Two carvedilol aqueous solutions and one carvedilol aqueous suspension for paediatric oral use (1mg/ml) were studied to determine their stability.
MethodAll samples were stored at 4, 25 and 40°C. Carvedilol content of each of the three formulations was tested using high performance liquid chromatography (HPLC). Each sample was analysed in triplicate at 0, 3, 7, 14, 28 and 56 days.
ResultsCarvedilol stayed stable in the acidic aqueous solution at the three different temperatures during the 56 days of the study. In the alkaline solution, carvedilol was stable during 56 days at 25°C, but only 28 days at 4 and 40°C. In the aqueous suspension, carvedilol was stable during 56 days at 4 and 25°C, but only 28 days at 40°C.
ConclusionsAll the formulations that were tested can be stored at 25°C for at least 56 days.
Se estudió la estabilidad de carvedilol (1mg/ml) en 2 soluciones acuosas y una suspensión acuosa para uso pediátrico.
MétodoLas formulaciones fueron almacenadas a 4, 25 y 40°C. El contenido de carvedilol de cada una de las 3 formulaciones fue analizado por cromatografía líquida de alta eficacia (HPLC). Cada muestra fue analizada por triplicado a tiempos 0, 3, 7, 14, 28 y 56 días.
ResultadosCarvedilol se mantuvo estable en la solución acuosa de pH ácido durante los 56 días del ensayo a las 3 temperaturas estudiadas. En la solución alcalina fue estable 56 días a 25°C, pero sólo 28 días a 4 y 40°C. En la suspensión acuosa carvedilol fue estable 56 días a 4 y 25°C, y sólo 28 días a 40°C.
ConclusionesTodas las formulaciones ensayadas pueden ser conservadas a 25°C al menos por un período de 56 días.
Although oral pharmaceutical forms such as tablets or capsules are accepted by adult patients without major problems, the development of easily accepted formulas for paediatric patients is a constant challenge for the formulation pharmacist. The development of multiple pharmaceutical forms for different ages is hardly viable on a commercial scale, especially for liquid formulations.1 Nowadays, we have both a limited number of clinically approved drugs and a limited availability of liquid formulations for paediatric patients. Doses for children are adjusted by body weight, which presents quite an inconvenience when the formulation is displayed only in solid dosage form. For this reason, preparing extemporaneous liquid formulations using tablets is one of the most common practices employed to adjust doses for paediatric patients.2
Carvedilol is a non-selective beta-adrenergic blocking agent used clinically for treating cardiovascular diseases (heart failure and hypertension).3 Its vasodilator properties are attributed to α1-blocking activity, as is its capacity to inhibit oxidative stress in coronary smooth muscle. Although carvedilol has been tested only in adults, several studies report that it is effective in children with heart failure.4–7 In addition, carvedilol displays poor aqueous solubility (10μg/ml to 25°C),8 which makes it difficult to formulate as an oral liquid solution.
The only available oral formulation of carvedilol is in dosage tablet form. Since an oral liquid carvedilol formulation is not available on the market, an extemporaneous liquid formulation can be beneficial, especially for patients who are unable to swallow tablets, who receive medicine nasogastrically or by gastrostomy. The availability of liquid formulations is essential to paedriatric pharmacotherapy; children younger than 7 years old are not usually able to swallow solid medicine.9 A liquid formulation would therefore favour proper dose adjustment and avoid nurses and caretakers having to mix the powdered tablets with water or another liquid to administer the dose. This practice increases the potential for dosage mistakes, and complicates treatment adherence. Yamreudeewong et al.10 and Allen Loyd11 prepared extemporaneous suspensions by reconstituting crushed tablets in deionized water and sorbitol 70% in the first case and with Ora Sweet® and Ora Plus® in the second case. These suspensions are prepared with resulting carvedilol concentrations of 0.625 and 1.25mg/ml, respectively. Concentrations with decimals, as mentioned previously, can lead to potential errors by nurses and caretakers when they calculate the necessary dosage according to body weight.
The purpose of this study was to develop two aqueous carvedilol solutions at different pH levels (4.2 and 8.2) and an aqueous suspension using the pure drug to eliminate the need for manipulating commercial tablets for oral administration in paedriatic patients. We then studied the stability over 56 days of formulations stored at 4, 25 and 40°C. Both the solutions and the suspension prepared in this study have a final carvedilol concentration of 1mg/ml. This concentration makes it easier to calculate dosage according to body weight than the concentrations used in previous studies.
