Most cases of fever of intermediate duration (FDI) in Spain are associated with infectious diseases (mainly Q fever and rickettsia infections). In clinical practice, the causal diagnosis of these entities is based on immunodiagnostic techniques, which are of little help in the early stages. Therefore, the aim of this study was to evaluate the usefulness of molecular techniques for the early diagnosis of Q fever and rickettsia diseases in patients with FDI. A PCR method was used to detect the presence of genetic material of Coxiella burnetii and Rickettsia spp. in blood specimens from 271 patients with FDI. The specificity of both techniques is high, allowing diagnosis in cases undiagnosed by specific antibodies detection. These data suggest that the use of molecular techniques, with proper selection of the study specimen, and using appropriate primers is a useful tool in the early diagnosis of the main causes of FDI, especially if serology is negative or inconclusive.
La mayor parte de los casos de fiebre de duración intermedia (FDI) en España corresponden a enfermedades infecciosas (principalmente fiebre Q y rickettsiosis). En la práctica clínica el diagnóstico causal de estas entidades se basa en el inmunodiagnóstico, con una escasa utilidad en fases precoces. Por ello, el objetivo de este trabajo fue la evaluación de la utilidad de técnicas moleculares en el diagnóstico precoz de fiebre Q y rickettsiosis en pacientes con FDI. Se estudió mediante PCR la presencia de material genético de Coxiella burnetii y Rickettsia spp. en muestras sanguíneas de 271 pacientes con FDI. La especificidad de ambas técnicas es elevada, permitiendo el diagnóstico en casos no diagnosticados mediante detección de anticuerpos específicos. Estos datos sugieren que el empleo de técnicas moleculares, con una adecuada selección de la muestra de estudio y el empleo de cebadores adecuados, es un elemento útil en el diagnóstico precoz de las principales causas de FDI, principalmente si la serología es negativa o no es concluyente.
Fever of intermediate duration (FID) is defined as an axillary temperature above 38°C with no obvious source of infection in non-immunocompromised patients without prior hospital admission which lasts more than one week and less than three weeks and which remains undiagnosed after initial assessment.1 The majority of these cases correspond to infectious diseases (primarily caused by Coxiella burnetii, Rickettsia spp., Brucella spp., cytomegalovirus [CMV] and Epstein–Barr virus [EBV]2). In clinical practice, diagnosis of the cause of such conditions is based on immunodiagnostics, which require at least 15–21 days from the onset of the infection until antibodies appear. Serological techniques are not therefore very helpful in the early diagnosis of these diseases. However, the identification of the main causative agents in the early stages is of great interest for a number of reasons: (a) these are frequently diseases with a considerable effect on the general condition which benefit from aetiological treatment, if there is any; (b) they sometimes present as severe or complicated clinical conditions requiring hospitalisation3; (c) the duration of the recommended empirical treatment is different in the two main forms (Q fever and murine typhus); and (d) in some cases of Q fever, despite adequate antimicrobial treatment, clinical manifestations persist, indicating the need to add corticosteroids.4
Therefore, the aim of this study was to evaluate the utility of molecular techniques (polymerase chain reaction [PCR]) for the early diagnosis of Q fever and rickettsial diseases in patients with FID.
Patients and methodsThe total study population consisted of 271 patients with FID criteria1 assessed over a period of 6 years (2004–2009) at the outpatient clinic of the Infectious Diseases and Tropical Medicine Unit (Unidad de Enfermedades Infecciosas y Medicina Tropical, UEIMT). Epidemiological and clinical data were collected and basic blood and urine analysis, chest X-ray and blood cultures were taken, as well as tests for the detection of antibodies against C. burnetii, Rickettsia typhi, CMV and EBV. Particular attention was given to determining the time since onset of the condition and the serological study and previous use of antimicrobial agents.
Serological diagnosis of C. burnetii or R. typhi infection was performed by indirect immunofluorescence (IIF) (Coxiella burnetii-Spot IF, Rickettsia mooseri Spot IF, BioMérieux, Marcy l’Étoile, France). The criteria used for diagnosis were as follows: (a) a single titre of IgM≥1/80 and IgG≥1/320 to C. burnetii antigens in phase II or a single titre of IgM≥1/40 and IgG≥1/160 to R. typhi; (b) seroconversion (from initial negative titres to positive titres at 2–3 weeks), or (c) a four-fold increase in the IgG titre between the acute-phase serum and the convalescence phase.
