrelux® | Sanochemia | 3 weeks at 25°C | Bovaira García et al., 2004,4 García Vázquez et al., 19977 | B | |
| Sirolimus | Rapamune® 1mg tablets | Wyeth Farma | 24h at 25°C | Silgado et al., 2006,2 Cuervas-Mons et al., 2004,6 Sala et al., 2004,5 Bovaira García et al., 20044 | D | |
| Somatrophin | Zomacton® 4mg | Ferring | 24–48h | Consult lab 16/02/09 | 917994780 | D |
| Somatrophin | Genotonorm kabipen® 12mg vial | Pfizer | 6 months at 25°C (including use). Do not re-refrigerate | Cuervas-Mons et al., 2004,6 Bovaira García et al., 20044 | Specific recommendation | |
| Somatrophin | Genotonorm miniquick® 0.2mg vial | Pfizer | 6 months at 25°C (including use). Do not re-refrigerate | Cuervas-Mons et al., 2004,6 Bovaira García et al., 20044 | Specific recommendation | |
| Somatrophin | Genotonorm miniquick® 0.4mg vial | Pfizer | 6 months at 25°C (including use). Do not re-refrigerate | Cuervas-Mons et al., 2004,6 Bovaira García et al., 20044 | Specific recommendation | |
| Somatrophin | Genotonorm miniquick® 0.6mg vial | Pfizer | 6 months at 25°C (including use). Do not re-refrigerate | Cuervas-Mons et al., 2004,6 Bovaira García et al., 20044 | Specific recommendation | |
| Somatrophin | Genotonorm miniquick® 0.8mg vial | Pfizer | 6 months at 25°C (including use). Do not re-refrigerate | Cuervas-Mons et al., 2004,6 Bovaira García et al., 20044 | Specific recommendation | |
| Somatrophin | Genotonorm miniquick® 1mg vial | Pfizer | 6 months at 25°C (including use). Do not re-refrigerate | Cuervas-Mons et al., 2004,6 Bovaira García et al., 20044 | Specific recommendation | |
| Somatrophin | Genotonorm miniquick® 1.2mg vial | Pfizer | 6 months at 25°C (including use). Do not re-refrigerate | Cuervas-Mons et al., 2004,6 Bovaira García et al., 20044 | Specific recommendation | |
| Somatrophin | Genotonorm miniquick® 1.4mg vial | Pfizer | 6 months at 25°C (including use). Do not re-refrigerate | Cuervas-Mons et al., 2004,6 Bovaira García et al., 20044 | Specific recommendation | |
| Somatrophin | Genotonorm miniquick® 1.6mg vial | Pfizer | 6 months at 25°C (including use). Do not re-refrigerate | Cuervas-Mons et al., 2004,6 Bovaira García et al., 20044 | Specific recommendation | |
| Somatrophin | Genotonorm miniquick® 1.8mg vial | Pfizer | 6 months at 25°C (including use). Do not re-refrigerate | Cuervas-Mons et al., 2004,6 Bovaira García et al., 20044 | Specific recommendation | |
| Somatrophin | Genotonorm miniquick® 2mg vial | Pfizer | 6 months at 25°C (including use). Do not re-refrigerate | Cuervas-Mons et al., 2004,6 Bovaira García et al., 20044 | Specific recommendation | |
| Somatrophin | Humatrope® 12mg cassette | Lilly | 6 days at 8–25°C, 72h at 25–30°C, 24h at 30–40°C | Bovaira García et al., 20044 | C | |
| Somatrophin | Humatrope® 24mg cassette | Lilly | 6 days at 8–25°C, 72h at 25–30°C, 24h at 30–40°C | Bovaira García et al., 20044 | C | |
| Somatrophin | Humatrope® 6mg cassette | Lilly | 6 days at 8–25°C, 72h at 25–30°C, 24h at 30–40°C | Bovaira García et al., 20044 | C | |
| Somatrophin | Norditropin® simplexx 10mg | Novo Nordisk Pharma | 24h at <25°C | Consult lab 05/03/09, Bovaira García et al., 20044 | 913349800 | D |
| Somatrophin | Norditropin® simplexx 15mg | Novo Nordisk Pharma | 24h at <25°C | Consult lab 05/03/09, Bovaira García et al., 20044 | 913349800 | D |
| Somatrophin | Norditropin® simplexx 5mg | Novo Nordisk Pharma | 24h at <25°C | Consult lab 05/03/09, Bovaira García et al., 20044 | 913349800 | D |
| Somatrophin | Nutropin® AQ 10mg (30IU) cassette | Ipsen Farma | Discard (no information available) | Consult lab 21/12/09 | 936858100 | E |
| Somatrophin | Omnitrope® 3.3mg/ml vial | Sandoz Farmaceutica | 1 day at 25°C and 15 days at 15°C, 2 weeks at 0°C or between −2°C and −5°C | Consult lab 23/3/09 | 917401280 | D |
| Pulmonary surfactant | Curosurf® 120 | Chiesi Spain | 24h at <25°C | Cobos Campos et al., 2006,3 Silgado et al., 2006,2 Bovaira García et al., 2004,4 Consult lab 17/02/09 | 934948000 | D |
