To evaluate the utility of a post-discharge pharmaceutical care programme.
MethodThree-month prospective study where patients were randomised into two groups according to whether or not they received verbal and written information about their treatment at hospital discharge. Treatment compliance was assessed by the Morisky–Green test at discharge and at 30–50days via a telephone interview, also collecting information on patient medication.
ResultsA total of 59 patients were included, 30 in the control group and 29 in the experimental group. 42.1±9.6days had elapsed between discharge and the telephone interview. While a higher percentage of patients were adherent to treatment at discharge in the control group (83.3 versus 62.1%, OR=0.33, 95% CI: 0.1–1.1, P=.07), in the telephone interview the percentage in the experimental group was greater (62.5 versus 88.5%, OR=4.6, 95% CI: 1.1–19.8, P=.03). The differences between the two groups for the rest of the variables (deaths, visits to emergency department and hospital readmissions) were not statistically significant. In the telephone interview, 70% of patients’ treatment was changed in some way since hospital discharge.
ConclusionA post-discharge pharmaceutical care programme is a tool to improve treatment compliance, which needs continuity due to the large number of treatment changes suffered by these patients.
Conocer la utilidad de un programa de información farmacoterapéutica al alta hospitalaria.
MétodoEstudio prospectivo de 3 meses de duración en el que se aleatorizó a los pacientes en 2 grupos según recibieran o no información verbal y escrita sobre su tratamiento al alta hospitalaria. La adherencia se evaluó mediante el test de Morinsky Green, tanto en el momento del alta, como transcurridos 30–50 días mediante entrevista telefónica. Además se recogió la información sobre la medicación del paciente en ese momento y los cambios respecto a la medicación al alta.
ResultadosSe incluyeron un total de 59 pacientes, 30 en el grupo control y 29 en el experimental. Entre el alta y la entrevista trascurrieron 42,1±9,6 días. Mientras que en el momento del alta el porcentaje de pacientes adherentes era mayor en el grupo control (83,3 frente a 62,1%, OR=0,33, IC 95%: 0,1 a 1,1, p=0,07), en la entrevista telefónica fue mayor en el grupo experimental (62,5 frente a 88,5%, OR=4,6, IC 95%: 1,1 a 19,8, p=0,03). Las diferencias entre ambos grupos en el resto de las variables (fallecimientos, visitas a urgencias y reingresos hospitalarios) no fueron estadísticamente significativas. En la entrevista telefónica un 70% de los pacientes sufrió algún tipo de cambio respecto al tratamiento al alta hospitalaria.
ConclusionesLa información al alta por parte del farmacéutico es una herramienta para mejorar la adherencia al tratamiento de los pacientes que debe tener una continuidad debido al elevado número de cambios de tratamiento que sufren estos pacientes.
Long-term adherence to treatment in chronic diseases falls around 50% in developed countries, with even lower rates in developing countries.1 Poor adherence to treatment is related to worsening condition of the disease, increased mortality, and increased health costs. In the United States, 33%–69% of medication-related hospitalisations are due to poor adherence to treatment, with an associated cost of 100 billion dollars per year.2 The repercussions of deficient adherence to treatment grow as the burden of chronic disease increases worldwide.1
The patient's type of disease, the type of treatment used, the health care team, and patient characteristics are all factors that influence adherence to treatment. Among these factors, the complexity of treatment is a decisive predictor. As more drugs are added to the treatment plan, with different dosages and methods of administration, patients are more likely to become confused or forgetful about taking their medications.3
In addition to these factors, polymedicated patients are generally older than 65 years, with greater morbidity and a higher probability of developing adverse drug reactions (ADRs), pharmacological interactions, and becoming forgetful or confused. Elderly people make up approximately 17% of the population, yet represent 70% of pharmaceutical costs.4 The causes that have been related to non-adherence with treatment in elderly patients are the number of doctors prescribing medication, polymedication, complexity of treatment regimens, depression, and cognitive deterioration.5
Given the multifactorial nature of the issue of adherence to treatment and the impact that this has on the health system, we need education programmes specifically designed to improve compliance with treatment. Among the measures taken, one of the strategies implemented in hospitals to improve patient adherence to treatment is the pharmacotherapy information given by the pharmacist to patients when discharged from the hospital.
