To describe and determine the extent of use of unlicensed, off-label and high-alert drugs in the general pediatric units of a university hospital in southern Brazil.
MethodsA cross-sectional study conducted from November 2007 to January 2008 involving patients up to 14 years of age. Intensive care and pediatric oncology unit patients were excluded. Classification according to the Food and Drug Administration approval criteria was performed using the DrugDex-Micromedex® and high-alert medications were classified according to the Institute for Safe Medication Practices.
ResultsDuring the study period, 342 prescriptions were analyzed. Analgesic drugs were the most frequently prescribed therapeutic class of drugs (26.9%) and antispasmodic drugs (31.5%) were the most frequently issued off-label drugs. About 12% of the prescriptions analyzed presented unlicensed drugs and 39% presented at least one off-label drug, especially in relation to its therapeutic indication (38.4%) and age (21.9%). Approximately 6% of the total (2026) were classified as high-alert medications, such as opioid analgesic drugs (35%). No association was observed between off-label use and high-alert drugs.
ConclusionFrequency of unlicensed and off-label drug prescriptions showed in the study is according to the literature and may be considered high. High-alert drugs, although low in frequency, can present risks due to the harmful effects they can produce in patients. Thus, the highlighted drugs in this study constitute a constant concern in hospitals.
Describir y determinar el alcance del uso de medicamentos de alto riesgo, no aprobados y en indicaciones no aprobadas en las unidades generales de pediatría de un hospital universitario del sur de Brasil.
MétodosEstudio transversal realizado entre noviembre de 2007 y enero de 2008 en el que participaron pacientes de hasta 14 años. Se excluyó a los pacientes de las unidades de oncología pediátrica y cuidados intensivos. La clasificación, según los criterios de aprobación de la Agencia de Alimentos y Medicamentos de EE. UU., se realizó usando DrugDex de Micromedex,® y los medicamentos de alto riesgo se clasificaron de acuerdo con el Instituto para las Prácticas de Medicación Seguras.
ResultadosDurante el periodo de estudio se analizaron 342 prescripciones. Los analgésicos fueron la categoría terapéutica más prescrita, con un 26,9%, y los antiespasmódicos, con un 31,5%, fueron los medicamentos más usados en indicaciones no aprobadas. Alrededor del 12% de las prescripciones correspondían a medicamentos no aprobados, y el 39% contenían al menos un medicamento para una indicación no aprobada, especialmente en relación con su indicación terapéutica (38,4%) y la edad (21,9%). Aproximadamente el 6% del total (2.026) de los fármacos se clasificaron como medicamentos de alto riesgo, y entre ellos destacaron los analgésicos opiáceos (35%). No se observó ninguna relación entre el uso de fármacos en indicaciones no aprobadas y los medicamentos de alto riesgo.
ConclusiónLa frecuencia de la prescripción de fármacos no aprobados y de medicamentos en indicaciones no aprobadas coincide con la hallada en la literatura, y puede considerarse alta. A pesar de su baja frecuencia, los medicamentos de alto riesgo pueden ser peligrosos, por los efectos perjudiciales que pueden causar en los pacientes. Por lo tanto, el uso de los fármacos en los que se centra este estudio constituye una alerta constante en los hospitales.
More than 35 years ago, the term therapeutic orphans was created in order to highlight the fact that children were not frequently included in clinical trials for the development of new drugs.1 Examples such as congenital malformations associated with thalidomide, in the 1960s, development of kernicterus (severe brain damage related to neonatal hyperbilirubinemia) with the use of sulfonamides in neonates, gray baby syndrome associated with the use of chloramphenicol in the neonatal period, and, more recently, cardiac arrhythmia with the use of cisapride in the treatment of gastroesophageal reflux, brought attention to the need to set norms in order to regulate the experimentation and trade of new drugs for children, ensuring safety, effectiveness and quality.1–3
Based on new research regulations, the European Medicines Agency (EMA) and the Food and Drug Administration (FDA) have encouraged the development of studies involving individuals of less than 18 years of age, searching for improved safety in drug use through the creation of adequate formulations and pharmacokinetic assays for this population.4–9 In Europe, only 35% of all commercially available drugs are estimated to be licensed for use on children. Likewise, in the United States, until 2003, only 20–30% of the drugs were approved for use on children.4,10
The high prevalence of prescriptions with unlicensed (11%) and off-label drugs (30–50%) in hospitalized children has been described in several studies and is considered common practice in hospitals.11–15 In general pediatric units, 16 to 62% of the drugs are estimated to be off-label or unlicensed.16 Likewise, the prescription of off-label and/or unlicensed drugs to children outside the hospital is high, ranging from 11 to 37%.12
In addition, some drugs are classified as high-alert medications, since they present reduced safety and, thus, higher susceptibility to inflict harm, such as mild-to-severe adverse reactions caused by medication misuse.17,18 Several of these drugs are also classified as unlicensed or off-label, which increase the risks when used in children. The study aimed to describe and determine the extent of use of unlicensed, off-label and high-alert drugs in the general pediatric inpatient unit of a university hospital in southern Brazil.
