To identify, classify and quantify the frequency of negative clinical adverse drug reactions (ADR) resulting in hospital admission from the emergency department (ED). To determine ADR preventability, identify ADR-related admission factors, calculate related costs and recognise which drugs are the most often involved.
MethodCross-sectional, prospective and observational study of patients who were admitted to hospital from the ED. We used the Dader method to detect ADR. We classified ADR in accordance with the Tercer Consenso de Granada (third Granada consensus), and calculated ADR preventability using the Schumock and Thornton scales (modified by Otero et al.), and ADR severity according to Schneider. We considered the direct costs generated during the hospital stay for the economic study. We analysed the correlation between ADR and age, sex, kidney and liver failure, and drug use. We used multiple logistic regression analysis to identify risk factors.
Results19.4% of admissions were the direct consequence of ADR, 65% of which were preventable. Antineoplastic therapy and immunosupressants caused 38% of ADR. 20.4% of admissions had to be transferred to the intensive care unit (ICU) or caused permanent damage. We found statistical significance between ADR and patients undergoing hormonal therapy, ‘high risk’ drugs and those admitted to the endocrinology department. The ADR-associated cost was €237,377.
ConclusionsADR-related admission is a problem with a high prevalence, and most cases are preventable.
Identificar, clasificar y cuantificar la frecuencia de los resultados clínicos negativos asociados al uso de medicamentos (RNM) como motivo de ingreso hospitalario desde el servicio de urgencias (SU). Determinar el carácter prevenible de los RNM, identificar factores que predisponen al ingreso por RNM, determinar los costes asociados y conocer qué medicamentos se ven implicados con mayor frecuencia.
MétodoEstudio transversal, prospectivo y observacional en pacientes que ingresaban desde el SU. Se aplicó el método Dáder para la detección de RNM. Los RNM se clasificaron según el Tercer Consenso de Granada, la evitabilidad se determinó aplicando el algoritmo de Schumock y Thornton, modificado por Otero et al. y la gravedad según Schneider. Para el estudio económico se consideraron los costes directos generados durante el ingreso. Se analizó la asociación entre los RNM y edad, sexo, insuficiencia renal y hepática, y consumo de medicamentos. Se utilizó la regresión logística múltiple para identificar los factores de riesgo.
ResultadosEl 19,4% de los ingresos fueron consecuencia directa de RNM, siendo evitables el 65%. El grupo de terapia antineoplásica e inmunosupresores causó el 38% de los RNM. El 20,4% de los ingresos requirieron traslado a la UCI o provocaron daño permanente. Se encontró una asociación estadísticamente significativa entre los RNM y los pacientes en tratamiento con terapia hormonal, fármacos de «alto riesgo» o ingresados en el servicio de endocrinología. El gasto ocasionado por los RNM fue de 237.377 €.
ConclusionesLos ingresos por RNM son un problema de elevada prevalencia, y la mayoría son evitables.
Adverse drug reactions (ADRs) are events which can affect the health of people who consume drugs for therapeutic, diagnostic or prophylactic purposes.1 An ADR, or a combination of multiple ADRs, can lead to treatment failure or even trigger new medical problems which may be as harmful as the original disease being treated.2
Morbidity associated with drug treatment is a severe public health problem that creates a significant demand for care and generates high healthcare costs. It is one of the leading causes of death in developed countries.3
According to data from various authors, the frequency with which ADRs appear is between 2.6% and 50%.4–6 Santamaría-Pablos et al. state that 16.6% of all hospital admissions are primarily caused by an ADR.7
Regarding mortality, a study carried out in the United States listed drug-induced iatrogenesis as between the fourth and the sixth most common cause of death in hospitals,8 meaning that between 43 000 and 98 000 patients die yearly as the result of a medication problem.9
Meanwhile, ADRs take a heavy toll on health and social resources, that increases both hospital stays and their associated costs.10,11 In the hospital context, Bates et al.10 and Classen et al.11 calculate that the direct costs generated by ADRs in hospitals in the United States range between 1.6 and 4bn dollars per year.
