Descriptive epidemiology of Yersinia enterocolitica infection in an area of Castellón (Spain) between 2006 and 2013 from Yersinia enterocolitica strains isolated in the area and confirmed by the Spanish national reference laboratory.
There were a total of 144 cases. The estimated incidence was 9.7 cases per 105 person-year. The age group most affected was 0–4 years (rate 110.3 per 105 p-y), with a maximum in infants aged 6–11 months of age (190.4 per 105 p-y). The average duration of the disease was 15.5 days. 7% of the patients were hospitalised. Only 2 outbreaks of a family nature related to the consumption of pork were detected. The temporal evolution reflects higher incidence during the winter season (January). The most common exposure factor among the cases was the consumption of dried pork sausage (50% of the cases interviewed). The 58 typed strains were all of the biotype 4, serotype O:3, except one O:9. We distinguished 21 pulsotypes grouped in 8 clusters with a similarity of 97%. Over a number of years, a substitution of some pulsotypes for others was observed.
Yersiniosis has a high incidence in our area, with a clear seasonality of winter predominance. It affects very young children, in particular. The strains are of the same serotype, but the variety of pulsotypes changed over time. As an exposure factor for further analytical studies, the consumption of some pork products is proposed, without ruling out other factors.
Epidemiología descriptiva de la infección por Yersinia enterocolitica en un área de Castellón (España) entre 2006 y 2013 a partir de las cepas de Yersinia enterocolitica aisladas en el área y confirmadas por el laboratorio de referencia nacional.
Total 144 casos. La incidencia estimada fue de 9,7 casos 105 persona-año. El grupo de edad más afectado fue el de 0–4 años (tasa 110,3 por 105 persona-año), con una máximo en lactantes de 6 a 11 meses de edad (190,4 por 105 persona-año). La duración media de la enfermedad fue de 15,5 días. El 7% de los pacientes fueron hospitalizados. Solo se detectaron 2 brotes, de carácter familiar relacionados con el consumo de carne de cerdo. La evolución temporal refleja mayor incidencia en invierno (enero). El factor de exposición más frecuente entre los casos fue el consumo de longaniza seca de cerdo (el 50% de los casos entrevistados). Las 58 cepas tipadas fueron todas del biotipo 4, serotipo O:3, excepto una O:9. Se distinguieron 21 pulsotipos agrupados en 8 clusters con similitud del 97%. A lo largo de algunos años se observó una sustitución de unos pulsotipos por otros.
La yersiniosis tiene una incidencia alta en nuestra área, con una estacionalidad clara de predominio invernal. Afecta sobre todo a niños muy pequeños. Las cepas son del mismo serotipo, pero la variedad de pulsotipos cambió a lo largo del tiempo. Como factor de exposición para ulteriores estudios analíticos se propone el consumo de algunos productos del cerdo, sin descartar otros factores.
Yersiniosis is a disease caused by the species Yersinia, including Yersinia enterocolitica (Y. enterocolitica) and Yersinia pseudotuberculosis. It is mainly considered a zoonosis which affects mammals (pigs, dogs, cats, etc.), birds and amphibians. However, with 6471 confirmed human cases (1.9 per 105 population), yersiniosis was the third most recorded zoonosis in the European Union in 2013.1 Yersiniosis has been a notifiable disease in Spain since 2014 and an average of 457 cases per year (1 per 105 population) were recorded in the period 2014–2015 according to data from the Centro Nacional de Epidemiología [Spanish Epidemiology Centre].2 In the Castelló and La Plana-Vila-real trust areas, an average of 17 cases per year (3.6 per 105) were reported for the same period.
Studies in Spain,3,4 Belgium5 and the United States6 in the 1980s discovered that the consumption or home preparation of pork products was the main cause of yersiniosis in humans, and that pigs could be the primary reservoir of this bacterium.7,8 Over the last ten years, new research has identified different aspects of the epidemiology of this disease that has been poorly understood to date, such as how it spreads, mechanisms of transmission, infective dose, etc.1,9,10 However, there have been few recent studies on the distribution and incidence of Y. enterocolitica in Spain.11
The group Estudio de la Diarrea Infecciosa en Castellón (EDICS) [Study of infectious diarrhoea in Castellón] has so far dealt with infections by Salmonella, Campylobacter, rotavirus and norovirus.12–14 This paper describes the epidemiology of Y. enterocolitica infection in an area with a high incidence of this infection over the eight years prior to the incorporation of yersiniosis as a notifiable disease in the Epidemiological Surveillance system. We describe (a) the incidence in that period, by age groups; (b) the epidemiological features of the cases; (c) the trends and seasonality; and (d) the microbiological traits of the strains over a period of three years.
Population and methodsEpidemiologyWe included all isolates of Y. enterocolitica obtained from stool samples at the Microbiology Laboratory of the La Plana Trust Hospital Universitario de la Plana in Castelló during the period 2006–2013. This is the only hospital belonging to La Plana Trust. There are no private hospitals. All the strains were sent to and confirmed by the reference laboratory of the Centro Nacional de Microbiología [Spanish Microbiology Centre] in Madrid.