MethodsA descriptive prospective cross-sectional study was conducted in the pediatric inpatient unit of Hospital de Clínicas de Porto Alegre (HCPA), a tertiary general public university hospital in southern Brazil. The pediatric inpatient unit is equipped with 71 beds for patients from 0-14 years of age assisted in clinical and surgical conditions. The study was based on the collection of variables related to the patients and the prescribed drugs available on clinical records and from information provided by the health care team.
SampleA population of 2,040 patients hospitalised in the general pediatric inpatient unit during a period from January to December 2006 was considered for the sample size measurement. For a P value lower or equal to 0.05 and an estimated frequency of off-label drug of 49.5%±5%,12 a sample of 323 patients was calculated with Epinfo, version 6.04. Some 342 patients from the total survey were considered and the prescriptions of these patients were analysed.
Patient-related variablesPrescriptions of patients from zero to 14 years of age, who were committed to the pediatric inpatient unit for different clinical and/or surgical conditions, for a minimum period of 24hours, were included. Prescriptions from patients in the intensive care (ITU), neonates and paediatric oncology units were excluded, as they presented different profiles from the general clinical practice prescriptions.
The collection was conducted from November 2007 to January 2008. The selection of patients hospitalised in paediatric units was carried out using the clinical records provided by the hospital's computerised system.. Patients’ information was gathered in special forms containing the variable's age, gender, weight, chronic diseases and reason for hospitalisation which were completed upon the examination of hospital records and specific prescriptions. For analysis reasons, due to the heterogeneity of ages, four age groups were created. These included infants from zero to under 2 years of age; pre-school children from 2 to under 7 years of age; school children from 7 to under 10 years of age and adolescents over 10 years of age.
Drug-related variablesThe collection of variables related to drugs started from the second prescription in effect on the first day of hospitalisation in the pediatric inpatient unit. In our institution, the first prescription basically includes antipyretics and analgesics until the patient waits for assessment teams or examinations. The decision to start from the second prescription was also taken to avoid bias in the prescription if the patient had been transferred from another unit of the hospital. The second prescription of the current day is made after the clinical evaluations of the unit team. The prescription was evaluated only once. Collected data referred to total prescribed drugs, pharmaceutical forms, administration forms, drug presentation and administration interval.
The study excluded prescription items related to blood products, oxygen, total parenteral nutrition, oral rehydration salts and topical products (lanette cream, hydrogel, almond oil) used during hospitalisation, as well as the electrolytes (0.9% sodium chloride solution and 5% glucose solution) of routine use in clinical assistance for the maintenance of peripheral venous access and drug administration.
Drugs were classified as licensed, unlicensed, and off-labeled,19 according to ATC's (Anatomical Therapeutic Chemical Classification) therapeutic classes and the FDA approval criteria, following the DrugDex-Micromedex® database. The medicines were classified according to FDA approval criteria and not according to the Brazilian criteria because the ANVISA (Agência Nacional de Vigilância Sanitária), the agency in charge of drug licensing in Brazil, does not provide these data; the marketing authorisation of medicines is not available in official formulary. The evaluation of a drug registration dossier is usually divided into three parts by ANVISA: pharmacotechnical, efficiency and security analysis.