Data published in the literature coincide in stating that ADR can be avoided in most cases. It is estimated that up to 80%12,13 of ADRs are avoidable or preventable. Gaining a better knowledge of ADRs and the factors that tend to cause them will therefore contribute to detecting them at an earlier point in time. As a result, patients will have fewer health problems and enjoy better quality of life.14
In general, results from different studies on ADR are difficult to extrapolate to the general public, as they are gathered from very concrete contexts with reduced number of patients. In contrast, hospital emergency departments (ED) allow us to study larger number of patients. Even more importantly, they provide data that approach the likely situation in the general population, which is a crucial step towards identifying and preventing drug-induced iatrogenesis.15,16
Although some studies have analysed adverse drug effects in patients attended to in the ED,5,13,17,18 few studies evaluate ADRs that result in admission to hospitals. Such studies would be extremely useful for detecting the most severe incidents that occur in the primary care setting, where more than 90% of all medications are prescribed.
The main aim of this study was to identify, classify and measure the frequency of the adverse drug reactions that lead to hospital admissions through the ED.
Our secondary objectives are to determine how preventable ADRs are, identify factors that make patients more likely to be admitted due to ADR, determine costs associated with admissions of this type and which medications are the most frequently involved.
MethodCross-sectional, prospective, observational study was done over a 12-month period.
The target population of this study included all patients admitted through the ED to any medical department in the hospital.
Inclusion criteria were as follows: patients admitted from the ED to any medical department in the hospital, as well as patients in the ED pending admission to the hospital in both the observation and treatment areas.
We excluded all patients who did not give their informed consent to participate in the study, patients who were admitted to a surgical department, and patients whose physical or mental states would not permit us to gather all information necessary in order to undertake the study.
We completed a pilot study to calculate the sample size; this allowed us to estimate the frequency with which patients were admitted due to ADR in our area. The results of our pilot study showed an ADR frequency of 20%. Considering a 95% confidence interval and a margin of error of 5%, we calculated that the sample size required in order to attain study objectives would consist of 246 patients. This figure was increased to 258 allowing for a 5% drop-out rate.
Patients were assigned on workdays through random daily selection from among all patients pending hospital admission. The pharmacist entered all details of patients who met inclusion criteria in an Excel spreadsheet. For the random assignment of patients we used the Excel program's “RAND” function. For cases in which one of the selected patients did not meet the inclusion criteria, we selected the patient corresponding to the next sequential number on the random assignment list. Patients who remained in the emergency department for several days were only included once in the selection process.
We used the Dáder Program for the implementation of Drug Therapy Follow-Up Method, adapted for the hospital setting, as a tool for detecting cases of ADR that resulted in hospital admission.19
In order to gather information, we held personal interviews with all of the patients within the first 48h of their hospital stay. The interviewer employed a questionnaire based on the Dáder method.19,20
In addition to information obtained in the interview, we systematically reviewed the entries for diagnostic test results, analytical data, and emergency department reports in order to gather all possible clinical history and drug therapy information.
We monitored the clinical progress of all patients until we were able to determine the definitive clinical cause of their admission to hospital.
All admissions suspected of being associated with an ADR were evaluated by both a doctor and a pharmacist. In cases in which the two evaluators were unable to agree on whether the hospital admission was caused by an ADR, an additional doctor was called upon to clarify the case.
The ADRs responsible for hospital admissions were classified according to the Tercer Consenso de Granada (Third Granada Consensus).1
Avoidability was determined by applying the criteria set forth in the algorithm proposed by Schumock and Thornton21 and modified by Otero et al.22 The degree of avoidability of the ADR was estimated using the Nelson and Talbert23 criteria as adapted by Otero et al.17
The severity of the ADR was analysed according to the Schneider scale.24
Once the ADR had been detected, we proceeded to identify the medications involved in the reactions. We did not analyse those drugs involved in the ADRs associated with an untreated health problem since establishing the exact active ingredient needed by the patient was impossible.
All drugs were classified according to whether or not those could be considered “high risk” drugs25 and whether they met criteria for being considered as drugs with a narrow therapeutic margin (NTM).
Economic StudyThe economic study was performed based on the ADR that caused the patient's hospital admission, including the direct associated costs. Data were provided by the hospital's accounting unit.
Considering the number of patients admitted to the ED per year and the ADR incidence rate determined by the study, we then estimated the annual economic cost generated by ADRs.
