To describe the epidemiological characteristics of a large tuberculosis outbreak in the university environment and the main risk factors associated with it.
MethodA descriptive analysis of the data collected from sick individuals and their contacts was made. For the contact tracing, the guidelines established in the Tuberculosis Program of the Autonomous Community of the Basque Country were followed. Six of the outbreak strains were sent to the National Centre of Microbiology for molecular typing.
ResultsThe total number of cases of the outbreak was 11. The rate of tuberculosis infection in the classroom of the index case, including the sick individuals, was 88.1% (59 infected and only 8 uninfected). The diagnostic delay of the index case was 260 days, and in the other 8 symptomatic cases it ranged between 10 and 70 days. The pattern obtained by the 2 genotyping techniques was identical in the 6 strains studied.
ConclusionsThe long diagnostic delay of the authentic index case, which was diagnosed in the contact tracing, and the poor ventilation conditions of the classroom, determined the high number of secondary cases associated with this outbreak.
Describir las características epidemiológicas de un importante brote de tuberculosis en el ámbito universitario y los principales factores de riesgo asociados.
MétodoSe realizó un análisis descriptivo de los datos recogidos de las personas enfermas y de los contactos. Para el estudio de contactos se siguieron las pautas establecidas en el Programa de Tuberculosis de la Comunidad Autónoma del País Vasco. Seis de las cepas del brote fueron enviadas al Centro Nacional de Microbiología para su tipado molecular.
ResultadosEl número total de casos del brote fue de 11. La tasa de infección tuberculosa en el aula del caso índice, incluidas las personas enfermas, fue del 88,1% (59 infectados y solo 8 no infectados). La demora diagnóstica del caso índice fue de 260 días, y en los otros 8 casos sintomáticos osciló entre 10 y 70 días. El patrón obtenido por las 2 técnicas de genotipado fue idéntico en las 6 cepas estudiadas.
ConclusionesLa gran demora diagnóstica del caso índice auténtico, que se diagnosticó en el estudio de contactos, y las malas condiciones de ventilación del aula determinaron el alto número de casos secundarios asociados a este brote.
Tuberculosis (TB) is a communicable disease that remains a major public health concern worldwide. Its incidence has decreased in the Basque Country by approximately 5% per year over the last 10 years, reaching a rate of 12.7 cases per 100,000 people in 2016. According to the National Epidemiological Surveillance Network, the overall rate in Spain was 10.8 per 100,000 in 2014.1
