Tuberculosis continues to be a major public health problem in Spain. The incidence of tuberculosis in the native population has declined steadily in recent years. Migration flows have changed drastically since the beginning of the 21st century, with Spain becoming a recipient country for immigrants. Because most of the immigrants comes from countries with high incidence of tuberculosis, the contribution of the migrant population to new cases of tuberculosis is higher in relative terms than its weight in the total population. Tuberculosis programmes must address the cultural, economic and medical aspects of the disease, and particularly target groups at risk, including the migrant population. In this paper, we will review the epidemiology and dynamics of tuberculosis in the migrant population, their differentiating clinical characteristics and the programmatic actions to address the problem.
La tuberculosis continúa siendo un problema de salud pública de primer orden en España. La incidencia de tuberculosis en la población autóctona ha disminuido progresivamente en los últimos años. Los flujos migratorios se han modificado drásticamente desde inicios del siglo xxi, cuando España ha pasado a ser un país receptor de inmigrantes. La mayor parte de los inmigrantes proceden de países con alta incidencia de tuberculosis, lo que ha supuesto que la contribución de esta población a los nuevos casos de tuberculosis sea relativamente superior respecto al peso que representan en el conjunto de la población. Los programas de lucha contra la tuberculosis tienen que abordar los aspectos culturales, económicos y médicos de la enfermedad, e incidir especialmente en los grupos de riesgo, entre los que destaca la población inmigrante. En este artículo revisaremos la epidemiología y la dinámica de la tuberculosis en la población inmigrante, sus características clínicas diferenciadoras y las acciones programáticas para abordar el problema.
Tuberculosis (TB) remains a primary health concern the world over. Although the World Health Organization (WHO) declared it a global public health emergency in 1993, the necessary efforts have perhaps not been dedicated to controlling it on a global scale.1 According to the WHO, there were 10.4 million cases of TB worldwide in 2015, 3.1% of which were diagnosed in the European region, and 1.8 million people died as a result of TB.2 It has been estimated that a third of the global population is infected with Mycobacterium tuberculosis (M. tuberculosis), primarily in developing countries.3
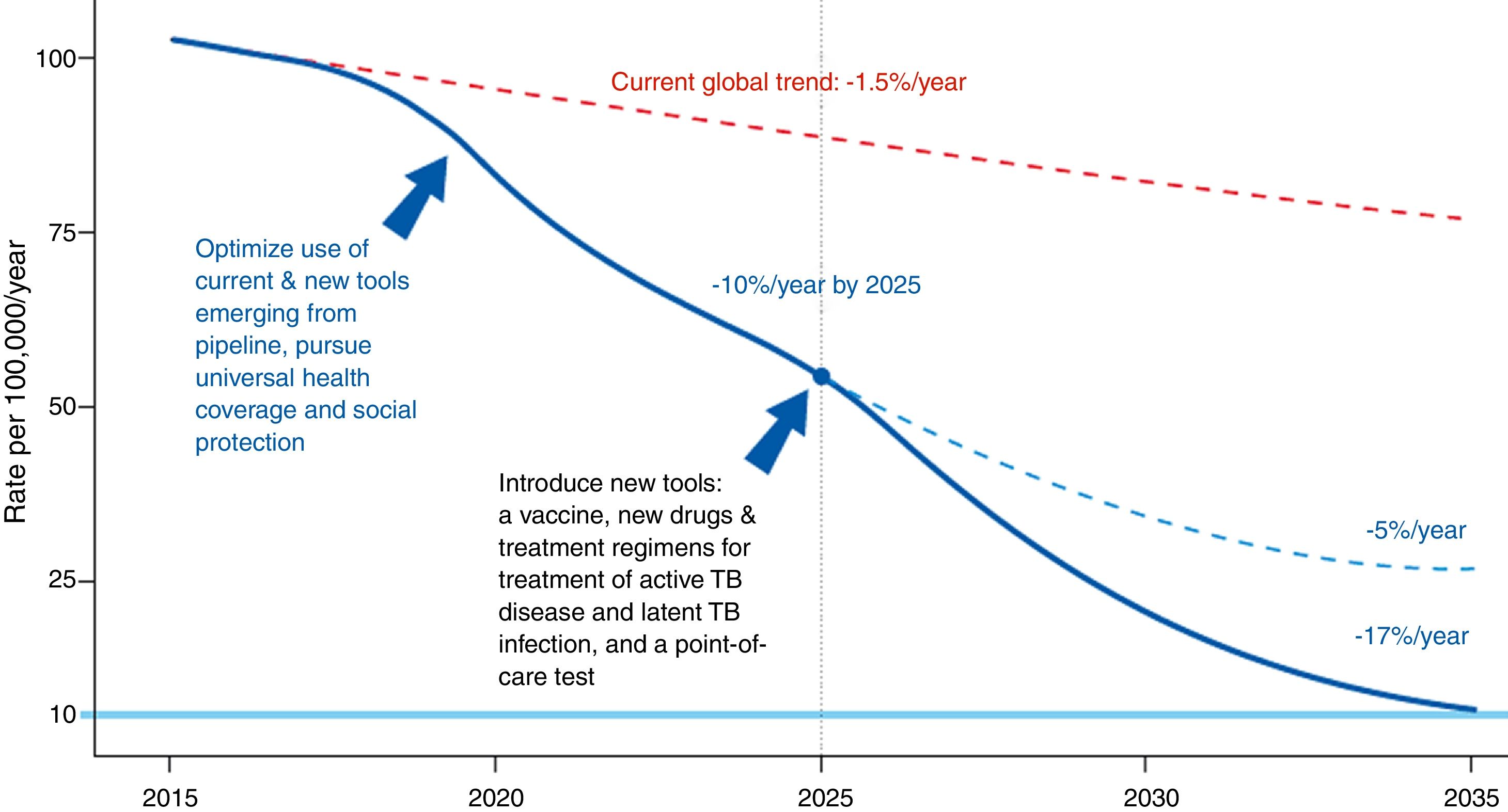
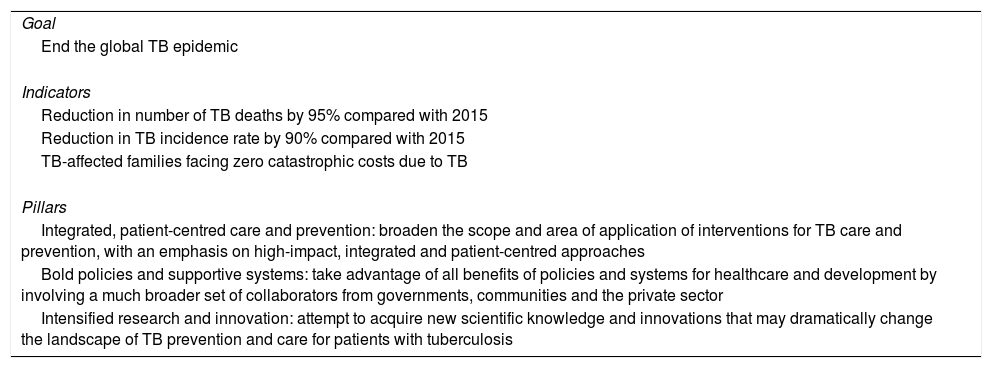
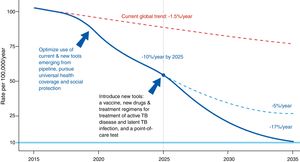
The Global Plan to Stop Tuberculosis 2006–2015 launched by the WHO ended with mixed results by region. The new plan proposed by the WHO is meant to end the global epidemic of TB by 2035 (Fig. 1). Table 1 shows this plan's objective, indicators and pillars.4 Reaching this goal requires special attention to immigrant health and cooperation between countries.
Evolution of the incidence of tuberculosis under different scenarios.4Source: WHO end TB strategy: objectives and indicators.