Material and methodsMaterialThe carvedilol solutions (1mg/ml; Parafarm batch: 040401) were prepared by solubilising 0.5% w/v of polyvinylpyrrolidone (PVP-K30) (D. Prest, batch: LTX61215) in 40ml of propylene glycol (Van Rossum SRL, batch: TRK01270909) at 50°C. Once PVP was dissolved, 0.1% w/v of carvedilol was added. It was then cooled and 10ml of glycerin was incorporated (D. Prest, batch: 090505), to which 0.1% w/v raspberry essence had been added previously. Next, 40ml of sorbitol (Van Rossum SRL, batch 808181760) was added and homogenised. Lastly, 0.3% w/v of saccharin sodium (Stanton, batch: 40704) was added and the volume was made up using distilled water (100ml). The resulting alkaline solution had a pH of 8.2 (F1). To obtain an acidic solution, 0.1% w/v of citric acid was added (Magel, batch: A06111101), resulting in a pH of 4.2 (F2). The suspension (F3) was prepared to yield the same carvedilol concentration (1mg/ml) in an aqueous suspension vehicle without sucrose (similar to Ora Plus®). This vehicle was prepared with xanthan gum 0.2% w/v, carboxymethylcellulose 0.025% w/v, glycerin 2% v/v, sorbitol 70% solution USP 5% v/v, saccharin sodium 0.2%, citric acid 0.1%, dibasic sodium phosphate 0.06%, methylparaben 0.1%, potassium sorbate 0.15% and simethicone 0.1% v/v. All of the trial formulations were stored in amber glass vials and kept refrigerated (4°C), at controlled room temperature (25°C) and heated (40°C).
Analytical methodCarvedilol content of the formulations was tested using high performance liquid chromatography (HPLC). The liquid chromatographic system consisted of an isocratic solvent delivery pump (Shimadzu LC-20AT) that pumped a mixture of (A:35;B:65) (A: acetonitrile and B: phosphate buffer, pH: 2.0) through a 15-cm×4.6-mm reverse-phase C-8 5μm column (RP-8 Microsorb-MV 100-5) at 1.0ml/min. The column was maintained at 55°C. Twenty microliter of this sample was introduced into the liquid chromatographic system using an injector (Rheodyne 7725i). The column effluent was monitored with a variable wavelength ultraviolet detector (Waters 486) at 285nm.
To establish the stability-indicating nature of the method, carvedilol (1mg/ml) was subjected to forced acidic degradation (1N HCl), basic degradation (1N NaOH) and oxidation (10% H2O2) at a temperature of 100°C for 1h. The analytical method validation was carried out according to the specifications in USP 31, chapter 1225.12