The prospective study was started in January 2007. The cases from 2006 were incorporated retrospectively; the following information was available for these cases: date of isolation, age, gender and place of residence. From 2007 onwards, the laboratory immediately reported each case to the Epidemiology Section of the Castelló Public Health Centre in order to survey the patient as soon as possible. The patients were interviewed by phone to obtain additional information about symptoms, onset, hospitalisation and duration of the disease, as well as some environmental and food exposure factors. They were asked about the other cases in the family context in order to detect outbreaks.
The incidence rates (cases/person-year [p-y]) were calculated by age group. As a denominator, we used the sum of the populations in the La Plana Trust area in each of the years of the study, as provided by the Conselleria de Sanitat [Regional Ministry of Health] of the Autonomous Region of Valencia. The age groups were as follows: 0–5 months, 6–11 months, 1 year, 2 years, 3 years, 4 years, 5–9 years, 10–14 years and >14 years. The monthly time series is described throughout the eight years of the study. The overall trend was calculated using simple linear regression from the monthly incidence rates and the seasonality by smoothing method with three-month moving averages. The pulsotypes of the isolated strains from March 2007 to February 2010 were studied (see microbiology section).
MicrobiologyFor the isolation of Yersinia in faeces we used Yersinia CIN Agar from bioMérieux (Marcy L’Étoile, France), incubated for 18–24h at 36°C. The formulation of the medium is that described by Schiemann in 1979.15 This medium contains as selective agents cefsulodin, triclosan and novobiocin, as well as cholate, deoxycholate and crystal violet; these agents inhibit the growth of Gram-positive microorganisms and most Gram-negative microorganisms. The presence of mannitol and neutral red allows the Yersinia colonies to be differentiated by colour (from dark pink to red). After the set incubation period, the colonies have a diameter of approximately 1mm.
Serotyping and pulsotyping was performed during the three-year period from March 2007 to February 2010, inclusive, at the Spanish Microbiology Centre in Madrid, a national reference centre for Enterobacteriaceae. For reasons of resource availability, this period is shorter than the full period of the entire study. Pulsed-field typing (Pulsed Field Gel Electrophoresis, PFGE) was performed by way of digestion with the NotI restriction enzyme, as used in the majority of published studies.16 The discrimination index was calculated and a dendrogram created.
To calculate the discrimination index, the Simpson diversity index (D) was used, which measures the likelihood that two randomly selected isolates from a sample belong to the same category (or the same type), using the formula D=∑ n(n−1)/N(N−1), where n=the total number of isolates of a particular type and N is the total number of isolates. The dendrogram was created using the Dice coefficient with a tolerance of 1%, applying the UPGMA (unweighted pair group method with arithmetic mean) algorithm (simple method of agglomerative hierarchical clustering).