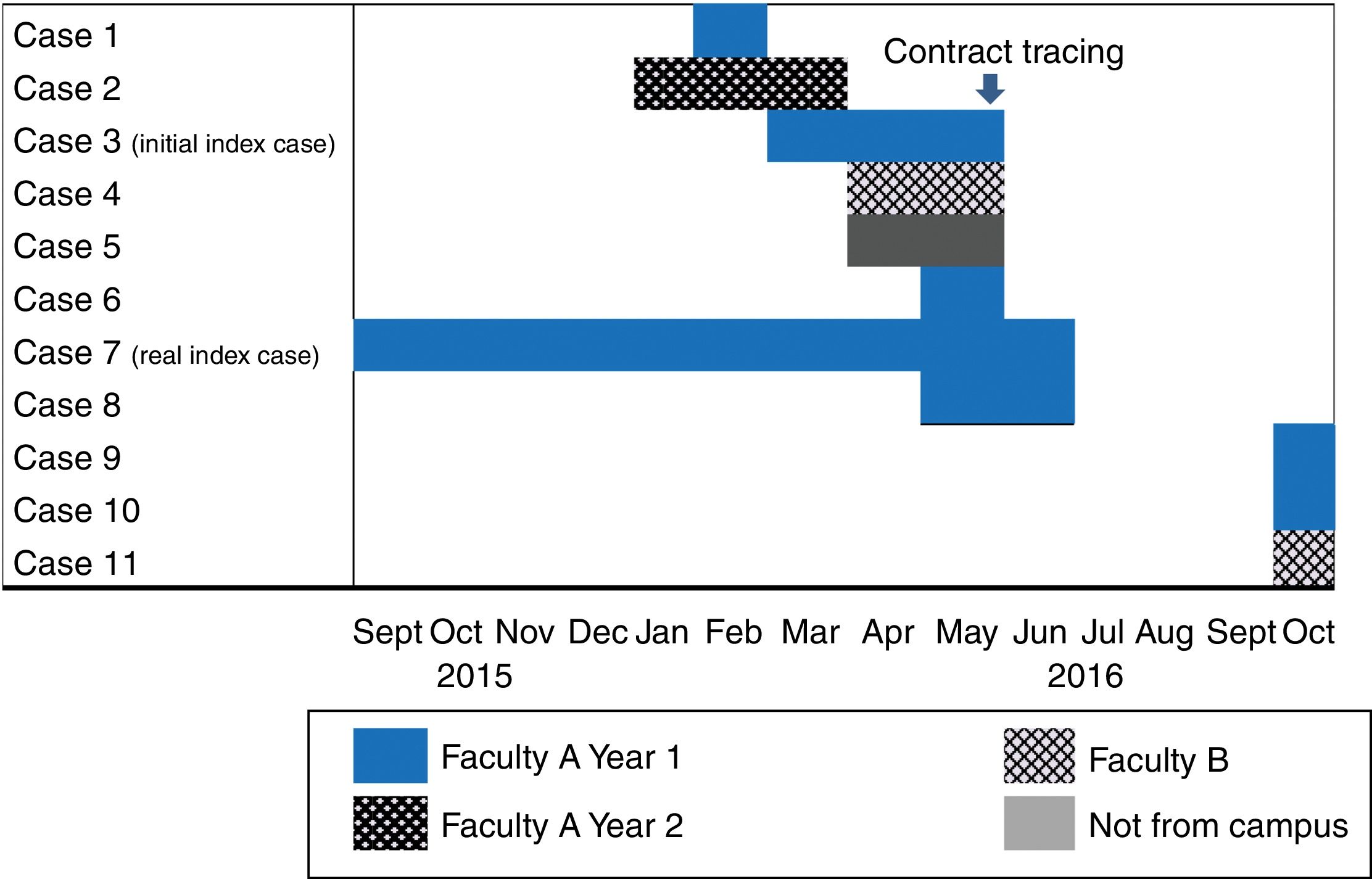
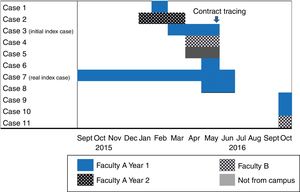
TB outbreaks are common in educational institutions, including universities.2–4 We will look at an outbreak that occurred on the Leioa university campus (Vizcaya) at the University of the Basque Country, which accounted for a total of 10 secondary cases over a period of one year, the largest outbreak ever recorded at a university in our country.
The initial index case was an 18-year-old student diagnosed in May 2016 with pulmonary TB with positive sputum smear and culture of bronchial washings, with cavitation observed on X-rays. No cases of tuberculous were detected in his family medical history. He was in the first year of an undergraduate degree taught in faculty A on the campus. In February 2016, another student from the same class had been diagnosed with pleural TB.
The week following diagnosis of the index case, contact tracing was implemented on university premises to identify all students and teaching staff involved in his class. A student who was ill and later diagnosed with pleural TB by his local hospital did not attend for screening.
In addition to these 3 cases, a student from a different course and a different degree, also at faculty A, had been diagnosed with bacilliferous pulmonary TB 2 months earlier. Contact tracing included a total of 40 people from the student's family, social and university environment but only one case of latent tuberculosis infection (LTBI), affecting a sexual contact, was detected.
During contact tracing, we learned that a female student from another faculty (B), on the same campus, had also been diagnosed with pleural TB by a hospital outside our autonomous community.
On discovering this microepidemic, contact tracing was extended to include students from other courses who shared at least one subject with the index case and students living at the same hall of residence.
In October 2016, after completing contact tracing, another student from faculty B but from a different degree course was diagnosed with pleural TB.
Contact tracing was carried out in accordance with the guidelines established in the Tuberculosis Control Program of the Autonomous Community of the Basque Country.5 The aim was to find the source of the outbreak, detect possible cases of active disease and start rapid treatment, where appropriate, and to diagnose and prescribe preventive treatment to all cases of LTBI.