Pharmacotechnical analysis includes the verification of all stages of manufacture of the drug and is performed by pharmacists, who rarely request ad hoc consultants. The same does not occur with regard to assessments of effectiveness and safety, conducted through analysis of preclinical studies and clinical trials.These are subdivided into phases I, II, III, and possibly IV, in the case of drugs already registered in other countries for which post-market pharmacovigilance data are available.
Drugs were also classified as high-alert medications, according to the Institute for Safe Medication Practices.18 According to the approval criteria, drugs for children were classified as: licensed – drugs approved in all their specifications for pediatric use; unlicensed – drugs not approved or contraindicated for pediatric use; or off-label – drugs prescribed in a non-standard manner, according to official drug compendiums, in relation to one or more parameters, such as dose, age, administration form, administration interval, drug presentation and use indication.19 This classification was based on information from the Drug-Dex Micromedex® tertiary source, which includes all drugs approved by the FDA and drugs in phase-3 studies performed by the agency. Thus, each prescription item was individually analysed, according to clinical diagnosis and use indication for each patient. When not explicit in the patients’ clinical records, some information such as use indication of a certain drug was requested from the medical team during the multidisciplinary round.
Drugs that are prescribed as “if necessary” and that presented an administration interval in the prescription were classified according to the indicated interval; drugs without an administration interval prescribed were classified as “others” for this variable.
Data arrangement and analysisAfter data collection, the variables of interest were entered in a database created in Epinfo, version 6.04. Database typing was performed in duplicate to minimise errors. Data analysis was performed using the same program and included measurements of central tendency and dispersion and prevalence ratio and chi-square test (x2). They were also processed in SPSS, version 17.0. Values of p ≤ 0.05 were considered significant for statistical analyses.
Ethical aspectsThe study project was approved by the Research Ethics Committee of the Hospital. The investigators signed a commitment term related to data utilisation for the study purposes, ensuring the ethical aspects, according to Resolution 196/96 and complementary norms of the National Health Council.
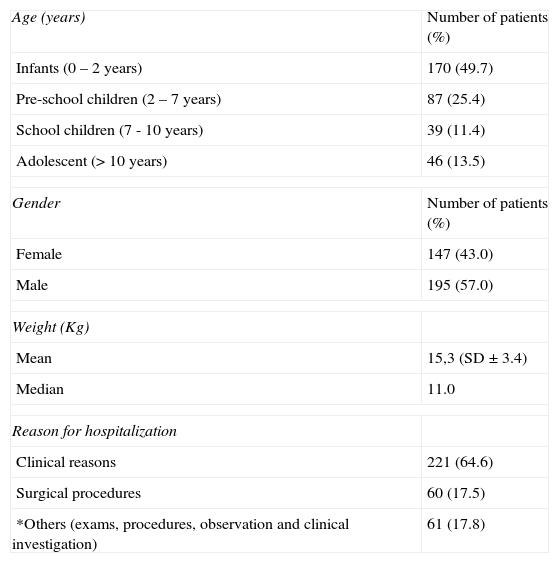
ResultsCharacteristics of patientsThe study analysed 342 prescriptions. Patients’ ages ranged from one month to 14 years of age with a mean of 2.0 years (SD ± 3.9 years). The group of infants was the most prevalent, constituting 49.7% of the patients. Males were most frequent, representing 57% of the hospitalised children. Table 1 shows other hospitalisation characteristics of the patients.
Characteristics of the patients (n = 342)
| Age (years) | Number of patients (%) |
| Infants (0 – 2 years) | 170 (49.7) |
| Pre-school children (2 – 7 years) | 87 (25.4) |
| School children (7 - 10 years) | 39 (11.4) |
| Adolescent (> 10 years) | 46 (13.5) |
| Gender | Number of patients (%) |
| Female | 147 (43.0) |
| Male | 195 (57.0) |
| Weight (Kg) | |
| Mean | 15,3 (SD ± 3.4) |
| Median | 11.0 |
| Reason for hospitalization | |
| Clinical reasons | 221 (64.6) |
| Surgical procedures | 60 (17.5) |
| *Others (exams, procedures, observation and clinical investigation) | 61 (17.8) |
Among the hospitalised patients in the period studied, 52% presented chronic diseases and approximately 12.6% presented more than one associated disease. Neurological diseases were the main baseline diseases of the hospitalised patients (39.2% of the cases), followed by respiratory diseases (19.8%).