The patients were classified into different groups: (a) “definite diagnosis of Q fever or rickettsiosis”, which were subdivided according to whether the initial serology was negative or positive; (b) “other definite diagnoses” (EBV and CMV infection, using the usual diagnostic criteria); (c) “incomplete” diagnosis”, in which a definite diagnosis could not be established because the patient did not meet initial diagnostic criteria and did not attend for follow-up; and (d) “no diagnosis”, if after all tests no definite diagnosis was reached.
An EDTA plasma sample was collected from each patient at the time of the consultation. The samples were kept frozen and 200μl was subsequently processed for extraction of DNA (QIAamp® DNA Mini kit, Qiagen, Germany) following the manufacturer's instructions. Molecular diagnosis was performed using polymerase chain reaction (PCR) amplification and detection of bacterial DNA for C. burnetii and Rickettsia spp., using in the first case primers that amplify the insertion segment IS11115 and using as a target for Rickettsia spp. the 23S-5S rRNA intergenic spacer region.6
ResultsOf the 271 patients with FID, Q fever was diagnosed in 86 cases (31.7%), murine typhus in 66 (24.3%), CMV infection in 16 (5.9%) and EBV infection in 13 (4.3%). No final diagnosis was reached in 64 patients, and 57 had an “incomplete diagnosis”. Therefore, half the patients with FID were diagnosed serologically with Q fever or murine typhus.
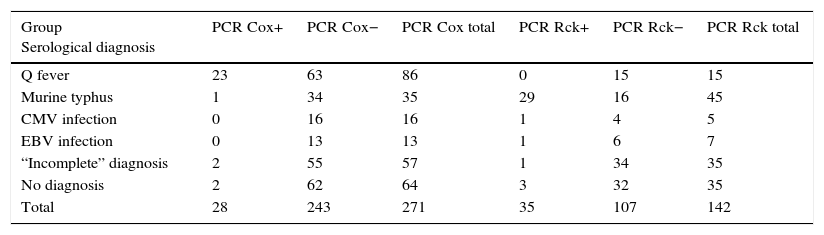
PCR was used to determine the presence of C. burnetii genetic material in 271 patients with different serological diagnoses (Table 1). There was a statistically significant association between the test being positive (molecular detection by PCR of C. burnetii) and the serological diagnosis of Q fever (Fisher's exact test, p<0.05). The characteristics of the test were as follows: (a) sensitivity: 26.7% (95% CI: 18.5–36.9%); (b) specificity: 97.9% (95% CI: 95.8–99.8%); (c) positive predictive value: 82.1% (95% CI: 64.4–92.2%); and (d) negative predictive value: 74.1% (95% CI: 68.2–79.2%).
Results of both PCR in the study groups.
| Group Serological diagnosis | PCR Cox+ | PCR Cox− | PCR Cox total | PCR Rck+ | PCR Rck− | PCR Rck total |
|---|---|---|---|---|---|---|
| Q fever | 23 | 63 | 86 | 0 | 15 | 15 |
| Murine typhus | 1 | 34 | 35 | 29 | 16 | 45 |
| CMV infection | 0 | 16 | 16 | 1 | 4 | 5 |
| EBV infection | 0 | 13 | 13 | 1 | 6 | 7 |
| “Incomplete” diagnosis | 2 | 55 | 57 | 1 | 34 | 35 |
| No diagnosis | 2 | 62 | 64 | 3 | 32 | 35 |
| Total | 28 | 243 | 271 | 35 | 107 | 142 |
CMV: cytomegalovirus; PCR Cox: PCR for Coxiella burnetii; PCR Rck: PCR for Rickettsia; EBV: Epstein–Barr virus.
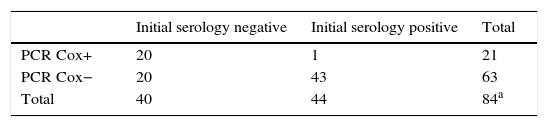
In order to identify the utility of molecular detection by PCR of C. burnetii in patients with Q fever, we re-examined the results according to the serology. The data are shown in Table 2a. Using Fisher's exact test, a significant association was found (p<0.001) between positive initial serology and a negative C. burnetii PCR.
Relationship between serological data and PCR results.
| Initial serology negative | Initial serology positive | Total | |
|---|---|---|---|
| PCR Cox+ | 20 | 1 | 21 |
| PCR Cox− | 20 | 43 | 63 |
| Total | 40 | 44 | 84a |
PCR Cox: PCR for Coxiella burnetii.