The objective of our study was to evaluate the usefulness of a pharmacotherapeutic information programme upon discharge of polymedicated patients and to profile the modifications to patient treatments 30–50 days after being discharged from the hospital.
Materials and MethodsStudy ScopeWe performed our study at the Unidad de Pacientes con Pluripatología y Atención Médica Integral (pluripathology and integrated health care unit) in the Servicio de Medicina Interna (internal medicine department) at the Hospital Universitario 12 de Octubre. This unit is orientated towards the treatment of pluripathological patients (polymedicated patients with a high frequency of medical visits and hospitalisations) with a mean age of 60–80 years, in coordination with Atención Primaria (primary care department).
Patient SelectionThe inclusion criteria used for our study were existing treatment previous to hospitalisation of at least 3 months duration, and 4 or more active ingredients prescribed at discharge from the hospital. The exclusion criteria used were transfer from an assisted living residence, dementia, and/or incapacitating psychiatric disease in the absence of a responsible caregiver during interviews, and a baseline Barthel index value below 20.
Instruments of Measure, Study Material, and Sources of InformationWe used the Morisky–Green test for evaluating adherence to treatment. This test is an indirect method of measuring adherence (validated Spanish version),6 consisting of 4 closed questions, with a wrong answer to any question indicating a non-compliant patient (Table 1).7
Morisky–Green Test.
| Have you ever forgotten to take your medicine? yes or no |
| Are you sometimes neglectful in regard to your medicine hours? yes or no |
| Do you skip you medicine hours when you are feeling well? yes or no |
| When you feel badly due to the medicine, do you skip it? yes or no |
| In order to be considered good adherence to treatment, the responses for all four questions must be correct (no, yes, no, no) |
The patient's condition was evaluated using the Barthel index, which is a scale that measures the capacity of a person to perform 10 basic activities in daily life, producing a quantitative estimate ranging from 0 to 100 from lower to higher independence.8
Pharmacotherapeutic information was provided both verbally and in written form to each patient upon discharge following the model of the Infowin® programme. We used structured interviews of the patient upon admission to the hospital, at discharge, and over the telephone, compiling information using a data entry form and storing it in an Access® database. We also used patient clinical histories and the pharmacotherapeutic history recorded by the pharmacy department.
Study DesignWe performed a controlled, randomised clinical trial with patients selected during a 3-month period (February–April 2008) during hospitalisation, based on the inclusion/exclusion criteria for the study.
Once patients were included in the study, they were first interviewed for information on their baseline characteristics and previous treatment received.
Upon discharge, patients were randomly distributed into 2 groups using a block method, in a proportion of 1:1. All patients were interviewed at discharge for information regarding treatment and adherence to treatment using the Morisky–Green test. Only patients in the experimental group received verbal and written pharmacotherapeutic information.
Some 30–50 days after discharge from the hospital, patient adherence to treatment was quantified through a telephone interview using the Morisky–Green test, and we also collected information regarding the medications taken by the patient at that point, changes from previous treatments, mortality rates, visits to specialist doctors, visits to primary care doctors, visits to the emergency room, and hospital readmissions.
Doctors in the Unidad de Pacientes con Pluripatología y Atención Médica Integral selected patients and collected medical backgrounds. The pharmacist performed interviews at patient discharge and over the telephone. The telephone interviewer was not aware of the patient's treatment group or previous adherence to treatment before discharge.
Study Variables and Statistical MethodThe primary study variable was the difference in adherence to treatment between the moment of discharge from the hospital and the telephone interview using the Morisky–Green test. Secondary variables were death rate, visits to the emergency room and readmissions to the hospital, the percentage of patients with modifications to treatment, and the causes for modifications. A modification was considered to be any drug suspension or addition that would imply different active ingredients, ignoring adjustments to dosages. We also ignored treatments prescribed for less than 7 days after discharge from the hospital when evaluating the percentage of active ingredients suspended.