Clinical hospitalisations were mainly caused by respiratory diseases (51.9% of cases), including 67 cases of bronchopneumonia (45.9%), and 19 cases of cystic fibrosis and mild respiratory dysfunction respectively (13%). Regarding surgical procedures, appendicectomy was performed in 23% of the hospitalised patients.
Infants were the most prevalent group with regards to chronic diseases (49.7%) as approximately 21% had already presented a neurological disease diagnosis before hospitalization and 5% also presented bronchopneumonia and associated recurrences. In addition, with regards to the reasons for hospitalisation, infants accounted for 53% of respiratory cases, including bronchopneumonia (40 of 67 cases), followed by mild respiratory dysfunctions and viral bronchiolitis.
Drugs and prescriptionsThe analysis recorded 2026 prescription items, with 5.9 mean items (range 1-24) per prescription (SD ± 2.9) and median of 5. The analysis also demonstrated that most of the prescribed drugs were part of the selection list of the hospital, except 49 (2.4%) of the items, which were not included in the standard drug list of the hospital.
The most frequent drug administration form was intravenous with 764 (37.8%) items, followed by oral administration with 752 (37.1%) items and then enteral feeding tubes with 263 (13%) items. Antibiotic and analgesic drugs were most frequently administered in the parenteral form during the acute phase of infections, such as in bronchopneumonia and cystic fibrosis, and during the postoperative period, changing to oral form, as soon as possible. In the case of feeding tubes, patients (most of whom had deglutition and cerebral paralysis problems) received drug administration through feeding tubes as an alternative form. Oral extemporaneous preparations were required for 79 (4%) items of prescriptions by hospital pharmacy. The most prescribed drugs included: paracetamol (14.7%), metoclopramide (10.1%), dipyrone (9.7%), ibuprofen (5.8%), salbutamol (3.2%), prednisolone (2.7%) and phenobarbital and valproic acid (2.2%).
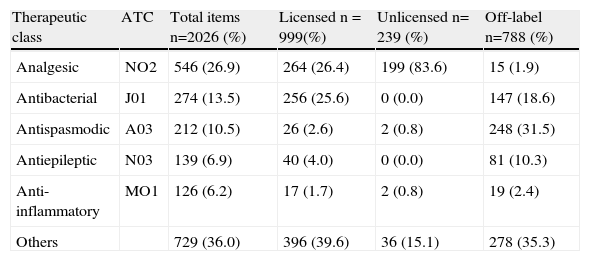
Off-label use, unlicensed and high-alert medicationsMost children (95.3%) were given off-label and unlicensed drugs. Considering the analysed items, 788 (38.9%) were classified as off-label and 239 (11.8%) as unlicensed drugs for pediatric use. The prevalence ratio of unlicensed or off-label drug was not higher in patients who received multiple drugs (0.94 CI 95% 0.74 – 1.19). Table 2 shows the most prescribed therapeutic classes according to the FDA approval classification.