MethodsFor contact tracing, the concentric circle approach was followed and students from the same class as the index case, with a mean daily contact time of 5h, 5 days a week, were included in the first circle. A Mantoux tuberculin skin test was performed with 2 TU of PPD RT23. LTBI was considered if the Mantoux skin test was positive (≥5mm of induration) 48h after administration, regardless of vaccination history. An interferon-gamma test (IGRA) (QuantiFERON-TB Gold) was also performed at the same time as the Mantoux test. Those with a negative result in the two tests were tested again 8 weeks after their last contact, with the Mantoux conversion considered when an increase in induration of ≥6mm from the previous test was observed. LTBI was considered if any of the tests were positive. All individuals had a chest X-ray.
The second circle comprised students from other courses who shared any subject with the index case, the teaching staff and students living at the same hall of residence. In this case, an IGRA was performed and those with positive results had a chest X-ray.
Medical staff specialising in Pneumology were responsible for diagnosing active or latent disease and, if applicable, for prescribing therapy.
All individuals affected were asked to complete an epidemiological survey on their everyday habits at the university in order to find an epidemiological link between them: means of transport, schedules, cafeteria, dining room, library, study rooms or other common rooms, memberships at leisure facilities, attendance at parties or other events.
Classroom ventilation conditions were also checked.
Available strains of Mycobacterium tuberculosis were sent to the Mycobacteria Laboratory of the National Centre of Microbiology for PCR-RFLP genotyping of the insertion sequence IS6110 and MIRU24-based genotyping.
Campus-related cases of TB diagnosed during the months following the outbreak were followed and strains were sent to the reference laboratory for genotyping, in case any more cases were associated with the outbreak.
The first patient that indicated the existence of the outbreak is considered the initial index case, and the patient who probably acted as the source of the outbreak is considered the real index case.
Treatment compliance was monitored in those individuals diagnosed with TB and those diagnosed with LTBI, and treatments were classified as completed, refused, lost to follow-up or withdrawn due to adverse effects.
A descriptive analysis of the data obtained for those individuals with TB and their contacts was performed. The attack rate for active and latent disease was estimated using the relative risk and its 95% confidence interval (CI) to assess differences between the different exposure groups. Agreement between the Mantoux test and IGRA was analysed using Cohen's kappa coefficient for those individuals with reported results for both tests. p-Values <0.05 were considered significant. Epi Info version 7.2.2.2 was used.
This study is a public health intervention developed within the context of the TB program of the Basque Country and is based on contact tracing results. Participants were informed verbally and verbal consent was obtained.
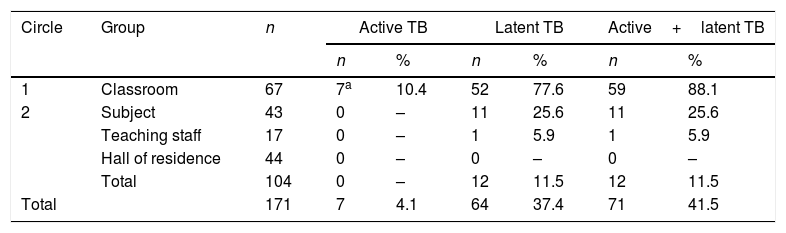
ResultsThe study comprised 64 contacts (45 male and 19 female) from the classroom of the index case, excluding the 3 cases of disease previously diagnosed in the classroom. Their mean age was 18 years. As a result of the study, 4 new cases were detected; therefore, the total number of sick individuals in the classroom, including the index case, was 7, giving an attack rate of 10.4%. The number of individuals with LTBI was 52, giving an LTBI rate of 77.6%, with only 8 out of a total 67 students not infected. The total attack rate for active or latent disease in the classroom was 88.1%. During the first contact tracing, a kappa coefficient of 0.446 was obtained for the 53 individuals with results for both tests (Mantoux test and IGRA): 32 individuals tested positive in both tests while 9 tested negative in both tests (agreement=77%; p<0.01), and the other 12 (8 IGRA [+] and Mantoux [−] and 4 IGRA [−] and Mantoux [+]) showed disagreement.