In total, 21 pulsotypes which were arbitrarily identified by numbers for this study were distinguished. They were then regrouped into other clusters with a similarity of 97%, which were identified with capital letters.
Because this was an official Epidemiological Surveillance study due to the suspected high incidence of diagnosed yersiniosis (notifiable disease since 2014), ethics committee authorisation was not required.
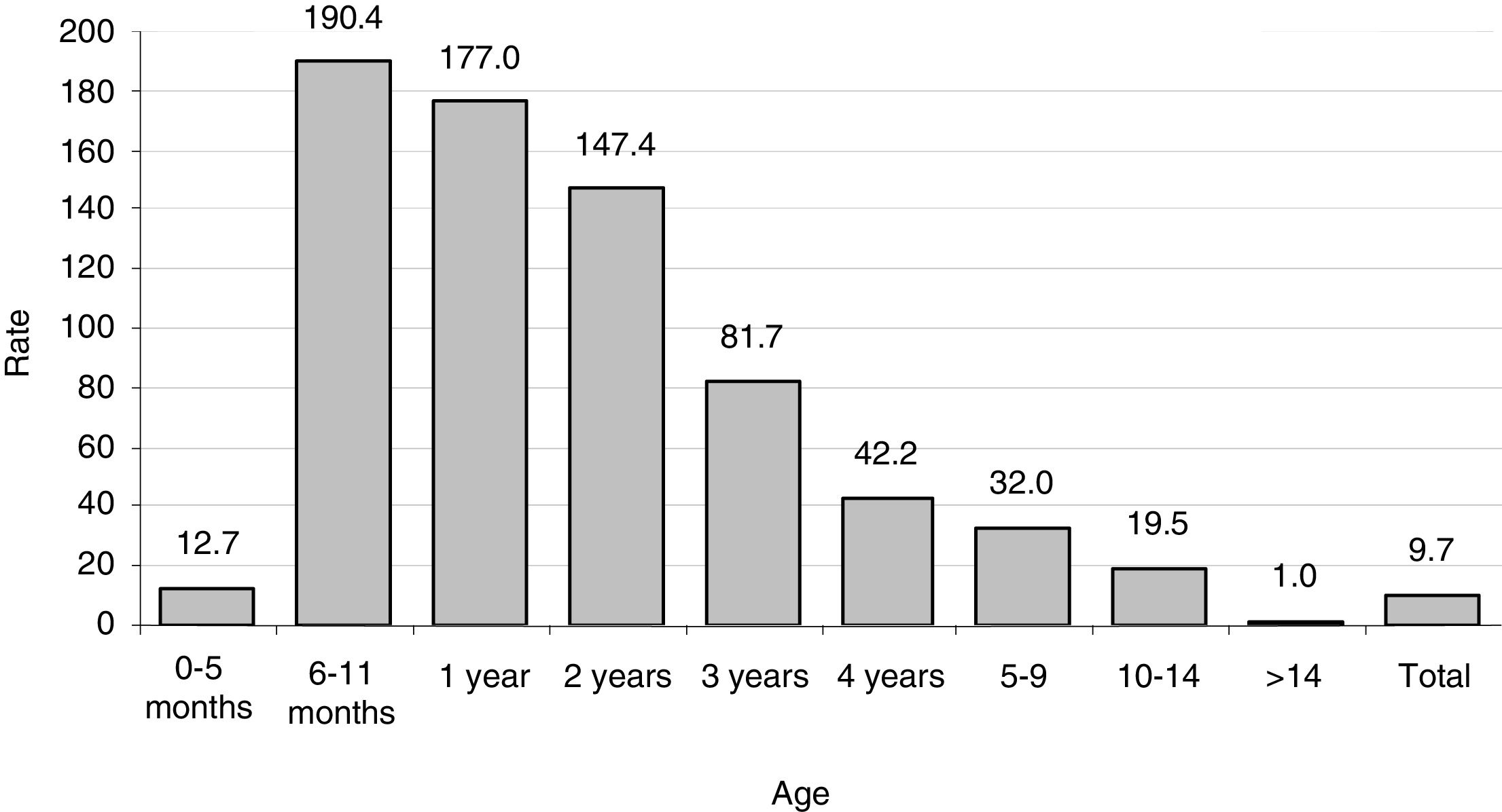
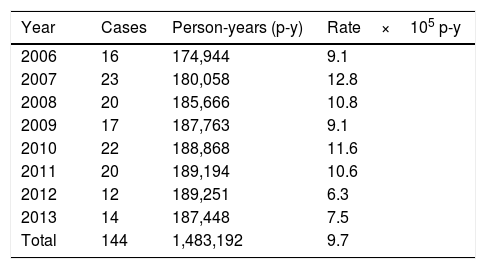
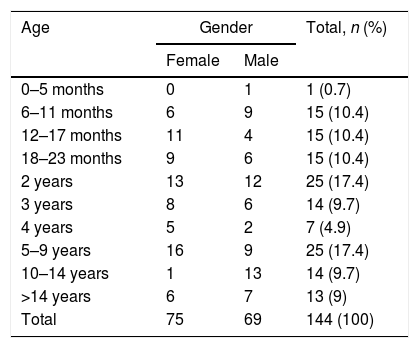
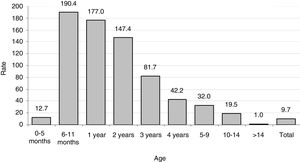
ResultsIncidence ratesThe population in the La Plana Trust area was 174,944 in 2006 and 187,448 in 2013. Over the entire period, there were 1,483,192 p-y of observation. There were 144 cases, with no substantial difference in terms of gender (75 females and 69 males). The estimated incidence rate was 9.7 cases per 105 p-y, with a maximum in 2007 (12.8) and a minimum in 2012 (6.3) (Table 1). There were large differences according to age (Table 2 and Fig. 1), with a rate of 110.3 in the 0–4 year age group. However, there were substantial differences within this group; the highest rates occurred at the age of 6–11 months, with 190 cases per 105 p-y. The rate decreased progressively with age to less than 1 per 105 over 14 years of age. In infants aged 0–6 months, the rate was much lower than the rest of the children (12.7 per 105 p-y).
Yersinia enterocolitica. Cases and annual and total rates in the La Plana (Castellón) Trust in the period 2006–2013.
| Year | Cases | Person-years (p-y) | Rate×105 p-y |
|---|---|---|---|
| 2006 | 16 | 174,944 | 9.1 |
| 2007 | 23 | 180,058 | 12.8 |
| 2008 | 20 | 185,666 | 10.8 |
| 2009 | 17 | 187,763 | 9.1 |
| 2010 | 22 | 188,868 | 11.6 |
| 2011 | 20 | 189,194 | 10.6 |
| 2012 | 12 | 189,251 | 6.3 |
| 2013 | 14 | 187,448 | 7.5 |
| Total | 144 | 1,483,192 | 9.7 |
Yersinia enterocolitica. Distribution of cases by age and gender. La Plana (Castellón) Trust in the period 2006–2013.
| Age | Gender | Total, n (%) | |
|---|---|---|---|
| Female | Male | ||
| 0–5 months | 0 | 1 | 1 (0.7) |
| 6–11 months | 6 | 9 | 15 (10.4) |
| 12–17 months | 11 | 4 | 15 (10.4) |
| 18–23 months | 9 | 6 | 15 (10.4) |
| 2 years | 13 | 12 | 25 (17.4) |
| 3 years | 8 | 6 | 14 (9.7) |
| 4 years | 5 | 2 | 7 (4.9) |
| 5–9 years | 16 | 9 | 25 (17.4) |
| 10–14 years | 1 | 13 | 14 (9.7) |
| >14 years | 6 | 7 | 13 (9) |
| Total | 75 | 69 | 144 (100) |
One hundred and five patients (73%) were interviewed by telephone. The most common symptoms they reported were: diarrhoea (98%), diarrhoea with visible blood (17%), pyrexia (72%); pyrexia >38C (26%) and vomiting (36%); the mean duration of the illness was 15.5 days (interquartile range 8–20 days) and 7% of the patients were admitted to hospital. Two family outbreaks were identified, one with four cases and the other with two. It was not possible to analyse Yersinia in the food, but it was found that the cases had consumed pork that had not been subjected to any health control and the relatives who did not get sick (3) had not.