Therapeutic classes related to the approval classification
| Therapeutic class | ATC | Total items n=2026 (%) | Licensed n = 999(%) | Unlicensed n= 239 (%) | Off-label n=788 (%) |
| Analgesic | NO2 | 546 (26.9) | 264 (26.4) | 199 (83.6) | 15 (1.9) |
| Antibacterial | J01 | 274 (13.5) | 256 (25.6) | 0 (0.0) | 147 (18.6) |
| Antispasmodic | A03 | 212 (10.5) | 26 (2.6) | 2 (0.8) | 248 (31.5) |
| Antiepileptic | N03 | 139 (6.9) | 40 (4.0) | 0 (0.0) | 81 (10.3) |
| Anti-inflammatory | MO1 | 126 (6.2) | 17 (1.7) | 2 (0.8) | 19 (2.4) |
| Others | 729 (36.0) | 396 (39.6) | 36 (15.1) | 278 (35.3) |
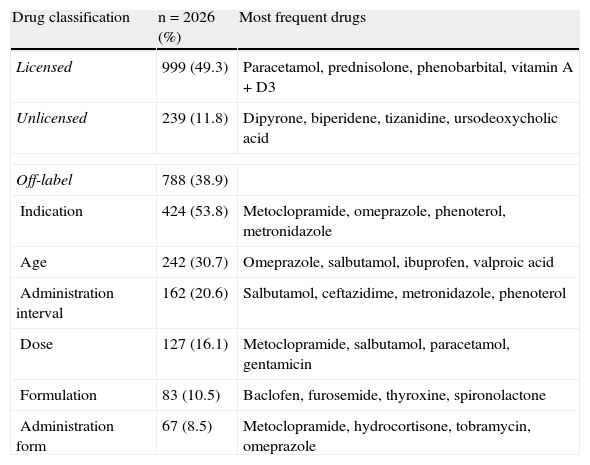
Analgesic (83.3%) and choleretic drugs (7.5%) were among the most frequently prescribed therapeutic classes of unlicensed drugs, particularly dipyrone (10% of total items prescribed) and ursodesoxycolic acid (0.9% of total items prescribed). Off-label drugs prescription in the studied population was more frequent in relation to its therapeutic indication (53.8%) and to age (30.7%). Table 3 shows the frequency of prescribed drugs according to the classification as licensed, unlicensed and off-labeled drugs, as well as the most frequent drugs in each classification.
Frequency of drugs prescribed according to classifications licensed, unlicensed and off-label
| Drug classification | n = 2026 (%) | Most frequent drugs |
| Licensed | 999 (49.3) | Paracetamol, prednisolone, phenobarbital, vitamin A + D3 |
| Unlicensed | 239 (11.8) | Dipyrone, biperidene, tizanidine, ursodeoxycholic acid |
| Off-label | 788 (38.9) | |
| Indication | 424 (53.8) | Metoclopramide, omeprazole, phenoterol, metronidazole |
| Age | 242 (30.7) | Omeprazole, salbutamol, ibuprofen, valproic acid |
| Administration interval | 162 (20.6) | Salbutamol, ceftazidime, metronidazole, phenoterol |
| Dose | 127 (16.1) | Metoclopramide, salbutamol, paracetamol, gentamicin |
| Formulation | 83 (10.5) | Baclofen, furosemide, thyroxine, spironolactone |
| Administration form | 67 (8.5) | Metoclopramide, hydrocortisone, tobramycin, omeprazole |
Dose, age and indication constitute the most frequent off-label uses, according to most studies performed with children. It was observed that indication (53.8%), age (30.7%) and administration interval (20.6%) were more frequent than the doses prescribed (16.1%) out of the recommended therapeutic range. In terms of indication, metoclopramide was prescribed in 47.8% of the items for the treatment of gastroesophageal reflux and prophylaxis of postoperative nausea and vomiting, a non-official use in children.
Similarly, fenoterol (6% of the items), prescribed to patients with cystic fibrosis, is not indicated for the treatment of obstructive respiratory diseases or asthma prophylaxis before respiratory physiotherapy. Salbutamol spray (21.9%) and valproic acid (15.7%) were the most prescribed drugs under the recommended age, 4 and 10 years old, respectively. Drugs for the treatment of respiratory diseases represented 5.4% of all prescribed off-label items. Salbutamol spray, for instance, was prescribed under the recommended age to approximately 15% of the children.
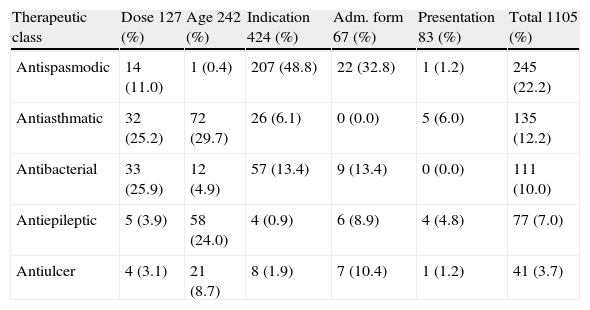
Also, it was the most frequently prescribed drug with a non-standardised administration interval (13.7% of the patients) and presented different administration intervals from those officially indicated, a practice that can lead to conditions of systemic toxicity. However, the study shows that most drugs were within the recommended therapeutic range. Regarding off-label drugs therapeutic classes, a higher frequency was observed for antispasmodic (31.5%), antiasthmatic (20%) and antibacterial drugs (18.6%). Table 4 shows the frequency of therapeutic classes related to the non-standardized items of the prescriptions; the administration interval not was considered in the table.