We performed statistical analyses on the data using SPSS® software version 17.0®. Qualitative variables were described as an absolute frequency and relative percentage. Quantitative variables were expressed as mean and corresponding ranges or 95% confidence intervals (95% CI), or using medians and interquartile ranges (IQR). We analysed the results using chi-square tests (alpha=0.05) for categorical variables, and Student's t-tests with a significance level of P<.05 for continuous variables. All statistical analyses were performed with the intent to treat.
We also performed a multivariate analysis using a logistic regression model, with adherence to treatment at the telephone interview used as the dependent variable. We also considered those variables with a P-value<.2 in the bivariate analysis as possible confounding variables, or when theoretical justification existed. Interaction terms were included in the model using criteria for statistical significance (P<.05). Confounding variables were considered when changes >10% were produced in the adjusted OR. The hierarchical principle was maintained in all cases.
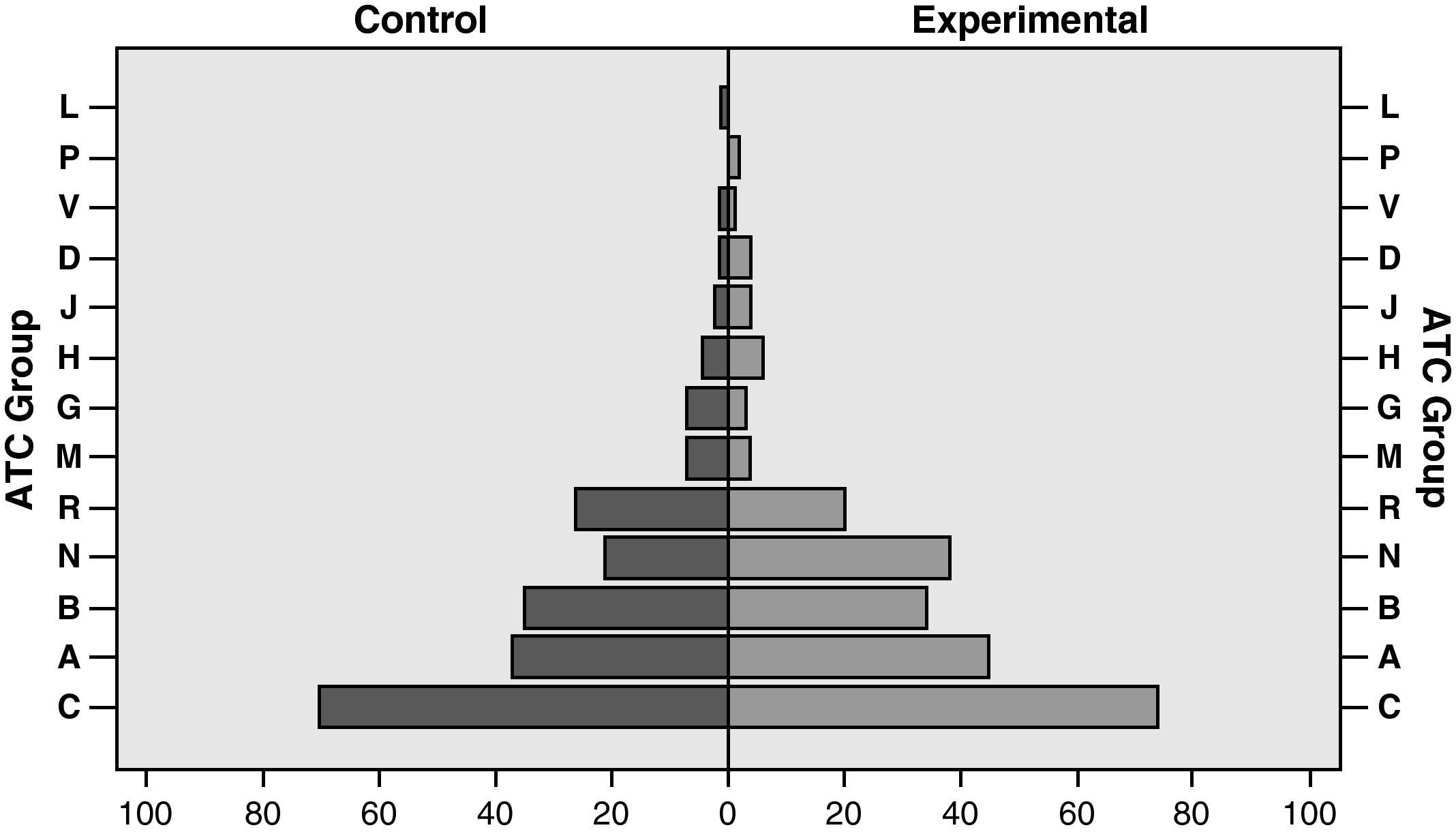
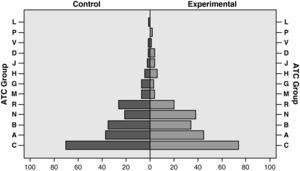
ResultsDuring the study period, 88 patients were admitted under the attending pharmacist. Fifty-nine (67%) of them complied with inclusion criteria, with 30 patients (50.8%) assigned to the control group and 29 (49.2%) to the experimental group. Baseline characteristics of the study population are summarised in Table 2, with no significant differences between the two groups. Nor were significant differences observed in the distribution of active ingredients at discharge from the hospital according to ATC classification (Fig. 1), the mean number of active ingredients, pharmaceutical forms, and number of daily doses between study groups between discharge from the hospital and the telephone interview (Table 2).
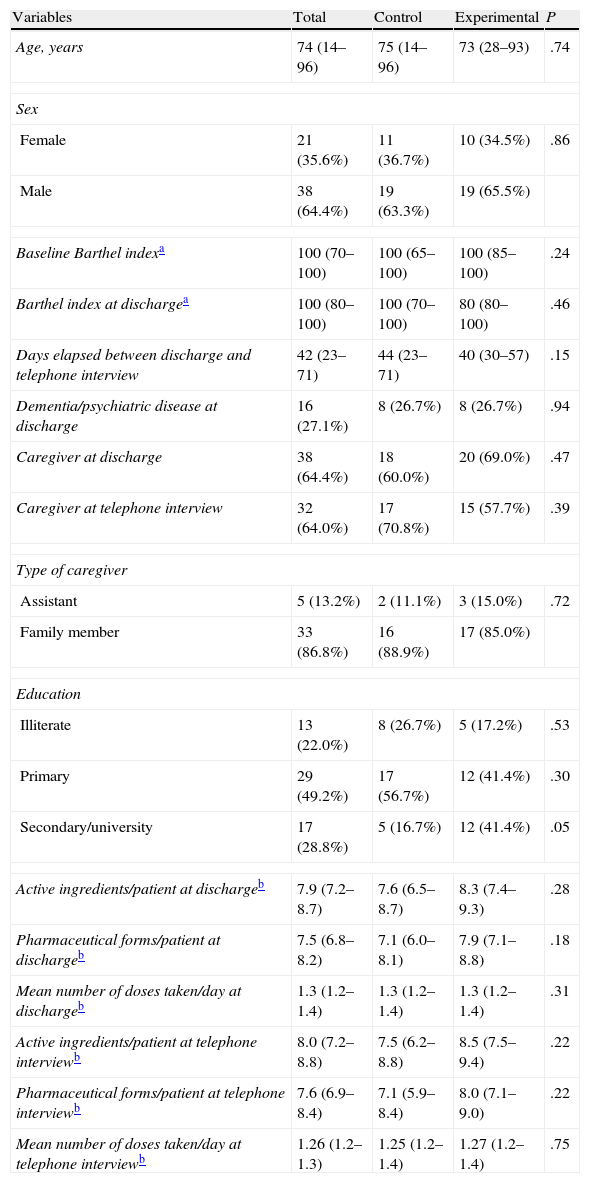
Baseline Characteristics of the Study Patients.