During the second contact tracing, 133 people were screened, including 104 who participated in the study and 29 (21.8%) who did not, all belonging to the group of students who only shared a subject with the index case. Of the 104 who participated in the study, 15 had LTBI, presenting an infection rate of 14.4%. No sick individual was detected. Among the 44 students who shared the same hall of residence as the index case, no infected person was detected. The attack rate for LTBI among students who shared a subject with the index case was 25.6%. Only one of the 17 professors included was infected (Table 1).
Active or latent tuberculosis according to exposure group.
| Circle | Group | n | Active TB | Latent TB | Active+latent TB | |||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| 1 | Classroom | 67 | 7a | 10.4 | 52 | 77.6 | 59 | 88.1 |
| 2 | Subject | 43 | 0 | – | 11 | 25.6 | 11 | 25.6 |
| Teaching staff | 17 | 0 | – | 1 | 5.9 | 1 | 5.9 | |
| Hall of residence | 44 | 0 | – | 0 | – | 0 | – | |
| Total | 104 | 0 | – | 12 | 11.5 | 12 | 11.5 | |
| Total | 171 | 7 | 4.1 | 64 | 37.4 | 71 | 41.5 | |
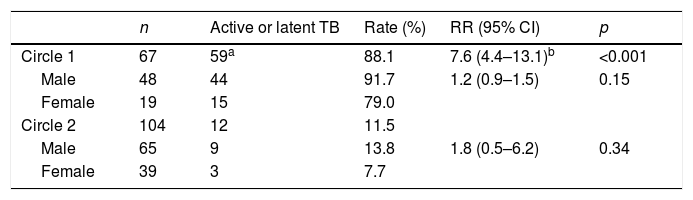
Of the 171 contacts studied, belonging to circle 1 was associated much more with a higher risk of active or latent TB than belonging to circle 2 (relative risk=7.6; 95% CI: 4.4–13.1). The rate of active or latent disease was lower for women, in both circles, but this difference was not statistically significant (Table 2).
After an individual assessment of exposure time, chest X-ray, symptoms and risk of disease, a total of 64 LTBI treatments were prescribed. Of these, 6 were refused, 6 were withdrawn as a result of medical advice due to toxicity, 3 were lost to follow-up and 49 were successfully completed (76.6%).
Among the cases detected during contact tracing, one had started with respiratory symptoms in September of the previous year, at the beginning of the course, and was therefore considered the real index case (Fig. 1). This was a case of bacilliferous TB that showed cavitations on the chest X-ray. This student took the same bus from the university, and at a similar time, as the first case from faculty B. He also had a friend who did not study at the university who had also been diagnosed with pleural TB at a private clinic in April.
No epidemiological links of interest were found between those cases detected in the classroom of the real index case in faculty A and the student from a different degree course within the same faculty. They did not attend classes at the same time, did not have the same professors and did not use the same leisure facilities outside the university. No link was found between the real index case and the last case diagnosed from faculty B either.
The ventilation conditions of the classroom used for the course attended by the index case were very poor, with no forced ventilation and no opening windows, despite having large windows.
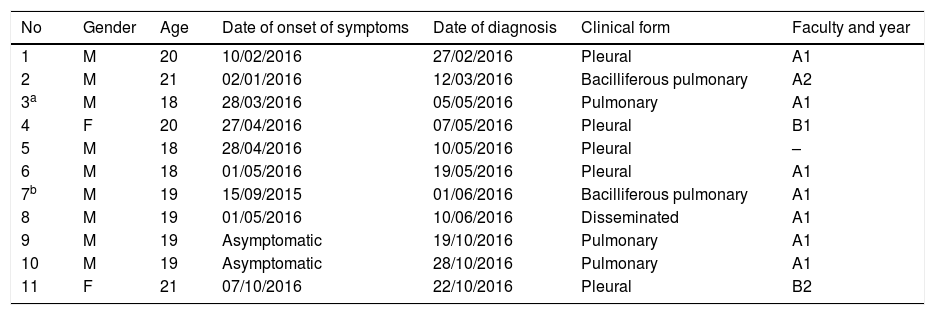
Patient characteristicsThere are 11 cases of TB linked to this outbreak: 2 females and 9 males aged between 18 and 21 years. These were diagnosed at 8 different public hospitals, 2 of which were outside our autonomous community, and one at a private hospital. The clinical form of the disease was pleural in 5 cases, pulmonary in 5 cases and disseminated, pleural, pulmonary and abdominal in one case. Two of the cases had positive sputum smear results and another two had positive sputum smear and culture in bronchial washings. Four were diagnosed during contact tracing, with two of these diagnosed during the second part of the study, which took place after the summer break. Diagnostic delay after the onset of symptoms for the 9 symptomatic cases ranged between 10 and 260 days, with a median of 18 days. None of the cases required hospitalisation (Table 3).