Distribution of therapeutics classes of drugs, according to their off-label use
| Therapeutic class | Dose 127 (%) | Age 242 (%) | Indication 424 (%) | Adm. form 67 (%) | Presentation 83 (%) | Total 1105 (%) |
| Antispasmodic | 14 (11.0) | 1 (0.4) | 207 (48.8) | 22 (32.8) | 1 (1.2) | 245 (22.2) |
| Antiasthmatic | 32 (25.2) | 72 (29.7) | 26 (6.1) | 0 (0.0) | 5 (6.0) | 135 (12.2) |
| Antibacterial | 33 (25.9) | 12 (4.9) | 57 (13.4) | 9 (13.4) | 0 (0.0) | 111 (10.0) |
| Antiepileptic | 5 (3.9) | 58 (24.0) | 4 (0.9) | 6 (8.9) | 4 (4.8) | 77 (7.0) |
| Antiulcer | 4 (3.1) | 21 (8.7) | 8 (1.9) | 7 (10.4) | 1 (1.2) | 41 (3.7) |
Considering the total number of prescribed items, 126 (6.2%) were classified as high-alert medications. The most prescribed high-alert drugs included: morphine (25.8%), chloral hydrate (15.8%), meperidine (9.2%), ketamine, codeine and promethazine (8.3%). No significant difference was observed in relation to the presence of high-alert medications in the prescriptions, considering the different age groups of the patients (x2 = 0.41; p= 0.815). Similarly, the chance of a drug being classified as high-alert was not higher for unlicensed and off-label drugs (1.11 CI 95%; 0.76 – 1.62). The ratio between polypharmacy and high-alert medications showed that there is less chance of potentially dangerous drugs being used with patients exposed to fewer number of drugs (0.38 CI 95%; 0.19 – 0.74).
DiscussionThe study attempted to describe the prescription profile of a general pediatric inpatient unit that assist patients between 1 month and 14 years old in southern Brazil.
Among the most prescribed therapeutic classes in general pediatric units, according to a study conducted by Hsien et al, the antibacterial drugs of systemic use accounted for 25% of the items, the drugs for the respiratory system represented 19% and the analgesic drugs represented 15% of items15. According to Santos et al, these classes represented 68.8%, 33% and 51.5%, respectively20. In the study, analgesic drugs were the most prescribed drugs, due to recommendation (if necessary) and to postoperative use, for approximately 27% of the items. Antibacterial drugs presented a lower percentage (13.5%) than that found in previously mentioned studies, possibly indicating a rational antibiotic therapy. This difference can also be attributed to the type of study and period of prescription analysis.
Paracetamol was mentioned in many studies as the most prescribed drug in pediatric units, ranging from 7 to 14% of the items.14,21 It is rarely prescribed as an off-label drug but some studies report its non-compliant use with official standards, especially in terms of dosage.12,20,22 An Italian study, comparing paracetamol dosages in two official references, verified a difference in the recommended dosages for children of less than 1 year of age, which could result in underdoses.22 Our findings showed that 7 patients were prescribed a dosage of paracetamol which was below that indicated in the literature (10–15mg/Kg/dose). This is possibly due to the non-adjustment of the dose based on body weight, since some patients came from other units, such as emergency and pediatric intensive care.