This was related to the time course of the disease, which was significantly lower in patients with positive PCR (7 days [5–12]) than those with negative PCR (14 days [4–90]). Moreover, 56% of patients with Q fever in whom C. burnetii PCR was negative had received antimicrobial treatment (doxycycline in 2 cases, quinolones in 6 cases and beta-lactams in 27 cases).
The PCR study to detect Rickettsia spp. genome was performed on 142 patients with different serological diagnoses (Table 1). There was a statistically significant association between the test being positive (molecular detection by PCR of Rickettsia spp.) and the diagnosis of murine typhus (Fisher's exact test, p<0.0001). The characteristics of the test were as follows: (a) sensitivity: 82.9% (95% CI: 67.3–91.9%); (b) specificity: 85.0% (95% CI: 77.1–90.6%); (c) positive predictive value: 64.4% (95% CI: 49.8–76.8%); and (d) negative predictive value: 93.8% (95% CI: 82.7–97.1%).
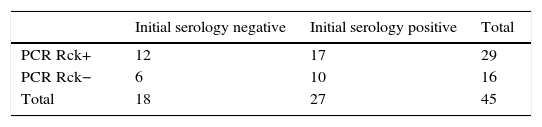
In order to identify the utility of molecular detection by PCR of Rickettsia spp. in patients with murine typhus, we re-examined the results according to the serology. The data are shown in Table 2b. There was no association between the presence of negative serology to R. typhi and a generic positive PCR for Rickettsia spp. (χ2 test, p=0.80). We found no differences in the time course of the disease according to the results of the PCR, in both cases being between four days and two weeks.
DiscussionThere have been very few studies in humans on the utility of C. burnetii PCR on blood specimens for the diagnosis of Q fever.7–10 Our study found similarities, but also some differences. As we have seen, the effectiveness of PCR is greater when it is performed in the early stages of the disease.7,8 The results also differ when using primers that amplify different sequences (e.g. htp AB or ompA) or different techniques (conventional PCR, nested PCR or real-time PCR).7–10 Our results are similar to those of other authors in terms of obtaining greater diagnostic effectiveness using plasma samples instead of serum samples.9 In our study, this technique shows a degree of high specificity in the diagnosis of acute Q fever in the early stages of the disease. A negative test in the early stages may be related to the common use of antimicrobial agents (including beta-lactams); this reinforcing the argument for avoiding using antibiotics in cases of FID. However, it is interesting to note that C. burnetii has peptidoglycan in its cell wall11 and is sensitive to ampicillin in vitro.12 It is therefore possible that beta-lactams are effective in the elimination of C. burnetii in blood and so capable of making the PCR negative. Nevertheless, the difficulty they have in penetrating the interior of the cells makes them ineffective in practice. The presence of C. burnetii genetic material in patients with positive serology may suggest the possibility of a chronic form developing.7,8
There is even less literature on the use of PCR with blood specimens for the early diagnosis of infections from the genus Rickettsia than for Q fever, and the number of patients studied is very small.13–15 In general, the data in the literature show low sensitivity, but there are also differences in terms of the disease stage. In our study, the use of generic primers meant we were not only able to detect R. typhi, but also other rickettsial diseases that can be present in our environment (e.g. R. felis and R. massiliae),16,17 so it was not surprising to find PCR-positive cases in patients with negative serology to R. typhi.
The combined use of these two PCRs allowed us to obtain a diagnosis in eight patients (4 cases of Q fever and 4 of rickettsiosis) who had negative serology.
In summary, our data suggest that the use of molecular techniques, with appropriate selection of the study specimen and the use of suitable primers, is a useful element in the early diagnosis of the main causes of FID, especially if the serology is negative or inconclusive. The use of other molecular techniques such as LAMP, which does not require the use of a thermocycler and is performed in 60min, could provide additional benefits.18–20
FundingThis study was funded in part by the INIA (Instituto Nacional de Innovación Agraria [National Institute for Agricultural Innovation]), FAU2006-00002-C04-01.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Both authors contributed equally to this article.
Please cite this article as: Bolaños-Rivero M, Carranza-Rodríguez C, Hernández-Cabrera M, Pisos-Álamo E, Jaén-Sánchez N, Pérez-Arellano J-L. Utilidad del diagnóstico molecular precoz de fiebre Q y rickettsiosis en pacientes con fiebre de duración intermedia. Enferm Infecc Microbiol Clin. 2017;35:655–658.