| Pulmonary surfactant | Curosurf® 240 | Chiesi Spain | 24h at <25°C | Cobos Campos et al., 2006,3 Silgado et al., 2006,2 Bovaira García et al., 2004,4 Consult lab 17/02/09 | 934948000 | D |
| Suxamethonium | Anectine® 100mg vial | Glaxo SmithKline | 2 weeks at 25°C | Cobos Campos et al., 2006,3 Silgado et al., 2006,2 Bovaira García et al., 2004,4 Cuervas-Mons et al., 2004,6 García et al., 20044 | B | |
| Temsirolimus | Torisel® 25mg vial | Wyeth Farma | 24h at 25°C; note batch no., expiry, temperature, exposure time and contact laboratory. (cadena_de_frio@wyeth.com) | Consult lab 20/2/09 | 913346400 | F |
| Tetracosactide | Nuvacthen Depot® | Defiante Farmaceutica | 1–3 months at <25°C | García Vázquez et al., 1997,7 Sala et al., 20045 | A | |
| Tetracosactide | Synacthen® | Novartis Farmaceutica | 3–4h at <25°C | Silgado et al., 2006,2 Cobos Campos et al., 2006,3 Cuervas-Mons et al., 20046 | D | |
| Thymoglobulin | Timoglobulina® 25mg vial | Genzyme | 24h at <37°C | Silgado et al., 20062 | D | |
| Thiotepa | Tioplex® 15mg vial | Addiene | 3 months at 25°C (Oncothiotepa) | Silgado et al., 20062 | A | |
| Tipranavir | Aptivus® 250mg soft capsules | Boehringer Ingelheim Spain | Storage: 60 days, 15–30°C. Once in use: 60 days at <25°C | Consult lab 21/12/09 | 934045100 | A |
| Thyrotropin alpha | Thyrogen® 0.9mg vial | Genzyme | 1–2 years | Cuervas-Mons et al., 2004,6 Cobos Campos et al., 2006,3 Sala et al., 2004,5 Silgado et al., 2006,2 Bovaira García et al., 20044 | A | |
| Tobramycin | Tobi® 300mg 56 amp. 5ml | Chiron Iberia | 1 month at 25°C | Cuervas-Mons et al., 2004,6 Silgado et al., 2006,2 Bovaira García et al., 2004,4 Sala et al., 20045 | A | |
| Topotecan | Hycamtin® 0.25mg capsules | Glaxo SmithKline | Note batch no., expiry, temperature, exposure time and contact laboratory | Consult lab 20/02/09 | 918070301 | F |
| Topotecan | Hycamtin® 1mg capsules | Glaxo SmithKline | Note batch no., expiry, temperature, exposure time and contact laboratory | Consult lab 20/02/09 | 918070301 | F |
| Toxin A, Cl. botulinum | Dysport® 500IU vial | Ipsen Pharma | 3 days | Silgado et al., 2006,2 Cuervas-Mons et al., 2004,6 Arrixaca, Sala et al., 20045 | C | |
| Botulinum toxin | Botox® 100U | Allergan Sau | 14 days at 25°C; 7 days at 30°C | Cuervas-Mons et al., 2004,6 Bovaira García et al., 20044 | B | |
| Botulinum toxin | Botulismus-antitoxin Behring® | Esteve | 1 week at 37°C | Bovaira García et al., 20044 | B | |
| Trabectedin | Yondelis® 0.25mg vial | Pharmamar | 72h at 25°C | Consult lab 25/10/07 | 918466000 | C |
| Trabectedin | Yondelis® 1mg vial | Pharmamar | 72h at 25°C | Consult lab 25/10/07 | 918466000 | C |
| Trastuzumab | Herceptin® 150mg vial | Roche Farma | 30 days at 40°C, 3 days at 50°C | Cobos Campos et al., 2006,3 Silgado et al., 2006,2 Cuervas-Mons et al., 2004,6 Arrixaca | A | |
| Trientine hydrochloride | Syprine® | Merck Sharp Dohme | 7 days | García Vázquez et al., 19977 | B | |
| Tuberculin | Tuberculina® PPD | Ucb Pharma | 6 months at 25°C, 2 weeks at 35°C | Cobos Campos et al., 2006,3 Silgado et al., 20062 | A | |
| Meningococcal C vaccine | Meningitec® prefilled syringe | Wyeth Farma | 24h at <25°C | Cobos Campos et al., 2006,3 Silgado et al., 2006,2 Bovaira García et al., 20044 | D | |
| Diphtheria, tetanus, pertussis vaccine | Boostrix® 0.5ml syringe | Glaxo SmithKline | Note batch no., expiry, temperature, exposure time and contact laboratory | Consult lab 25/08/09 | 918070467 | F |
| DTP and hepatitis B vaccine | Tritanrix® | Glaxo SmithKline | 2 weeks at 21°C; 1 week at 37°C. Although, as it is a vaccine, it is recommended to be discarded | Bovaira García et al., 20044 | E | |
| DtaP vaccine | Infanrix® | Glaxo SmithKline | 2 weeks at 21°C; 1 week at 37°C. Although, as it is a vaccine, it is recommended to be discarded | Cobos Campos et al., 2006,3 Silgado et al., 2006,2 Bovaira García et al., 20044 | E | |
| Haemophilus influenzae b vaccine | Hiberix® | Glaxo SmithKline | 2 weeks at 21°C; 1 week at 37°C. Although, as it is a vaccine, it is recommended to be discarded | Silgado et al., 20062, Cuervas-Mons et al., 2004,6 Bovaira García et al., 20044 | E | |