Salbutamol is also mentioned as an off-label drug in terms of age, which increases the chances of developing adverse reactions in children, being used mainly in primary care units.12,23,24 The off-label use of salbutamol, both in terms of age and administration intervals, can lead to problems in handling asthmatic patients, which can be harmful due to the use of overdoses and the development of future adverse effects.21,22,24
Regarding the prescription of unlicensed drugs, in Brazil dipyrone is largely used and freely traded with no need for a doctor's prescription. It is available in solid and oral liquid formulations, suppositories and injections, and is part of the country's list of essential drugs. However, it is carefully administered in hospitals as a postoperative analgesic drug and as an antipyretic drug in cases of refractory fever to other anti-thermal drugs.11,25
The frequency of off-label and unlicensed drug use found in this study is according to the results of other studies performed in general pediatric units. Di Paolo et al. reported a use of 25% of off-label drugs and 24% of unlicensed drugs in pediatric units of a Swedish hospital. However the study included, besides general pediatric units, intensive care units, neonatology and surgery units, which may explain the high prevalence of unlicensed drugs.14 T¿Jong et al. identified in general pediatric units and a neonatology unit of a Dutch hospital that 28% of the prescribed drugs were unlicensed and 44% were of off-label and that patients under 6 months old presented a higher chance of using these drugs.26
In India, Jain et al. reported a high prevalence of off-label drugs (50.6%) in children in general pediatric units.27 A German study verified that 61% of the patients in general pediatric units received at least one off-label or unlicensed drug and that 34% of the drugs were off-label in terms of age for patients receiving cardiovascular drugs.15 In Brazil, a cohort study of 272 children of general units, from zero to 16 years of age, in a university hospital, identified that approximately 22% of them received at least one unlicensed drug and 60% received at least one off label drug during the hospitalisation period. With regard to off-label drugs (39.6%), approximately 17.7% were prescribed with off-label doses and administration frequencies.20
The use of high-alert medications was also evaluated in pediatric prescriptions as there are few studies on the use in this population and many factors contribute to more effects in children than in adults, such as the calculation of doses based on the body weight, the need for drug dilutions and the patients’ hepatic and renal immaturity.28 According to the Institute of Health Care Improvement, around 58% of the problems caused by drugs in hospitals are caused by high-alert medications.29,30 These drugs, which are frequently used in emergency and intensive care units, present off-label usage in terms of dosages, administration forms, formulation and age. This occurs mainly due to the absence of an official standard, which makes the tendency for error and adverse reactions higher.3,28,30
High-alert medications include anesthetic, antiarrhythmic, antithrombotic, chemotherapeutic, hypoglycemic, opioid analgesic, benzodiazepinic and neuromuscular blocking drugs.18 The frequency of high-alert medications in pediatric prescriptions was higher in terms of opioid analgesic drugs (35%) prescribed during and after surgical procedures. There are no studies reporting the use of high-alert medications with off-label use drugs. In addition, the current study did not show any significant association between the use of off label drugs and unlicensed drugs with high-alert medications. On the other hand, the use of polypharmacy favors the use of high-alert medications, which can lead to adverse reactions in children.17,28
In Brazil, ANVISA is particularly concerned with the high prevalence of off-label drugs use in the country. The agency is seeking to identify these drugs through pharmacovigilance programs and notification of adverse reactions. Also, in relation to high-alert drugs, ISMP-Brazil (Institute for Safe Medication Practices of Brazil) is developing a system of medication errors and adverse reactions notification, aiming to have a better view of the Brazilian reality and, through these indicators, to establish strategies to prevent problems related to these drugs in the health system.
The cross-sectional model of the study could answer the proposed questions. However, for the results evaluation, it should be noted that the study was conducted in a university hospital that is a reference for the treatment of several diseases, with an intense development in clinical research. The limitations of this study may be related to the single source of inquiry used in the verification of drug approval criteria. In addition, in the absence of data on the records, the multidisciplinary group interference might have influenced the answers of the prescribers.
Despite the initiatives of drug agencies to encourage the research and development of drugs for use in children, ensuring safety and effectiveness, there is actually a very real prevalent use of potentially unsafe drugs, mainly off-label, in hospitals and primary care units. The use of these drugs without official approval should be prevented or controlled using protocols, since long-term effects of these medications are unknown in patients under 18 years of age. There are few studies on the use of off-label and/or unlicensed drugs in Brazil and there is no study which reports the use of high-alert medications.
Results of this study, combined with data already published, support the need to elaborate lists of priority drugs to be included in researches and studies aiming to establish effectiveness and safety for their use in populations not yet investigated. Thus, understanding clinical practice needs and with incentives from drug labs, further studies may contribute to the reduction of the high prevalence of unlicensed and off label drug use that is observed. The development and implementation of drug usage protocols in pediatric units may be useful to minimize risks.
Conflict of interestThe authors have no conflict of interest to declare.