In the cost description, we distinguished between patients with unpreventable and preventable cases of ADR. All costs were analysed and compared according to the ADR classification and degree of severity.
Statistical AnalysisWe completed a descriptive analysis of patient characteristics with respect to the explanatory variables considered in the study. We found the mean plus standard deviation for continuous variables, and determined absolute and relative frequencies for discrete variables.
A bivariate analysis was completed with ADR as the dependent variable and with age, sex, reason for ED visit, liver and kidney failure, number of medications, therapeutic group, pharmacological subgroup, use of “high-risk” medications, consumption of NTM drugs and clinical department responsible for the patient as independent variables.
To examine the association between discrete variables, we used a chi-squared test or Fisher's exact test when the theoretical frequency in any of the cells was less than 5. Continuous variables were compared using the Mann–Whitney test.
We designed a backward multiple logistic regression model; the maximal model was created using those variables that were significant in the univariate analysis. The maximum number of variables introduced was 8 events per variable. Results from the model were expressed as odds ratio and confidence interval. Model discrimination was performed using the area under the ROC curve, and it was calibrated using the Hosmer Lemeshow test.
Values of P<.05 were considered statistically significant. All comparisons were two-tailed.
Statistical data processing was performed using SPSS (Statistical Package for Social Sciences) software version 11.5 for Windows.
We used Excel 2000 for graphical analysis.
Ethical AspectsWe obtained informed consent from all participants in the study before inclusion.
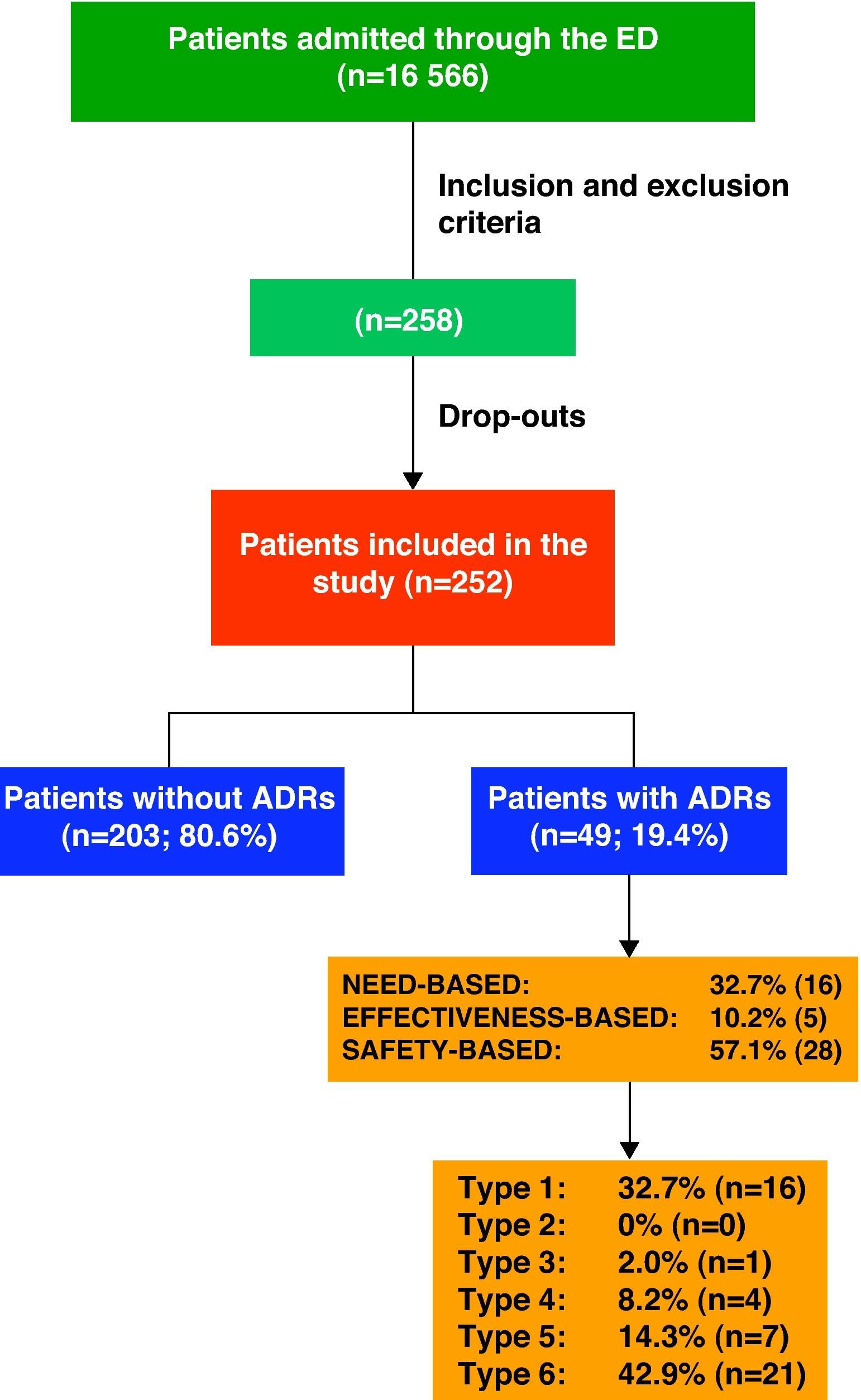
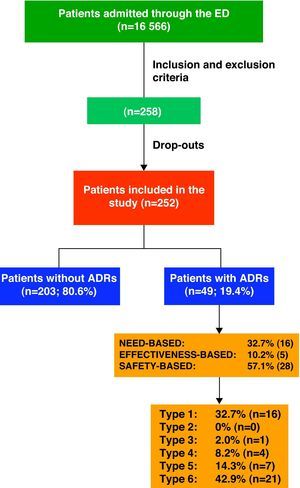
ResultsDuring the study period, the hospital ED attended to a total of 152 403 patients, 10.87% being admitted (16 566 patients).
Keeping inclusion and exclusion criteria in mind, we initially selected 258 patients; however, the final population consisted of 252 cases as the drop-out rate was 2.3%. We had to exclude 6 patients either who did not wish to participate or who could not be evaluated due to lack of information.
Description of the PopulationOf the 252 patients analysed, 59.5% (150) were male and 40.5% (102) were female. The mean age of the population was 68.2±16.1 years, range 21–96.