Aliquots were collected from each container on days 0, 3, 7, 14, 28 and 56. These were diluted with HPLC mobile phase and immediately analysed.
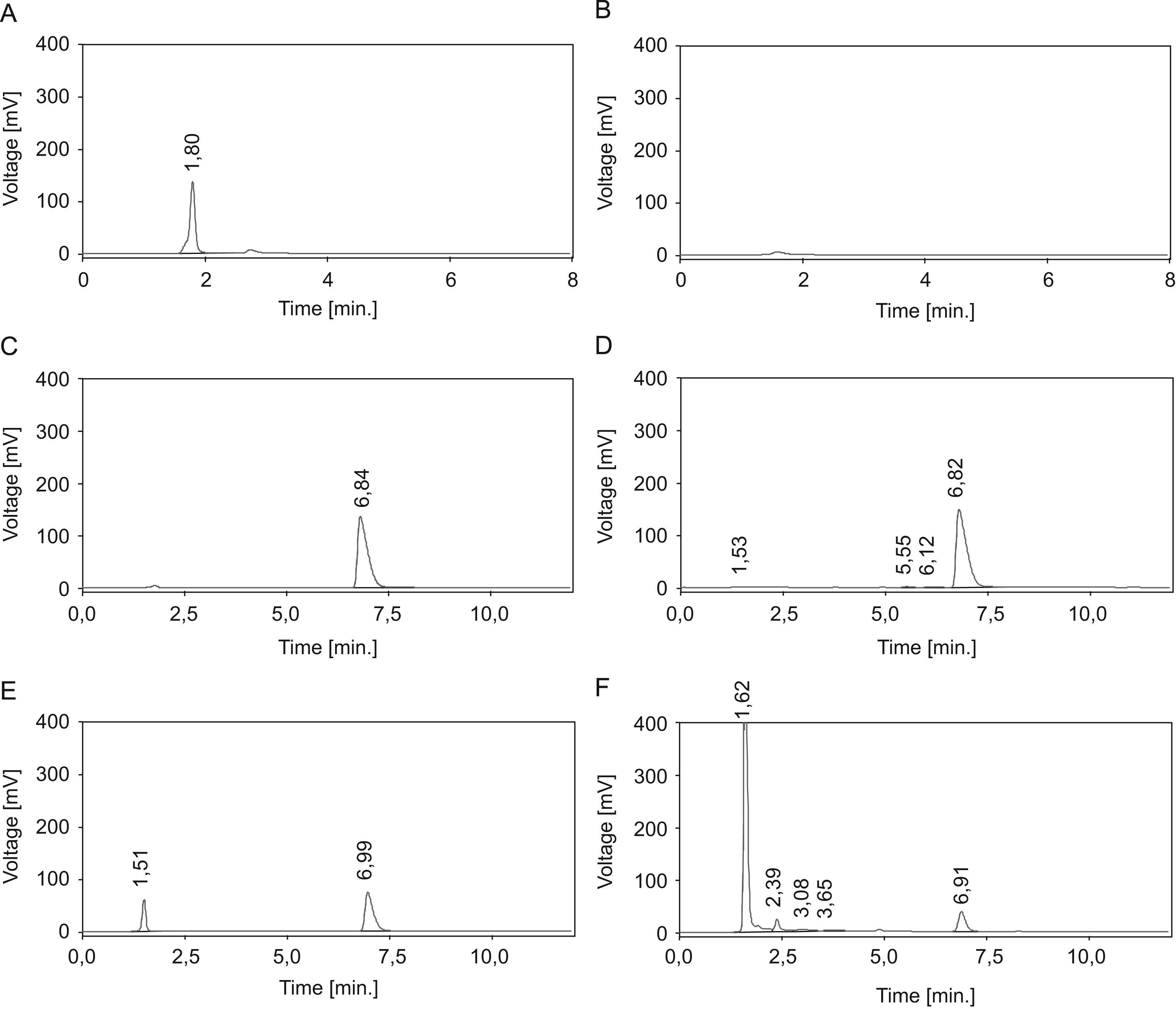
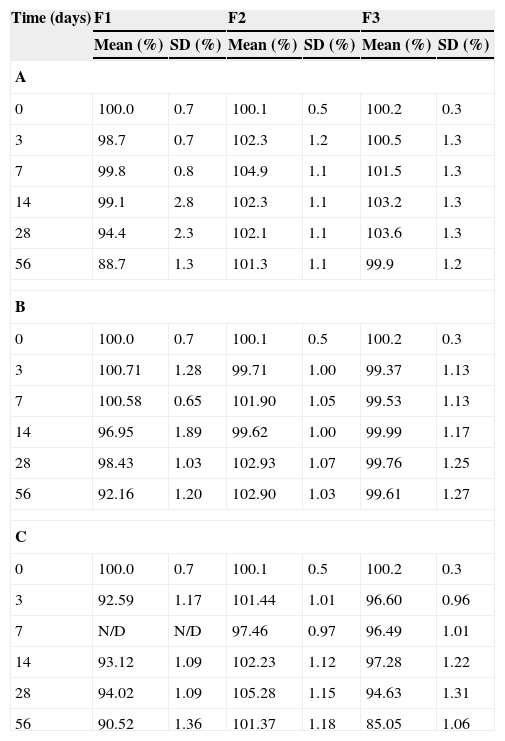
ResultsFigure 1 shows the chromatograms corresponding to the solution without carvedilol (A), suspension without carvedilol (B), standard carvedilol solution (40μg/ml) (C), carvedilol subjected to acidic degradation (D), carvedilol subjected to alkaline degradation (E) and carvedilol subjected to oxidation (F). Carvedilol retention time is 6.8min. Figure 1 shows that there is no interference in carvedilol's standard retention time, degradation products or solution and suspension controls. The linearity can be established by the relationship between the concentrations and areas on the standard chromatogram, and is shown by a linear regression model whose intercept (y-axis) is 10.244 with a slope of 54.208 and R2=0.9999. The relative error and the CV (coefficient of variation) were lower than 10%. The method precision is 95.4±0.29%, and it is linear in the 20–64μg/ml range of concentrations: with an accuracy of 98.15±2.98%. The method was able to separate the carvedilol peak from the degradation peaks. To assess carvedilol stability in paedriatic oral formulations, we prepared two aqueous solutions with different pH levels (4.2 and 8.2) and one aqueous suspension. In several cases, the drugs have less stability in solutions than in suspensions. This is because drug hydrolysis rates in aqueous solutions are greater than in solid state.11 In our case, the percentage of remaining carvedilol fell bellow 90% in 56 days in the F1 sample. In F2 and F3 the percentage of remaining carvedilol stayed above 99% during the 56 days of the study (Table 1A). A pronounced effect on the solutions can be observed in solutions with an approximately medium pH, since carvedilol is more stable in an acidic pH (F2) than an alkaline one (F1). At room temperature, all the three formulations kept the percentage of remaining carvedilol above 90% during the 56 days of the study (Table 1B). The aqueous suspension is the only formulation in which the percentage of remaining carvedilol fell below 90% at 40°C (Table 1C).