| Hepatitis B vaccine | Twinrix® adult prefilled syringe | Glaxo SmithKline | Note batch no., expiry, temperature, exposure time and contact laboratory | Consult lab 25/08/09 | 918070467 | F |
| Pneumococcal conjugate vaccine | Prevenar® prefilled syringe | Wyeth Farma | 24h at ≤25°C. Although, as it is a vaccine, it is recommended to be discarded | Silgado et al., 2006,2 Cuervas-Mons et al., 2004,6 Bovaira García et al., 20044 | E | |
| Rabies vaccine | Rabipur® | Novartis Farmaceutica | 1 year at 25°C; 3 months at >37°C; 1 month at >55°C. Although, as it is a vaccine, it is recommended to be discarded | Consult lab 01/04/06 | 933064200 | E |
| Varicella vaccine | Varilrix® | Glaxo SmithKline | 1 week at 21°C. Although, as it is a vaccine, it is recommended to be discarded | Bovaria García et al., 2004,4 Silgado et al., 20062 | E | |
| Hepatitis A vaccine | Havrix® 1440 | Glaxo SmithKline | 2 weeks at 21°C; 1 week at 37°C. Although, as it is a vaccine, it is recommended to be discarded | Cuervas-Mons et al., 2004,6 Bovaira García et al., 20044 | E | |
| Hepatitis A vaccine | Havrix® 720 | Glaxo SmithKline | 2 weeks at 21°C; 1 week at 37°C. Although, as it is a vaccine, it is recommended to be discarded | Silgado et al., 2006,2 Cuervas-Mons et al., 2004,6 Bovaira García et al., 20044 | E | |
| Meningococcal C vaccine | Menjugate® | Esteve | 6 months at 30°C, 1 week at 40°C (without reducing expiry period). Although, as it is a vaccine, it is recommended to be discarded | Cobos Campos et al., 2006,3 Bovaira García et al., 20044 | A | |
| Hepatitis B vaccine | Engerix-B® 10mcg | Glaxo SmithKline | 2 weeks 21°C, 1 week 37°C | Cobos Campos et al., 20063 | B | |
| Hepatitis B vaccine | Engerix-B® 20mcg | Glaxo SmithKline | 3 weeks 21°C, 1 week 37°C | Cobos Campos et al., 20063 | B | |
| Tetanus and diphtheria vaccine | Diftavax® 40/4IU adult | Sanofi Pasteur Msd | 14 days at 25°C | Silgado et al., 20062 | B | |
| Tetanus and diphtheria vaccine | Ditanrix® 20/2IU adult | Glaxo Smithk | 2 weeks at 21°C, 1 week at 37°C | Cobos Campos et al., 20063 | B | |
| Inactivated polio vaccine | Vacuna Poliomelitica Berna® | Berna Biotech Spain | Temperature ≤25°C: <24h: reduce expiry period by 2 months >24h and <72 h: reduce expiry period by 4 months>72h and <120h: reduce expiry period by 6 months>120h: discard | Cobos Campos et al., 20063 | D | |
| Vinblastine | Vinblastina® 10mg vial | Stada | 21 days at 15°C and 14 days at 25°C | Consult lab 20/2/09 | 934738889 | B |
| Vincristine | Vincristina® 2mg vial | Pfizer | 24h at 25°C | Cobos Campos et al., 2006,3 Cuervas-Mons et al., 20046 | D | |
| Vinorelbine | Navelbine® 20mg capsules | Pierre Fabre Iberica | 1 month at <25°C, 15 days at <30°C, can be frozen | Consult lab 20/2/09, Silgado et al., 2006,2 Bovaira García et al., 20044 | 934833000 | A |
| Vinorelbine | Navelbine® 30mg capsules | Pierre Fabre Iberica | 1 month at <25°C, 15 days at <30°C, can be frozen | Consult lab 20/2/09, Silgado et al., 2006,2 Bovaira García et al., 20044 | 934833000 | A |
| Vinorelbine | Navelbine® 50mg vial | Pierre Fabre Iberica | 1 month at <25°C, 15 days at <30°C, <48h at >30°C or exposed to the light. Can be frozen | Cuervas-Mons et al., 2004,6 Silgado et al., 20062 | A | |
| Voriconazole | Vfend® 40mg/ml oral suspension | Pfizer | 30 days at 25°C (temperature excursions are cumulative, so contact the laboratory for an individual evaluation) | Consult lab 21/12/09 | 914909900 | A |
| Hyaluronic acid viscoelastic | Provisc® | Alcon Cusi | 12h at 25°C | Consult lab 26/08/09 | 934977000 | D |
The number of drugs in each classification was as follows: (A) 65, (B) 47, (C) 30, (D) 47, (E) 12, and (F) 22. There were also specific recommendations for each category, as follows, provided that the time and temperature limits stipulated for each category were not exceeded:
- •
A, B, and C: Establish a control and monitoring system by labelling (Fig. 1).
- •
D: Continue in use if it was a one-off break in the cold chain and did not exceed the established maximum time. Otherwise, dispose of or return the drug to the laboratory.