The main reasons for patient visits to the ED were respiratory problems (39.3%), gastrointestinal complaints (13.5%) and fever (8.7%).
The mean number of different illnesses each patient presented was 3.2±1.9. The main chronic diseases that patients suffered prior to hospitalisation were hypertension (69.0%), heart disease (heart failure, ischaemic heart disease, atrial fibrillation or cardiomyopathy [39.3%]) and diabetes mellitus (29.4%). Only 7.5% of these patients did not suffer from any chronic diseases.
Regarding medication taken by patients prior to hospitalisation, the mean number of drugs per patient in the total study population was 5.7±3.6; range 0–17. Only 7.9% of the study patients were not on any medications.
The main therapeutic groups for the drugs taken by patients upon admission were digestive and metabolic treatment (90.5%), followed by cardiovascular treatment (65.9%).
Once the decision had been made to admit patients, they were assigned to different medical departments within the hospital, mainly internal medicine (24.21%), cardiology (15.48%) and respiratory medicine (13.89%).
ADR Classification and Drugs InvolvedOf the 252 patients included in the study, 19.4% (n=49) were admitted as a direct result of an ADR.
Fig. 1 shows the ADRs that caused hospitalisations grouped by ADR classifications and types.
We considered 65% (32) of the hospitalisations due to ADR as preventable. Of these, 68.7% (22) arose through a medical error, including both prescription errors and medication follow-up errors; 31.3% (10) were provoked by the patients themselves.
Main medication errors (ME) due to the patient not receiving needed treatment accounted for 21.9% of the total (7 cases); 18.8% (6) were due to poor treatment compliance; 15.6% (5) were due to the dose, frequency or route of administration being contraindicated by the patient's medical condition, and 9.4% (3) were due to contraindicated self-medication and lack of prophylactic treatment.
According to the Otero et al.17 adaptation of Nelson and Talbert's criteria,23 41.67% (15) of the ME were preventable and 58.33% (21) could have been prevented.
With regard to ADR severity, 77.6% (38) were level 4 (requiring additional treatment, longer stay or hospital admission); 20.4% (10) were level 5 (requiring ICU treatment or causing permanent damage to the patient; and 2% (1) were level 6 (fatal).