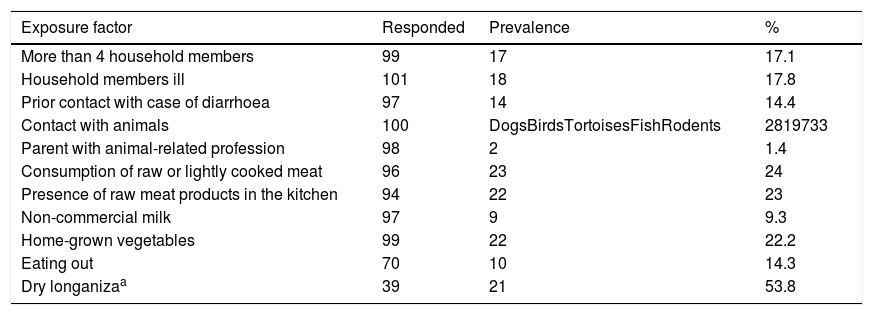
The prevalence of exposure factors in the approximately 100 cases from whom this information was obtained is shown in Table 3. Most had a prevalence of less than 20%. Exposure to meat products, vegetables and animals were the most prevalent factors. Among the items considered, only the consumption of dry longaniza (a regional type of meat product, generally made from minced pork) reached a prevalence of 50%.
Prevalence of exposure factors in the Yersinia enterocolitica patients interviewed. La Plana (Castellón) Trust.
| Exposure factor | Responded | Prevalence | % |
|---|---|---|---|
| More than 4 household members | 99 | 17 | 17.1 |
| Household members ill | 101 | 18 | 17.8 |
| Prior contact with case of diarrhoea | 97 | 14 | 14.4 |
| Contact with animals | 100 | DogsBirdsTortoisesFishRodents | 2819733 |
| Parent with animal-related profession | 98 | 2 | 1.4 |
| Consumption of raw or lightly cooked meat | 96 | 23 | 24 |
| Presence of raw meat products in the kitchen | 94 | 22 | 23 |
| Non-commercial milk | 97 | 9 | 9.3 |
| Home-grown vegetables | 99 | 22 | 22.2 |
| Eating out | 70 | 10 | 14.3 |
| Dry longanizaa | 39 | 21 | 53.8 |
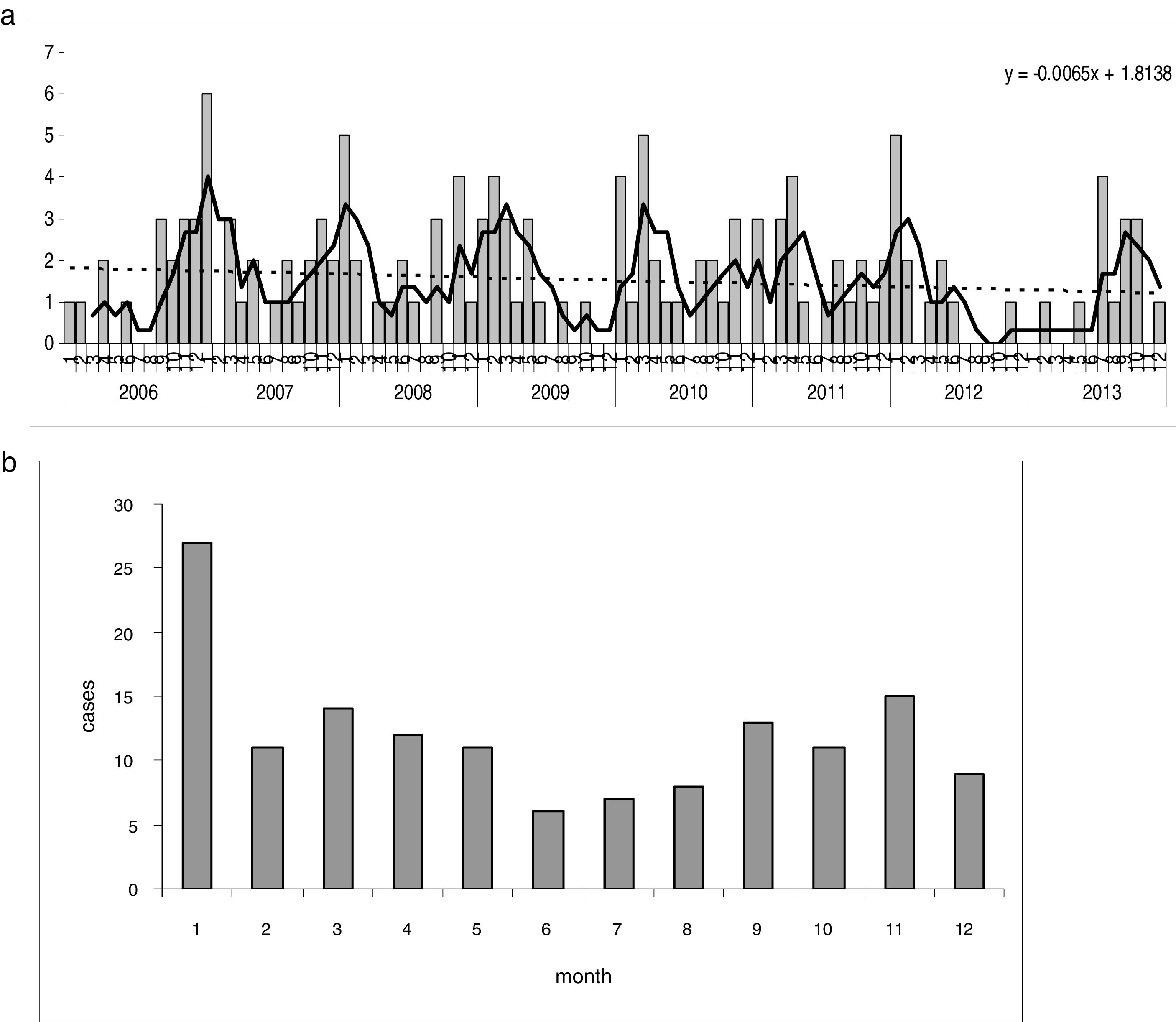
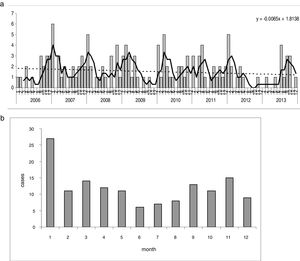
The seasonal changes over the eight years show some stability in the first few years, with rates of nine to 12 cases per 105 p-y, and a decrease in the last two years of the series (Table 1), which explains the slightly decreasing trend. There is a very striking seasonal pattern, with irregular peaks found each autumn and spring, except in the transition from 2012 to 2013 Fig. 2a). The lowest rates were regularly found in the warm months, from June to August; January was the month with the highest incidence (Fig. 2b).
Yersinia enterocolitica. Seasonal pattern. (a) Monthly incidence (cases) over the entire period. Trends and seasonality. Dotted line=adjustment by simple linear regression; continuous line=seasonality, moving averages (3 months). (b) Seasonality represented by cumulative monthly histogram of the 8 years.
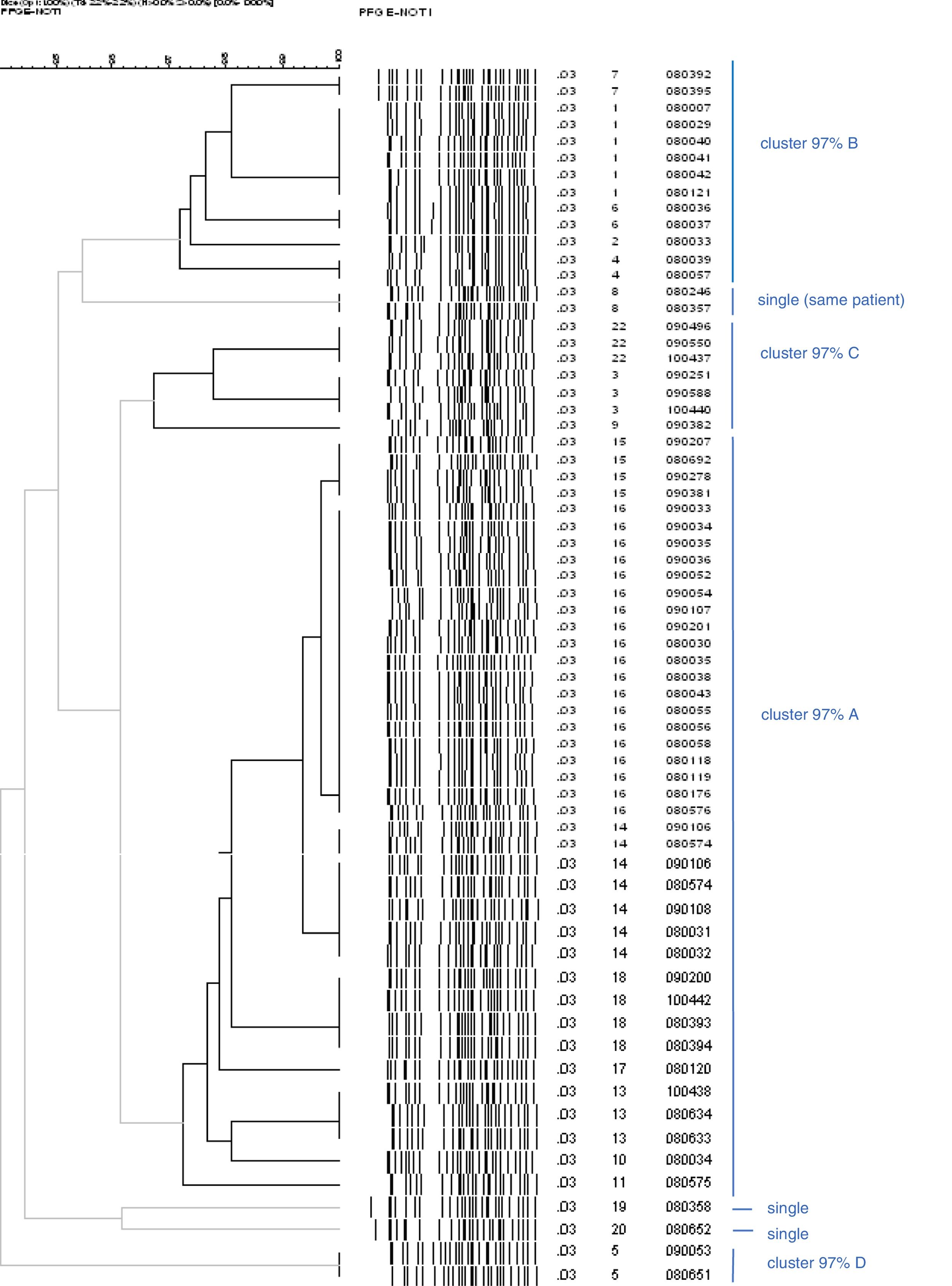