- •
E: Dispose of or return the drug to the laboratory.
There were 31 drugs that could not be included in any category, so it is recommended to check the specific information regarding room temperature stability in Table 2.
The information obtained is available on the hospital intranet, in the “manuales de procedimiento” section of the pharmacotherapy portal. There is also a version on the Internet, in the free access pharmacy department portal (see URL at the end of the article).11
Discussion and ConclusionsThe methodology used to conduct this review was similar to that used in the aforementioned studies.2–10 However, there are differences in the information given by certain pharmaceutical laboratories. This may be because the drug companies involved have carried out further stability studies during the time between the completions of both reviews.
One problem found was the differences in stability data for the same active ingredient, depending on the laboratory that undertook the study. We agree with Cobos Campos et al.3 who suggested that laboratories should conduct their stability studies under the same temperature conditions to standardise them and facilitate comparison. However, it is important to consult the information available at each centre for the drug product in question.
It was deemed necessary to have stability data for all drugs to be incorporated in the hospital guide upon inclusion, and for the laboratory to provide degradation tables, as a query made when the chain is broken is urgent, and the laboratory cannot guarantee a drug outside its recommended conditions.
When returning drugs to the laboratories, an agreement can be reached with the manufacturing laboratory, especially when the drug cost is high. The manufacturing cost is often significantly less than the most expensive selling price, and in our experience the contribution of the industry in these cases has been very adequate.
One limitation of this review is that the majority of the data found do not specify the possibility of re-refrigerating after breaking the cold chain, or whether these data are valid if there is a second break in the cold chain. For such a case, the manufacturing laboratory should be consulted.
Another limitation is that action has been formalised only for exceeding the maximum proper conservation temperature, but not for freezing.
Lastly, it is important to note that the information contained in this paper is intended as a reference tool for rapid action in those special situations when the cold chain is broken and it is necessary to know whether a drug may be used or not. If in doubt, we recommend contacting the manufacturing laboratory specifying the concrete conditions to which the product has been subjected. Due to the importance of establishing the period of time the drugs have been out of the recommended temperature range and the temperature reached, this information should not be given to other hospital departments, so as to prevent any bad conservation practice. However, it was considered appropriate to have access to it in the “manuales de procedimiento” section of the pharmacotherapy department portal and to inform other pharmacy departments to facilitate action in the case of a break in the cold chain.To summarise, one of the priority functions of a pharmacist is to ensure the correct storage of medicines.12 This requires the availability of updated information on the validity times of thermolabile drugs when the cold chain is broken. It is the responsibility of the pharmacist to establish guidelines for action when incidents occur. This information must be available and updated in the hospital pharmacy department to ensure that appropriate measures are taken.
Conflicts of InterestThe authors affirm they have no conflict of interest.
Please cite this article as: Periáñez Parraga L, et al. Medicamentos termolábiles. Protocolo de actuación en la rotura de la cadena de frío. Farm Hosp. 2011;35(4):190.e1–190.e28.