| Variables | Total | Control | Experimental | P |
| Age, years | 74 (14–96) | 75 (14–96) | 73 (28–93) | .74 |
| Sex | ||||
| Female | 21 (35.6%) | 11 (36.7%) | 10 (34.5%) | .86 |
| Male | 38 (64.4%) | 19 (63.3%) | 19 (65.5%) | |
| Baseline Barthel indexa | 100 (70–100) | 100 (65–100) | 100 (85–100) | .24 |
| Barthel index at dischargea | 100 (80–100) | 100 (70–100) | 80 (80–100) | .46 |
| Days elapsed between discharge and telephone interview | 42 (23–71) | 44 (23–71) | 40 (30–57) | .15 |
| Dementia/psychiatric disease at discharge | 16 (27.1%) | 8 (26.7%) | 8 (26.7%) | .94 |
| Caregiver at discharge | 38 (64.4%) | 18 (60.0%) | 20 (69.0%) | .47 |
| Caregiver at telephone interview | 32 (64.0%) | 17 (70.8%) | 15 (57.7%) | .39 |
| Type of caregiver | ||||
| Assistant | 5 (13.2%) | 2 (11.1%) | 3 (15.0%) | .72 |
| Family member | 33 (86.8%) | 16 (88.9%) | 17 (85.0%) | |
| Education | ||||
| Illiterate | 13 (22.0%) | 8 (26.7%) | 5 (17.2%) | .53 |
| Primary | 29 (49.2%) | 17 (56.7%) | 12 (41.4%) | .30 |
| Secondary/university | 17 (28.8%) | 5 (16.7%) | 12 (41.4%) | .05 |
| Active ingredients/patient at dischargeb | 7.9 (7.2–8.7) | 7.6 (6.5–8.7) | 8.3 (7.4–9.3) | .28 |
| Pharmaceutical forms/patient at dischargeb | 7.5 (6.8–8.2) | 7.1 (6.0–8.1) | 7.9 (7.1–8.8) | .18 |
| Mean number of doses taken/day at dischargeb | 1.3 (1.2–1.4) | 1.3 (1.2–1.4) | 1.3 (1.2–1.4) | .31 |
| Active ingredients/patient at telephone interviewb | 8.0 (7.2–8.8) | 7.5 (6.2–8.8) | 8.5 (7.5–9.4) | .22 |
| Pharmaceutical forms/patient at telephone interviewb | 7.6 (6.9–8.4) | 7.1 (5.9–8.4) | 8.0 (7.1–9.0) | .22 |
| Mean number of doses taken/day at telephone interviewb | 1.26 (1.2–1.3) | 1.25 (1.2–1.4) | 1.27 (1.2–1.4) | .75 |
The mean period between discharge and the telephone interview was 44 days (95% CI: 23–71 days) in the control group and 40 days (95% CI: 30–57 days) in the experimental group (P=.15). Telephone interviews were held with a total of 50 patients: 24 (80.0%) of them were in the control group and 26 (89.7%) in the experimental group (P=.03). Nine patients did not complete the telephone interview, 3 died, 5 were hospitalised at the time of the interview, and one was lost due to admission to an assisted-living residence.
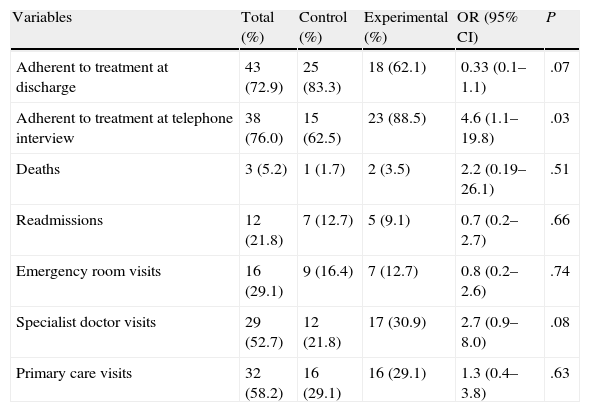
The percentage of patients adherent to treatment plans at discharge was 83.3% in the control group and 62.1% in the experimental group (OR=0.33, 95% CI: 0.1%–1.1%, P=.07), according to the Morisky–Green test. At the time of the telephone interview, this percentage changed to 62.5% in the control group and 88.5% in the experimental group (OR=4.6, 95% CI: 1.1%–19.8%, P=.03). The differences between the two groups at the moment of the telephone interview for the rest of the variables recorded (deaths, emergency room visits, medical visits, and hospital readmissions) were not statistically significant (Table 3).