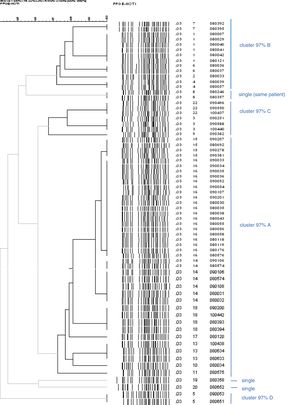
Of the 58 strains typed, all were biotype 4, serotype O:3, except one O:9 belonging to a female patient from Romania. The patterns found by PFGE had a high degree of similarity in serotype O:3. Strain O:9 had a very different pattern. The discrimination index found was 72.3%. Among the strains analysed from 58 patients, 21 pulsotypes were distinguished. Of them, 19 (33%) fitted in the same pulsotype of the most numerous cluster; 29 (50%) were grouped into 10 clusters of two to five cases, and 10 (17%) were single strains. These pulsotypes were regrouped according to similarity of 97% (Fig. 3, bold lines). One of the clusters – 97% covered 60% of the strains (35 of 58), the next 19% (11 strains), the next 10% (six strains), the next 5% (three strains) and finally, three single strains remained (pulsotypes 8, 19 and 20 in Fig. 3). Cluster A, the largest, was present throughout the three years, although to a lesser extent at the end; cluster B circulated and was predominant in the first half and cluster G in the last six months. The presence of the rest was lower and only in the middle part of the period Fig. 4a and b).
Dendrogram which only includes the strains of Y. enterocolitica O:3, excluding the only O:9 in order to see the similarity between them more clearly. The lines in bold indicate the strains 97% related. There are more strains than patients because there are some from the same patient, whose pulsotype did not vary: cluster 97% A (digits 12 and 21 were not used in the numbering of pulsotypes).
The basic features of the epidemiology of Y. enterocolitica infection in a Spanish Mediterranean coast trust area over the eight years prior to it being included as a notifiable disease to be reported to the Epidemiological Surveillance system have been described. The following aspects are particularly noteworthy:
EpidemiologicalThe incidence rates are in the high range of those reported in Europe and in Spain,1,17 and the seasonal changes showed a slightly decreasing trend, along with marked seasonal variation, with peaks in the cool and cold months (especially January) and low incidence in summer. In the years following the inclusion of yersiniosis as a notifiable disease, there is no indication of an increase in notifications in our Health Trust area. On the contrary, notifications decreased. Between 2014 and 2017, there were 35 declared cases (minimum six in 2014 and maximum 10 in 2015, with rates of 3.2×105 and 5.4×105, respectively).
The general clinical signs were those of mild gastroenteritis, although the average duration was two weeks, much longer than expected with common diarrhoea.
There were large differences in the distribution by age. More than 90% of the cases were under the age of 15 years and 64% were under five years. However, within this group of small children, there was a great deal of heterogeneity in the distribution and risks of infection, but with a very particular pattern: a peak in the second six months of life and then gradual decrease in risk.
The exposure factors considered had a relatively low prevalence (≤25%), except the consumption of dry longaniza (a generally pork-based product), which was consumed by more than half of the respondents. The consumption of pork products and, to a lesser extent, contact with domestic animals such as dogs and cats7,18–20 should be the subject of a specific study with a control group. A very high prevalence (93%) of Y. enterocolitica has been estimated in tonsils of pigs slaughtered in farms in south-east Spain,21 indicating their importance as a reservoir in Spain, as in other European countries, such as Finland and Poland.10,22
MicrobiologicalAll except one of the strains analysed were biotype 4 and serotype O:3, which has been detected in Spain since the end of the 1980s23 to the present day17 and is the most common in Europe.1 At this level of microbiological analysis, there is a very great deal of homogeneity, and there is little utility in discriminating between patients. However, within this same serotype, the analysis of the pulsotypes revealed a level of variability that enables the individual epidemiological analysis and the time series to be improved.