End TB strategy (2035).
| Goal |
| End the global TB epidemic |
| Indicators |
| Reduction in number of TB deaths by 95% compared with 2015 |
| Reduction in TB incidence rate by 90% compared with 2015 |
| TB-affected families facing zero catastrophic costs due to TB |
| Pillars |
| Integrated, patient-centred care and prevention: broaden the scope and area of application of interventions for TB care and prevention, with an emphasis on high-impact, integrated and patient-centred approaches |
| Bold policies and supportive systems: take advantage of all benefits of policies and systems for healthcare and development by involving a much broader set of collaborators from governments, communities and the private sector |
| Intensified research and innovation: attempt to acquire new scientific knowledge and innovations that may dramatically change the landscape of TB prevention and care for patients with tuberculosis |
Over time, migration movements in Spain have been influenced by the global economic crisis and numerous armed conflicts. It has been estimated that in 2015 there were close to 244 million international migrants and 66% of them migrated to developed countries.5 Although this decade started with historically high figures for the immigrant population (5,747,734 foreigners were recorded in a 2010 census, amounting to 12.2% of the Spanish population), the global economic crisis has brought about a decrease in the arrival of immigrants and has even created a negative migration balance in some regions of Spain (source: INE). Although most migration is voluntary, recent years have witnessed an increase in forced migration due to natural disasters, armed conflicts and political persecution.6 International protection was granted to 333,400 asylum seekers in the European Union in 2015, representing a 72% increase compared to 2014. In Spain, 15,755 people sought asylum in 2016. This figure was unprecedented. More than half of asylum seekers and refugees come from 4 countries: Syria (28%), Afghanistan (14%), Iraq (9%) or Nigeria (8%).7
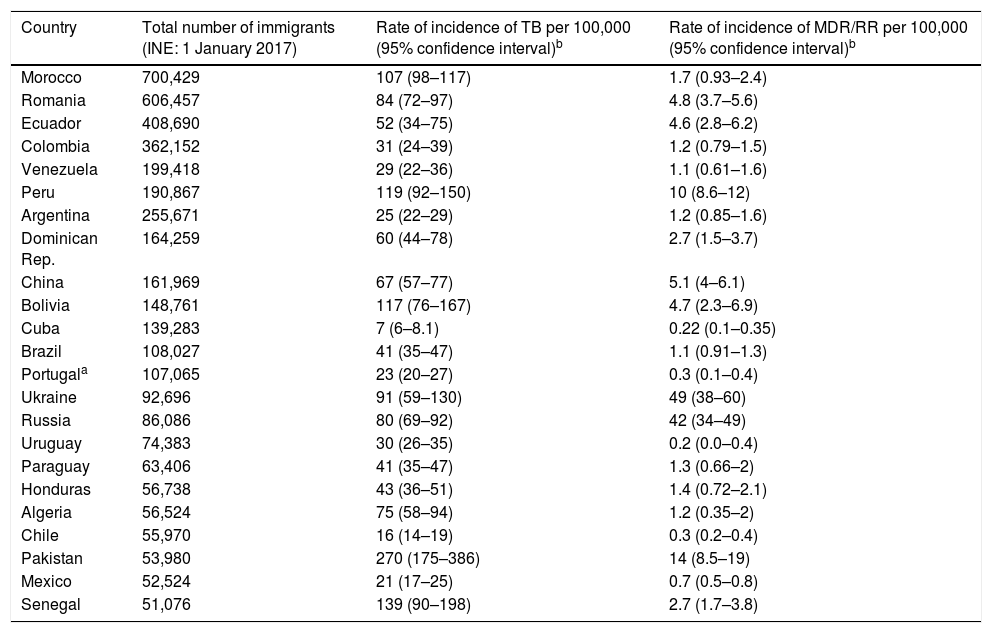
With regard to TB, the significance of the origin of the people who come to Spain to seek new opportunities lies in the rate of incidence of TB in their countries of origin, as it will determine the number of recently acquired latent infections. For their part, the strains circulating in the countries of origin of the immigrant population may have different degrees of virulence and different patterns of resistance than TB strains in host countries2 (Table 2). Consequently, it is clear that the evolution of TB in Spain is going to be marked by migration movements and by measures that shall be taken as part of programmes to control TB, especially in populations with increased susceptibility to acquiring the infection and developing the disease, including the immigrant population.