Primary Study Results.
| Variables | Total (%) | Control (%) | Experimental (%) | OR (95% CI) | P |
| Adherent to treatment at discharge | 43 (72.9) | 25 (83.3) | 18 (62.1) | 0.33 (0.1–1.1) | .07 |
| Adherent to treatment at telephone interview | 38 (76.0) | 15 (62.5) | 23 (88.5) | 4.6 (1.1–19.8) | .03 |
| Deaths | 3 (5.2) | 1 (1.7) | 2 (3.5) | 2.2 (0.19–26.1) | .51 |
| Readmissions | 12 (21.8) | 7 (12.7) | 5 (9.1) | 0.7 (0.2–2.7) | .66 |
| Emergency room visits | 16 (29.1) | 9 (16.4) | 7 (12.7) | 0.8 (0.2–2.6) | .74 |
| Specialist doctor visits | 29 (52.7) | 12 (21.8) | 17 (30.9) | 2.7 (0.9–8.0) | .08 |
| Primary care visits | 32 (58.2) | 16 (29.1) | 16 (29.1) | 1.3 (0.4–3.8) | .63 |
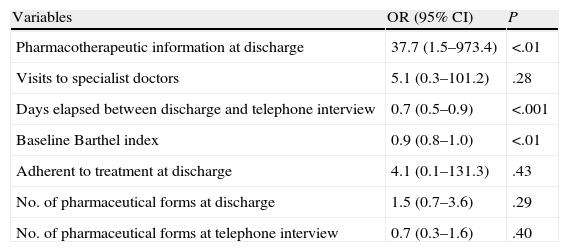
Table 3 shows the OR values for adjusted variables from the logistic regression model with their respective 95% CI values. The information provided at discharge, adjusted for possible confounding factors considered in the model (Table 4), has an OR of 37.7 (95% CI: 1.5–973.4) in favour of considering that a patient is adherent. The multivariate analysis indicated a clearly significant relationship between the number of days elapsed between discharge and the telephone interview and a reduced adherence to treatment (Table 4).
Multivariate logistic regression analysis of adherence at the telephone interview.
| Variables | OR (95% CI) | P |
| Pharmacotherapeutic information at discharge | 37.7 (1.5–973.4) | <.01 |
| Visits to specialist doctors | 5.1 (0.3–101.2) | .28 |
| Days elapsed between discharge and telephone interview | 0.7 (0.5–0.9) | <.001 |
| Baseline Barthel index | 0.9 (0.8–1.0) | <.01 |
| Adherent to treatment at discharge | 4.1 (0.1–131.3) | .43 |
| No. of pharmaceutical forms at discharge | 1.5 (0.7–3.6) | .29 |
| No. of pharmaceutical forms at telephone interview | 0.7 (0.3–1.6) | .40 |
In the interview, 70% of patients had some type of change to their treatment with respect to when they were discharged from the hospital:
- –
Some type of drug was added to the treatment in 62% of patients, with a mean of 2.23 (95% CI: 1.68–2.78) active ingredients added to initial treatment. The primary care or specialist doctor prescribed these changes in 80% of cases, primarily for treating previous conditions (65.1%). Of the active ingredients added, 33.3% were substitutes for previous treatments, with 57.1% from a different therapeutic group than the initial drug. The reasons for substitution were improvement (4.8%), adverse reaction (23.8%), worsening (33.3%) and other causes (38.1%).