Cases of tuberculosis per date of diagnosis.
| No | Gender | Age | Date of onset of symptoms | Date of diagnosis | Clinical form | Faculty and year |
|---|---|---|---|---|---|---|
| 1 | M | 20 | 10/02/2016 | 27/02/2016 | Pleural | A1 |
| 2 | M | 21 | 02/01/2016 | 12/03/2016 | Bacilliferous pulmonary | A2 |
| 3a | M | 18 | 28/03/2016 | 05/05/2016 | Pulmonary | A1 |
| 4 | F | 20 | 27/04/2016 | 07/05/2016 | Pleural | B1 |
| 5 | M | 18 | 28/04/2016 | 10/05/2016 | Pleural | – |
| 6 | M | 18 | 01/05/2016 | 19/05/2016 | Pleural | A1 |
| 7b | M | 19 | 15/09/2015 | 01/06/2016 | Bacilliferous pulmonary | A1 |
| 8 | M | 19 | 01/05/2016 | 10/06/2016 | Disseminated | A1 |
| 9 | M | 19 | Asymptomatic | 19/10/2016 | Pulmonary | A1 |
| 10 | M | 19 | Asymptomatic | 28/10/2016 | Pulmonary | A1 |
| 11 | F | 21 | 07/10/2016 | 22/10/2016 | Pleural | B2 |
UPV Outbreak, 2016.
The diagnostic delay for the real index case was 260 days. Symptoms first appeared at the start of the university year with a non-specific cough, coughing up phlegm and an episode of rib cage pain. The patient saw his Primary Care physician for the first time in January and was diagnosed as having an upper airway infection. The patient went back to the doctor with the same symptoms twice, at the end of January and in May. No antibiotic treatment was prescribed. He was finally diagnosed during contact tracing. His chest X-ray showed cavitation and sputum smear microscopy showed abundant bacilli.
All cases completed their treatment satisfactorily with cure.
Microbiological studyAll of the strains that were isolated were susceptible to all first-line drugs. Available strains were sent to the mycobacteria reference laboratory of the National Centre of Microbiology: three of the cases from the classroom of the real index case from faculty A, including that of the real index case, the two strains isolated from those cases from faculty B and the strain isolated from the friend of the real index case who did not study on campus. The pattern obtained by the 2 genotyping techniques was identical in the 6 strains studied. The first results were received in July, with 5 identical strains. The strain of the last case diagnosed was sent to the laboratory later in January and was confirmed to also belong to the outbreak.
DiscussionIn this outbreak, the most plausible hypothesis is that the source of infection was the student from the classroom of the initial index case from faculty A, who was diagnosed during contact tracing. This student had baciliferous TB and showed symptoms from the start of the university year and probably infected his classmates, the other student from the same faculty but a different degree course, his friend from outside the university and the two students from faculty B, one of whom used to take the same bus at the same time. We cannot rule out the possibility that some cases in the outbreak were tertiary cases since there was one secondary case, which was also bacilliferous, and two cases with positive sputum smear and culture of bronchial washings.
The main determining factor for this outbreak within the university environment has, without a doubt, been the significant diagnostic delay of the real index case. This is a factor that is frequently mentioned in outbreaks in school environments, especially in countries with a low incidence of TB.6–9 In recent years, outbreaks in Spain have also been described in which diagnostic delay has been a significant contributing factor.10–14 A progressive decrease in the incidence of this disease in our country makes diagnosis increasingly rare. Therefore, strategies are required to remind both professionals and the population in general that TB is still a public health concern in our country. Professionals should suspect TB if a patient has a cough and is coughing up phlegm for more than 15 days.