We confirmed the hypothesis that in the area of our study there was a higher incidence of Y. enterocolitica infection than reported in other studies. It is slightly higher than that estimated by Marimón et al.11 in San Sebastián in 2012–2014 (7.2 per 105) and much higher than the rate in the European Union in 2013.1 This same report gives the highest incidence rates in Finland (10.1) and Lithuania (8.8). Spain had a moderate incidence (1.8) and the United Kingdom and Ireland had very low rates (both 0.1). In Germany in the period 2001–2008, the average annual rate was 7.2 per 105.24 In the USA, the incidence is smaller than that reported in most of Europe, with annual figures of less than 1 per 105.25–27
Focusing on children under five years of age, again our results are almost double those in Germany, with 58 per 105 per year in children aged 0–4 years and 108 per 105 in one-year-olds,23 or in the USA, with 41.5 per 105 in African–American children.27 In San Sebastian, the group of one-to-two-year-olds was the most affected with a rate of 235.8 per 105 in the period 2012–2014.11 The difference between children under and over the age of six months is not usually reported, so our observation of the great contrast found in this small but important age group cannot be compared with the literature consulted. It is undeniable that the type of diet and care, and the possible presence of maternal antibodies, make a difference in early life.
Data on the notification of outbreaks caused by Y. enterocolitica in the medical literature are limited with regard to the number of cases and reports notified.28 The two investigated by us were small outbreaks (family outbreaks) and were identified thanks to the special attention paid to this disease during the study period. The consumption of pork products was behind these outbreaks.
The discreet downward trend appears in a more general context of a decrease in the incidence observed not only in Spain11,17 but also in the USA26 and Europe.1 Nonetheless, differences in reporting, isolation and characterisation of strains mean it is very difficult to make reliable comparisons between countries and regions.29
Seasonal variation has long been recognised30 and occurs in Europe.1 However, it does not happen everywhere, or with the same epidemiological profile.11 According to one study, in Germany, the months with the lowest incidence were March and April.23 In the USA, the seasonal pattern is different according to racial group27 or the period studied.26 The seasonal variation was more evident when examining the monthly series of each year sequentially (Fig. 2a) than when grouping every year in a 12-month diagram (Fig. 2b).
In microbiology terms, biotype 4 and predominant serotype O:3 is the same as in Europe, and except in rare cases (such as O:9 in one patient) it is not of any utility to discriminate strains in outbreaks.31 The patterns obtained by PFGE suggest a very interesting epidemiological pattern. They had an acceptable discrimination index.32 The predominance of one or other pulsotypes over several years in the endemic background indicates the introduction, presence and replacement of some by others over the months. Along with this there were some very uncommon pulsotypes. This information can be applied to detect and investigate possible foci of infection, or, conversely, to rule out cases apparently related by conventional epidemiology. Perhaps the new methods of whole genome sequencing will provide greater opportunities for the study of Y. enterocolitica.33
This study has a number of limitations. There is bound to be a patient selection bias considering the severity and duration of the disease and the older age. The proportion of stool cultures requested from the most severe, long-lasting cases and the child population was higher than from the rest. It is a general problem of passive surveillance observational studies that they underestimate incidence in diseases with a broad clinical spectrum. Even so, the percentage of serious cases admitted to hospital was very low. Regarding the exposure factors, it is important to stress that this was not a case–control study; hence there was no analysis to identify “risk” factors. This was a descriptive epidemiological surveillance study, and it should be considered as such; it is normal for the epidemiological surveys for this activity to include a section for “exposure” factors in order to provide useful information for further analytical studies with comparison groups.
In terms of the strengths of the study, this was a study in a small area where a thorough investigation of cases with positive stool culture, which were confirmed as Y. enterocolitica, was carried out. The microbiological analysis techniques and epidemiological method applied locally were consistent throughout the period, enabling us to draw valid conclusions about the time series. The isolates were all confirmed by the Enterobacteriaceae reference centre at the Spanish Microbiology Centre.
ConclusionsThe incidence of Y. enterocolitica gastroenteritis in the La Plana Trust area (Castellón) is high, almost exclusively involving serotype O:3. Within the great variety of circulating strains identified, 1/3 belonged to the same pulsotype, but a phenomenon of substitution of predominant pulsotypes was observed in the period studied. Epidemiologically, it has a cyclical pattern, predominantly in winter (particularly prevalent in January). It mainly affects 0–4-year-old children, although in this age group there are notable differences, with minimal risk in infants under the age of six months, peak incidence rate in infants aged 6–11 months, and subsequent marked gradual decline; this aspect is worthy of more in-depth investigation. The strains analysed by the molecular techniques used here showed a high degree of similarity, which limits their application in the detailed epidemiological analysis. The techniques applied were those available during the study period. However, there are now techniques with greater discriminative capacity, and these can be expected to be of greater practical use in the identification of transmission chains or foci of infection.32–34
Conflicts of interestThe authors declare that they have no conflicts of interest.