- –
At least one drug was suspended in 58% of patients, constituting a total of 13% of treatments prescribed at discharge. 75% of these decisions were made by the primary care or specialist doctor. The reasons for suspension were improvement (30.2%), adverse reaction (18.6%), worsening (9.3%), intolerance to the medication (2.3%), and other (41.9%).
We observed no significant differences between the two groups in the variables regarding causes for modifying treatment after discharge.
DiscussionUsing an indirect method for evaluating adherence to treatment in our study (the Morisky–Green test), we observed an improvement in the percentage of patients that adhered to treatment in the experimental group compared to the control group. However, we did not observe any differences in other variables measured (deaths and health costs). Regarding treatment modifications, 70% of patients suffered some type of change to the originally prescribed treatment within 30–50 days of being discharged from the hospital, considering a change to have occurred only when the suspension or addition of a drug implied a change in the active ingredients taken. We did not consider changes those between brand names and generic medications.
This high percentage of patients with modified treatment could be explained by the adjustments made to medication prescriptions due to the clinical state of the patient. However, we must also point out that 16.2% of drugs added and 25.7% of drugs suspended were not due to medical decisions. Patient initiative was the cause for 14% of suspended treatments. The majority of them were due to causes independent of improvement or worsening of the underlying disease. As such, these results indicate a lack of compliance with treatment recommendations by the patient, which could have repercussions in the efficacy of treatment.
Although our study results did not find differences in the number of hospitalisations, deaths, and health resources used, the limited sample size may have negatively affected the statistical power for observing differences between the two groups. Several different studies have reported improved adherence to treatment and reduced hospitalisations in patients included in pharmaceutical information programmes at discharge.9–13 Even so, one of the fundamental conclusions of these studies is the need for continuity in communication with patients. For example, the study by López Cabezas et al.,9 which evaluated the effectiveness of an education programme for patients with heart failure, with controls at 2, 6, and 12 months, demonstrated a loss of statistical significance as the length of time since hospital intervention grew longer. Our results support these findings given the large number of changes to treatment plans observed within a short period after discharge, as well as the relationship observed between the number of days elapsed between discharge and the telephone interview and the percentage of patients adhering to treatment.
In addition to the small sample size, one of the limitations in our study was the method of obtaining information, since the personal and telephone interviews lacked objectivity in patient responses, which may have influenced the results. Even so, the randomised design of the study and the multivariate analysis allowed for improved control of the biases originally present in the information collecting process.
Although the Morisky–Green test used for evaluating adherence is a comparative method, this would have ideally been combined with another indirect method such as counting the surplus medications left over, which we did not carry out due to logistical reasons and patient unavailability. Additionally, the quantification of adherence would have been improved with repeated interventions and measurements during a longer period of time. The lack of this continuity required a reduced period of time between discharge from the hospital and the telephone interview due to the high number of changes to treatment plans. The resulting discord in the multivariate analysis in associating the Barthel index with adherence to treatment (Table 4) could be explained by the influence of caregivers, the subjectivity of the index, and the absence of measurements taken during the telephone interview.
One of the possible keys for optimising treatment results of the information provided by the pharmacist to patients at discharge is greater collaboration between primary care and community pharmacies. In the United States, several different studies have shown improved adherence to treatment in patients with hypertension, dyslipidaemia, and depression when involving community pharmacists in patient education programmes.14–17 Studies performed in Spain have evaluated the efficacy of different education strategies, producing a variety of results but no solid conclusions.18,19 Currently, the region of Madrid is developing a programme with the objective of educating elderly polymedicated patients, with the participation of primary care doctors and nursing staff and in collaboration with community pharmacies.20
As such, our study has concluded that pharmacotherapeutic information provided by the pharmacist to patients at discharge is an effective method for improving adherence to treatment, although patient follow-up must have a high level of continuity due to the numerous changes produced in treatment plans shortly after discharge. Elderly, polymedicated patients are ideal candidates for any pharmacotherapeutic information programme at discharge. Further studies that evaluate educational programmes at discharge should involve end variables.
Conflict of interestThe authors declare that they have no conflicts of interest.