Immigrants in Spain from countries outside the European Union, rate of incidence, rate of incidence of MDR/RR by country (countries with communities of more than 50,000 people in Spain).
| Country | Total number of immigrants (INE: 1 January 2017) | Rate of incidence of TB per 100,000 (95% confidence interval)b | Rate of incidence of MDR/RR per 100,000 (95% confidence interval)b |
|---|---|---|---|
| Morocco | 700,429 | 107 (98–117) | 1.7 (0.93–2.4) |
| Romania | 606,457 | 84 (72–97) | 4.8 (3.7–5.6) |
| Ecuador | 408,690 | 52 (34–75) | 4.6 (2.8–6.2) |
| Colombia | 362,152 | 31 (24–39) | 1.2 (0.79–1.5) |
| Venezuela | 199,418 | 29 (22–36) | 1.1 (0.61–1.6) |
| Peru | 190,867 | 119 (92–150) | 10 (8.6–12) |
| Argentina | 255,671 | 25 (22–29) | 1.2 (0.85–1.6) |
| Dominican Rep. | 164,259 | 60 (44–78) | 2.7 (1.5–3.7) |
| China | 161,969 | 67 (57–77) | 5.1 (4–6.1) |
| Bolivia | 148,761 | 117 (76–167) | 4.7 (2.3–6.9) |
| Cuba | 139,283 | 7 (6–8.1) | 0.22 (0.1–0.35) |
| Brazil | 108,027 | 41 (35–47) | 1.1 (0.91–1.3) |
| Portugala | 107,065 | 23 (20–27) | 0.3 (0.1–0.4) |
| Ukraine | 92,696 | 91 (59–130) | 49 (38–60) |
| Russia | 86,086 | 80 (69–92) | 42 (34–49) |
| Uruguay | 74,383 | 30 (26–35) | 0.2 (0.0–0.4) |
| Paraguay | 63,406 | 41 (35–47) | 1.3 (0.66–2) |
| Honduras | 56,738 | 43 (36–51) | 1.4 (0.72–2.1) |
| Algeria | 56,524 | 75 (58–94) | 1.2 (0.35–2) |
| Chile | 55,970 | 16 (14–19) | 0.3 (0.2–0.4) |
| Pakistan | 53,980 | 270 (175–386) | 14 (8.5–19) |
| Mexico | 52,524 | 21 (17–25) | 0.7 (0.5–0.8) |
| Senegal | 51,076 | 139 (90–198) | 2.7 (1.7–3.8) |
INE: Spanish National Statistics Institute; MDR/RR: multi-drug-resistant or rifampin-resistant tuberculosis; TB: tuberculosis.
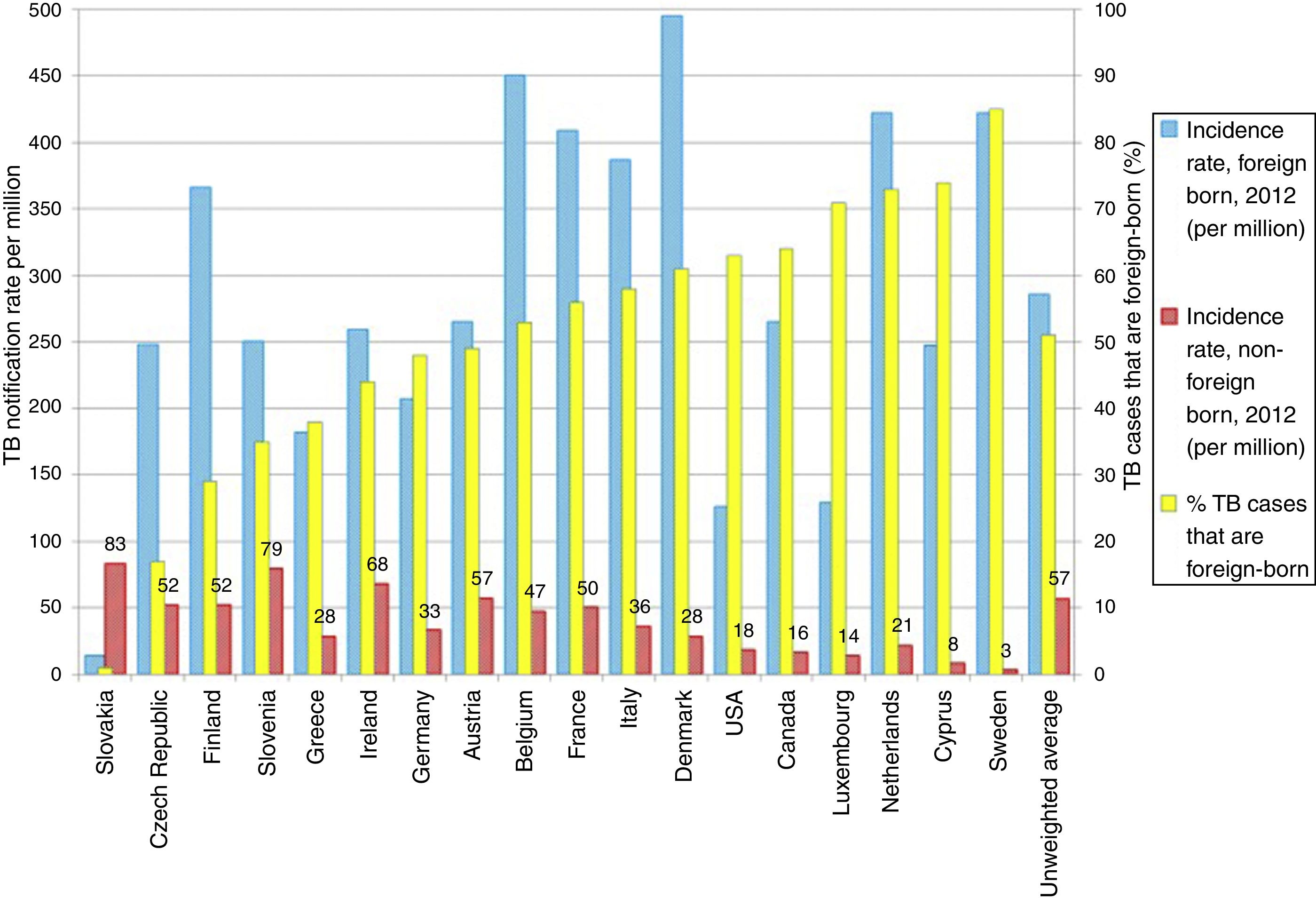
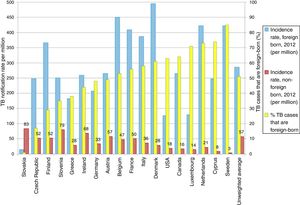
TB in developed countries has exhibited an abrupt decline in the last quarter century, with incidences below 40 cases per 100,000 inhabitants.8 At present, TB in countries with a low incidence (<100 cases per million inhabitants) is primarily diagnosed in immigrants from countries with a high incidence (>100 cases