Mean percentage of initial carvedilol concentration at 4°C (A), 25°C (B) and 40°C (C), for all conditions (n=3).
| Time (days) | F1 | F2 | F3 | |||
| Mean (%) | SD (%) | Mean (%) | SD (%) | Mean (%) | SD (%) | |
| A | ||||||
| 0 | 100.0 | 0.7 | 100.1 | 0.5 | 100.2 | 0.3 |
| 3 | 98.7 | 0.7 | 102.3 | 1.2 | 100.5 | 1.3 |
| 7 | 99.8 | 0.8 | 104.9 | 1.1 | 101.5 | 1.3 |
| 14 | 99.1 | 2.8 | 102.3 | 1.1 | 103.2 | 1.3 |
| 28 | 94.4 | 2.3 | 102.1 | 1.1 | 103.6 | 1.3 |
| 56 | 88.7 | 1.3 | 101.3 | 1.1 | 99.9 | 1.2 |
| B | ||||||
| 0 | 100.0 | 0.7 | 100.1 | 0.5 | 100.2 | 0.3 |
| 3 | 100.71 | 1.28 | 99.71 | 1.00 | 99.37 | 1.13 |
| 7 | 100.58 | 0.65 | 101.90 | 1.05 | 99.53 | 1.13 |
| 14 | 96.95 | 1.89 | 99.62 | 1.00 | 99.99 | 1.17 |
| 28 | 98.43 | 1.03 | 102.93 | 1.07 | 99.76 | 1.25 |
| 56 | 92.16 | 1.20 | 102.90 | 1.03 | 99.61 | 1.27 |
| C | ||||||
| 0 | 100.0 | 0.7 | 100.1 | 0.5 | 100.2 | 0.3 |
| 3 | 92.59 | 1.17 | 101.44 | 1.01 | 96.60 | 0.96 |
| 7 | N/D | N/D | 97.46 | 0.97 | 96.49 | 1.01 |
| 14 | 93.12 | 1.09 | 102.23 | 1.12 | 97.28 | 1.22 |
| 28 | 94.02 | 1.09 | 105.28 | 1.15 | 94.63 | 1.31 |
| 56 | 90.52 | 1.36 | 101.37 | 1.18 | 85.05 | 1.06 |
F1: alkaline carvedilol solution; F2: acidic carvedilol solution; F3: carvedilol suspension.
Extemporaneous formulations tend to be one of the most commonly used alternatives when adequate forms for paediatric use or for patients with trouble swallowing tablets are not available. Suspensions, unlike solutions, need to be shaken to ensure content uniformity.13 Generally, no stability data are available for use in hospitals. The results of our study agree with those obtained by Yamreudeewong et al., for controlled room temperature (25°C) and refrigeration (4°C), in that carvedilol concentration in an extemporaneous suspension remained above 90% at controlled room temperature and refrigerator temperature for 8 weeks. Rizwan et al.14 observed that carvedilol is more susceptible to degradation in an alkaline medium than an acidic one. This behaviour agrees with our results—we observed that F1 (alkaline pH) shows a greater drop in the percentage of remaining carvedilol than F2 at all study temperatures.
Stability data at controlled room temperature are of real importance, since it is not necessary to use special storage conditions during distribution and storage of the formulation at the hospital.
In conclusion, oral liquid carvedilol formulations for paediatric use can be kept at 25°C (room temperature) for at least 56 days without adverse effects.
The authors acknowledge the financial support for this work provided by UBACyT 2008–2010 (Grant B003).