A total of 46 drugs were involved in the 33 ADR cases having to do with effectiveness and safety. In 66.6% of the cases (22), only 1 medication was involved; in 24.2% (8) 2 medications and in 10.1% (3) 3 medications were involved.
Fig. 1 shows the indications for the 46 medications that were involved. Antineoplastic and immunosuppressive drugs (L) caused 38% of the cases of ADR, followed by cardiovascular drugs (C) at 14%.
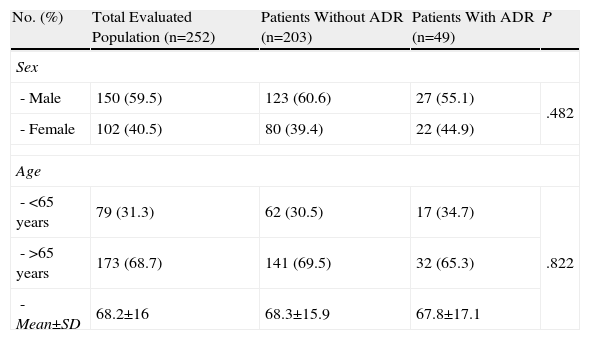
Statistical Analysis of Risk FactorsBivariate AnalysisTable 1 shows the patients’ socio-demographic characteristics according to whether or not they suffered an ADR. Upon analysing the risk of hospitalisation due to ADR by patient age (>65 years) and sex, we found no statistically significant differences between the groups, with values of P=.822 and P=.482 respectively.
Sample Distribution According to Socio-Demographic Characteristics.
| No. (%) | Total Evaluated Population (n=252) | Patients Without ADR (n=203) | Patients With ADR (n=49) | P |
| Sex | ||||
| - Male | 150 (59.5) | 123 (60.6) | 27 (55.1) | .482 |
| - Female | 102 (40.5) | 80 (39.4) | 22 (44.9) | |
| Age | ||||
| - <65 years | 79 (31.3) | 62 (30.5) | 17 (34.7) | .822 |
| - >65 years | 173 (68.7) | 141 (69.5) | 32 (65.3) | |
| - Mean±SD | 68.2±16 | 68.3±15.9 | 67.8±17.1 | |
When analysing the reasons for patient visits to the ED, according to whether or not the visit was caused by an ADR, we found statistically significant differences among patients with a biochemical disorders (14.3% vs 3.4%, P=.008) and those with respiratory problems (18.4% vs 44.3%, P=.001).
The percentage of patients with respiratory failure was greater in those admitted due to an ADR than in the general population (36.7% vs 29.6%, P=.629). Regarding liver failure, no significant differences were found between the two groups (6.1% vs 9.4%, P=.584).
Mean medication consumption among patients without ADRs was 5.6±3.6; among patients with ADRs, it was 6.0±3.8 (P=.215).
When analysing drug consumption by therapeutic groups and the reason for hospital admission, we observed that those patients admitted as a direct consequence of an ADR were mainly undergoing hormone therapy (30.6% vs 16.5%, P=.022) and taking antineoplastic drugs (24.5% vs 10.8%, P=.012). In contrast, respiratory drug consumption was higher in those patients admitted for a reason other than ADRs (24.6 vs 8.2%, P=.012).
Furthermore, when analysing the use of the different pharmacological subgroups according to whether or not the patient experienced an ADR and if it was preventable, we found statistically significant differences in consumption of thyroid drugs (23.5% vs 4.9%, P=.003) and systemic corticosteroids (41.2% vs 11.3%, P=.001) for the unpreventable ADR and non-ADR groups, respectively. There were also significant differences in the use of cytostatic drugs among patients with both preventable and unpreventable ADRs (18.4% vs 6.4%, P=.019), and in use of antiplatelets among patients with preventable ADRs (43.6 vs 24.6%, P=.018).
At least one “high risk” medication was being taken by 44.4% of the patients. The percentage of patients being treated with this drug group was higher in the group of patients admitted due to ADR (59.2%) than in the other patients (40.9%), and this difference is statistically significant (P=.021).
Furthermore, 53.6% (135) patients were on NTM drugs. Some 63% of patients on NTM drugs were hospitalised due to an ADR, compared to 51.2% (P=.130) that were admitted for reasons other than ADR.