To José Antonio López-Portolés for his indispensable collaboration in the identification of pulsotypes during his work at the Spanish Microbiology Centre, ISCIII (Instituto de Salud Carlos III [Carlos III Health Institute]) in Madrid.
Please cite this article as: Yagüe-Muñoz A, Arnedo-Pena A, Herrera-León S, Meseguer-Ferrer N, Vizcaíno-Batllés A, Romeu-García MÀ, et al. Epidemiología descriptiva de infección por Yersinia enterocolitica en un área de alta incidencia durante 8 años, 2006-2013. Proyecto EDICS. Enferm Infecc Microbiol Clin. 2019;37:441–447.






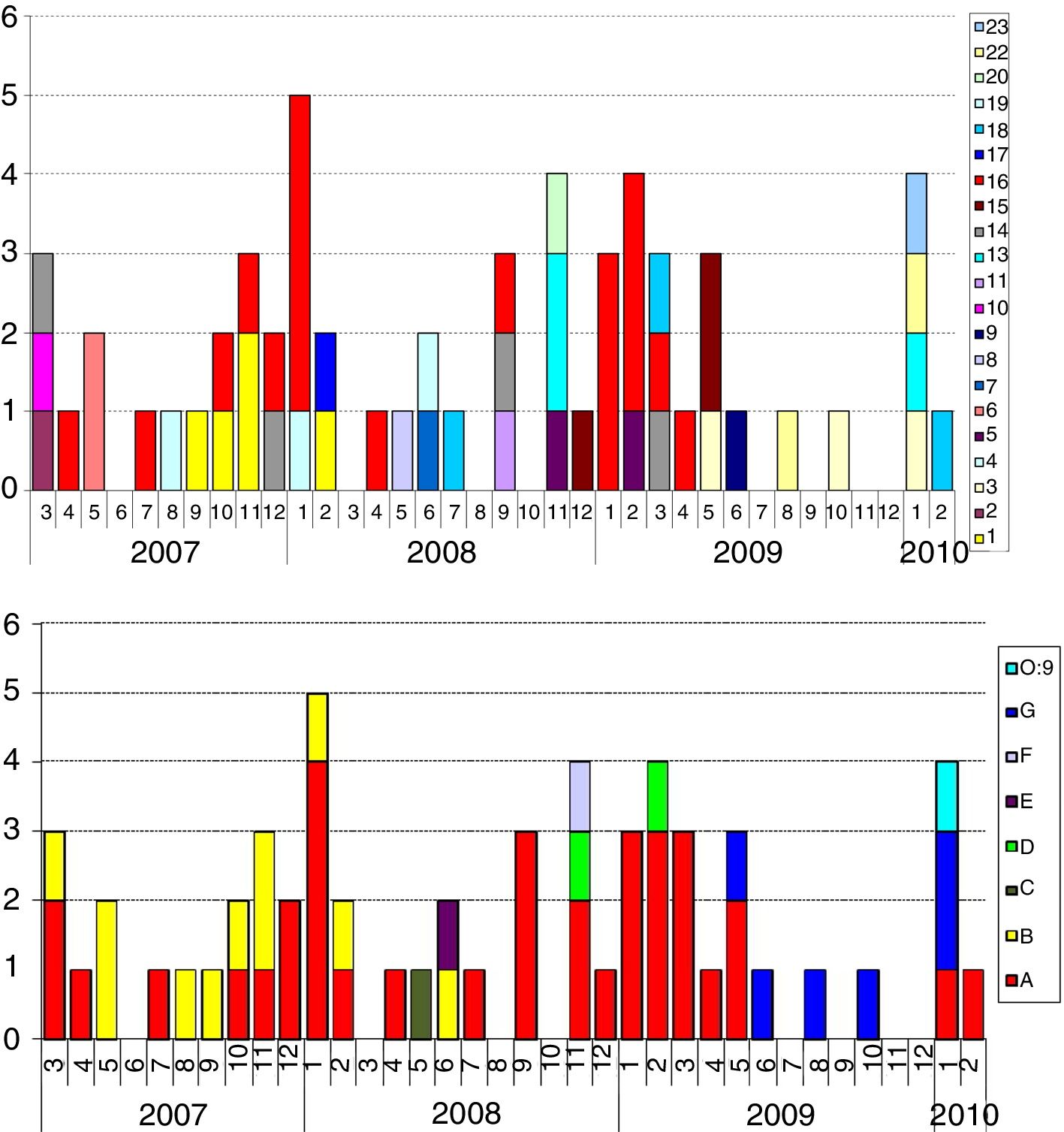
![Yersinia enterocolitica. Monthly incidence according to pulsotypes of the 58 cases in which this information is available in the 3-year period. (a) Pulsotypes (21 cluster) [numbers 12 and 21 were not used]. (b) Pulsotypes grouped by similarity of 97% (cluster 97%). Yersinia enterocolitica. Monthly incidence according to pulsotypes of the 58 cases in which this information is available in the 3-year period. (a) Pulsotypes (21 cluster) [numbers 12 and 21 were not used]. (b) Pulsotypes grouped by similarity of 97% (cluster 97%).](https://static.elsevier.es/multimedia/2529993X/0000003700000007/v1_201907280622/S2529993X19301133/v1_201907280622/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