The second factor that promoted transmission of the TB bacillus in the classroom was poor ventilation. Natural ventilation is natural air movement to achieve dilution and removal of infectious droplet nuclei. For this reason, ventilation through open windows is a very effective environmental control measure for TB prevention. This is not the first outbreak described in the school setting in which this factor has been a determining factor for its spread.15,16 Therefore, we must ensure that educational authorities and the population in general are aware of the importance of this effective measure.
The high prevalence of infection and disease observed in this outbreak indicates that, after a long period of exposure in a crowded environment, the rate of infection and disease can be extremely high.17–19 In a review of 95 contact tracing studies from low- and middle-income countries, the prevalence of TB found among sexual contacts was 51.5%.20
Given the high risk of the group studied, the team responsible for contact tracing decided to perform Mantoux tests and IGRA on the sexual contacts of those individuals in circle 1 in order to increase sensitivity. Agreement observed between the two tests performed on sexual contacts of those individuals in the first circle is similar to that obtained in a study specifically conducted for this purpose,21 which was 72% among high-risk contacts with a kappa coefficient of 0.473.
In our TB control program, contact tracing of pleural cases is only recommended when such cases involve children. We must review this point because, if we had carried out contact tracing when the first pleural case was diagnosed, two months prior to diagnosis of the initial index case, we could have avoided cases of disease and undoubtedly infection.
One of the problems with TB contact tracing is usually poor compliance with the treatment prescribed to infected people. The Galician TB program reported 77.3% LTBI treatment compliance, while the Barcelona TB program reported 68.9% compliance.22,23 Only 61% of the 50,000+ people prescribed LTBI treatment over an 8-year period in Texas complied with their treatment.24 In our case, LTBI treatment compliance was good.
The molecular epidemiology study of the outbreak strains was a determining factor in seeing whether cases that were not from the same classroom as the index case belonged to the outbreak or not, and its usefulness has also been described in the study of other outbreaks reported in schools in Spain.25
The outbreak had a major impact and generated great concern among the university's education community and their families. To minimise this concern and ensure that the study could be conducted without issues, good coordination between the public health team responsible for the study and the university authorities was critical.
The cases were diagnosed at 8 different health centres, which hindered the flow of information, but this was not relevant for the purpose of outbreak management. Coordination of the 5 laboratories that stored the strains associated with the outbreak prior to dispatch to the reference laboratory was very effective.
This investigation had several limitations. The first limitation was the difficulty of identifying actual contact-persons. The lists provided by the university authorities included students enrolled in any subject taught as part of the course studied by the index case, but it was not possible to distinguish who actually attended class regularly. The reason some students did not take part in the study was probably because they did not attend class on a regular basis.
Furthermore, contact tracing at this age is complicated because individuals have multiple interrelationships and take part in mass-attendance social activities in which it is difficult to identify contacts.8,26 This may be why we were unable to determine epidemiological links between the real index case and any of the secondary cases.
In our autonomous community, molecular characterisation of TB strains is not carried out systematically and we therefore do not know if this group of cases is an isolated case or if it belongs to a larger cluster. It would be interesting to find out whether this strain is responsible for other clusters of cases.
Conflicts of interestNone declared.
We would like to thank: The case management nurses and staff from the Pneumology Departments of the Basque Country who helped manage the cases and contacts in each area;
The Álava and Guipúzcoa Epidemiology Departments and the Epidemiology Departments of Navarra;
The Microbiology Laboratories of the Osakidetza Hospitals, Hospital Complex of Navarra and the Medikosta Laboratory;
And the Dean's Office of the Faculty of Science and Technology of UPV, which helped organise contact tracing.
Please cite this article as: Castells Carrillo C, San José Rodríguez S, López Aranaga I, Ciruelos Ayuso E, Garrós Garay J, Jiménez Pajares MS, et al. Demora diagnóstica como principal factor contribuyente a un importante brote de tuberculosis en una universidad. Enferm Infecc Microbiol Clin. 2019;37:496–501.