When analysing the risk of being admitted for ADR according to the medical department, we only find statistically significant differences for patients who were admitted to the endocrinology department (6.1 vs 0.5%, P=.024).
Multivariate AnalysisThe variables introduced in the maximal regression model were hormone therapy, treatment with respiratory system drugs, treatment with antineoplastic drugs and immunosuppressive drugs, treatment with antiplatelet drugs, admission to the endocrinology department, and treatment with high-risk drugs. Although they were statistically significant, we excluded the variables that had the highest P-values, since bivariate models did not show a clear correlation with dependent variables; as well as variables not considered particularly relevant from a clinical point of view; and variables that were highly similar to another variable.
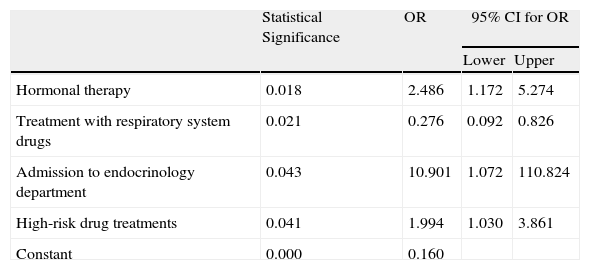
The variables shown to contribute to the presence of ADR were hormone therapy, respiratory treatment drugs, admission to the endocrinology department and treatment with high-risk drugs (Table 2).
Variables Incorporated in the Multiple Logistic Regression Model.
| Statistical Significance | OR | 95% CI for OR | ||
| Lower | Upper | |||
| Hormonal therapy | 0.018 | 2.486 | 1.172 | 5.274 |
| Treatment with respiratory system drugs | 0.021 | 0.276 | 0.092 | 0.826 |
| Admission to endocrinology department | 0.043 | 10.901 | 1.072 | 110.824 |
| High-risk drug treatments | 0.041 | 1.994 | 1.030 | 3.861 |
| Constant | 0.000 | 0.160 | ||
CI: confidence interval; OR: odds ratio.
In the Hosmer Lemeshow test, chi-squared was equal to 1.584 (statistical degree of confidence=4, statistical significance=0.812).
The area below the ROC curve was equal to 0.688.
The total cost of hospital admissions caused by ADRs was €237 377 with a mean estimated cost per admission of €4844.
Considering a 19.4% incidence rate for ADR-induced hospital admissions through the ED, the estimated annual cost due to ADR was €15 568 952.
A breakdown of data by whether or not ADR is preventable shows that 59.6% of costs were considered preventable. The mean cost of hospitalisation due to preventable ADR is €4418.1, while the mean cost of an unpreventable ADR is €5646.9.
Analysis by ADR type showed that the mean cost of safety-based ADRs (€6118) was far higher than that of need-based (€2994) and effectiveness-based ADRs (€3634).
Considering ADR costs according to severity, we estimated a mean cost of €342/admission for level 4 episodes, €438/admission for level 5 episodes, and €726/admission for level 6 episodes.
DiscussionAlthough numerous studies evaluate ED visits due to ADR,5,13 few studies focus specifically on detecting and analysing the number of hospital admissions that such episodes produce. This observational, descriptive prospective study was designed to exclude admission to surgical departments through the ED and it included an interview between the pharmacist and the patient. The study detected a high number of ADR-induced hospital admissions, with figures are similar to those obtained by Queneau et al.18 and García et al.,13 who followed the same methodology and found incidence rates of ADR-induced hospitalisation of 13% and 16.1% respectively.
Of the total ADR-induced admissions, 63.5% were preventable, which shows the importance of developing prevention strategies. Our results are within the range shown by previously published studies (37.9–88.9%).7,13,18
Omission of a drug needed in order to treat a health problem was the main cause of the preventable ADRs detected in the study (21.9%). These data coincide with results published in 2006 by Sotoca-Momblona and Siso-Almirall,26 who identified omission as the main cause of medication errors (34.5%). In addition, incorrect administration of the drug (dose, frequency or route) accounted for 15.6% of the preventable ADRs. According to some authors,27 incorrect administration is the most frequent cause of ADR in hospitalised patients. It is generally attributed to a prescribing doctor's lack of knowledge of drug characteristics and/or the importance of adjusting the dose based on the patient's individual situation. One useful measure for preventing such errors could be the use of interconnected databases containing patients’ clinical, analytical and drug treatment data.
Regarding the effect of risk factors on the incidence rate of ADR-induced hospitalisations, the multiple regression analysis showed that the only influential variables were an admission to the endocrinology department, hormone therapy, treatment with respiratory system drugs, and treatment with “high-risk” drugs.
The risk factor in patients admitted to the endocrinology unit may be due to a high percentage of these patients being treated with high risk drugs (oral anti-diabetic and/or insulin agents). This information is extremely important because although there have been numerous studies analysing ADR-induced admissions to different medical units, we have not found any study focusing on the endocrinology unit. This could be an interesting future line of study.
The most common causes of ADR among hormonal therapies were in the corticosteroid group, which coincides with the studies published by Forsters28 in 2006 and Zhang et al.29 in 2007. These two studies associated corticosteroids with 9.1% and 10.1% of studied cases of adverse drug reactions, respectively. The inherent risk associated with hormonal therapy makes dosage adaptation and continuous patient monitoring necessary. This pharmacological group's potential pharmacological and pharmacodynamic interactions require the development and implementation of measures to prevent ADR. These may include action protocols.
We also found a statistically significant association between the use of high-risk medications and the possibility of presenting an ADR that would require hospitalisation. These results show that we must develop systems to provide close and continuous monitoring of these drugs. Future studies on how to systematically prevent ADRs in the healthcare sector by using pharmacotherapeutic monitoring programmes may be of interest. On this subject, the ISMP recommends that a pharmacist reviews and validates prescriptions (checking dosage limits and treatment durations), and elaborates protocols.30,31Permanent damage occurred or ICU treatment was required in 20% of the cases of ADR, and death in 2%, which clearly demonstrates the high morbidity associated with drug treatment. These figures are consonant with published data in the literature (6%–24%).32–34
Another relevant aspect of this study is the economic impact associated with drug treatment morbidity and mortality. The mean cost of the ADR-induced hospitalisations was €4844.
In 2001, Sent et al.35 estimated a cost of $6685 per hospital admission due to ADR. In the previous year, Suh et al.36 calculated a mean cost per ADR-induced hospital stay of $5456. These results are likely similar to our most because studies accounted for all costs associated with the entire hospital stay, as ours did. In contrast, the 1999 study performed by Climente et al37 in a Spanish public hospital with a capacity of 550 beds estimated a mean cost of €769 (128 201 pesetas). This substantial difference is due to the fact that they only included the costs for the hospital stay and diagnostic tests and it did not account for medication, consultation with other specialists, ED stays, medical material and meals.
One limitation of this study which we should point out is that we did not know patients’ complete drug treatment history, and therefore we were unable to analyse what drugs were involved in need-based ADRs, as it was impossible to establish exactly which active ingredient the patient needed. In addition, we did not evaluate treatment compliance and knowledge of drug treatment as risk factors having an effect on ADR-induced hospitalisation. Furthermore, although some ADR-induced admissions were the result of self-medication, we did not study factors involved in self-medication.
We believe that our study's strong point is that our efforts to gather drug treatment information from both patients who experienced an ADR-induced hospitalisation and those who were admitted for other reasons produced an in-depth analysis of ADRs and their magnitude.
In conclusion, data from the study show that ADRs cause a high percentage of hospital admissions, and are preventable in most cases. These hospital admissions are mainly connected to safety-based ADRs, followed by need-based and then effectiveness-based ADRs. Risk factors that contribute to ADR-induced hospitalisations are being admitted to the endocrinology department and receiving hormonal therapy, respiratory system drugs and high-risk drugs. The results of this study confirm that the health risks of ADR are considerable and their economic burden is heavy. It restates the need for designing, developing and implementing effective healthcare practices intended to promote safe use of medications, with special emphasis on the at-risk populations identified by this study.
Conflict of InterestThe authors have no conflicts of interest to declare.
Please cite this article as: Pérez Menéndez-Conde C, et al. Resultados negativos asociados al uso de medicamentos que motivan ingreso hospitalario. Farm Hosp. 2011;35:236–43.