per million inhabitants) and in patients with immunosuppression, such as transplant recipients, oncology/haematology patients, patients with immunosuppression caused by drugs and patients with immunosenescence.8 Some European countries with a low incidence of TB have experienced a less-abrupt-than-expected drop in their rates of TB. This has been found to be closely linked to the increase in the number of immigrants from countries with a high incidence of TB (Fig. 2).9 According to data from the European Centre for Disease Prevention and Control, more than 25% of cases of TB in Europe are diagnosed in individuals born in foreign countries. France, Germany, Spain and the United Kingdom account for approximately 75% of all these cases.8 In recent years, the number of cases of TB in the European Union/European Economic Area decreased by 5% annually; however, the number of cases of TB in immigrants increased by 6.8%.10 In Spain, during the period 2007–2013, 49,222 cases of TB were reported; of these, 30.6% (15,058) were diagnosed in patients not born in Spain. In general, the incidence of cases of TB in the immigrant population is linked to the incidences of TB in the country of origin.11
One of the most strongly debated subjects is the possibility of native cases increasing as a result of interaction with the immigrant population. However, many studies have shown that, although secondary cases may occur following contact with immigrants with TB, this does not represent a major public health problem, and in general transmission of TB from immigrants to the native population is limited.12,13 A molecular epidemiology study conducted in Italy showed that of 1080 cases (614 native and 466 immigrant) of TB with a positive culture, 12.9% of native cases were due to transmission by the immigrant population.14 Moreover, transmission from native patients to the immigrant population has also been documented.15 In a dynamic, modern and integrationist society, the flow of TB infections is bidirectional and depends heavily on the interaction of immigrants in the host community.13
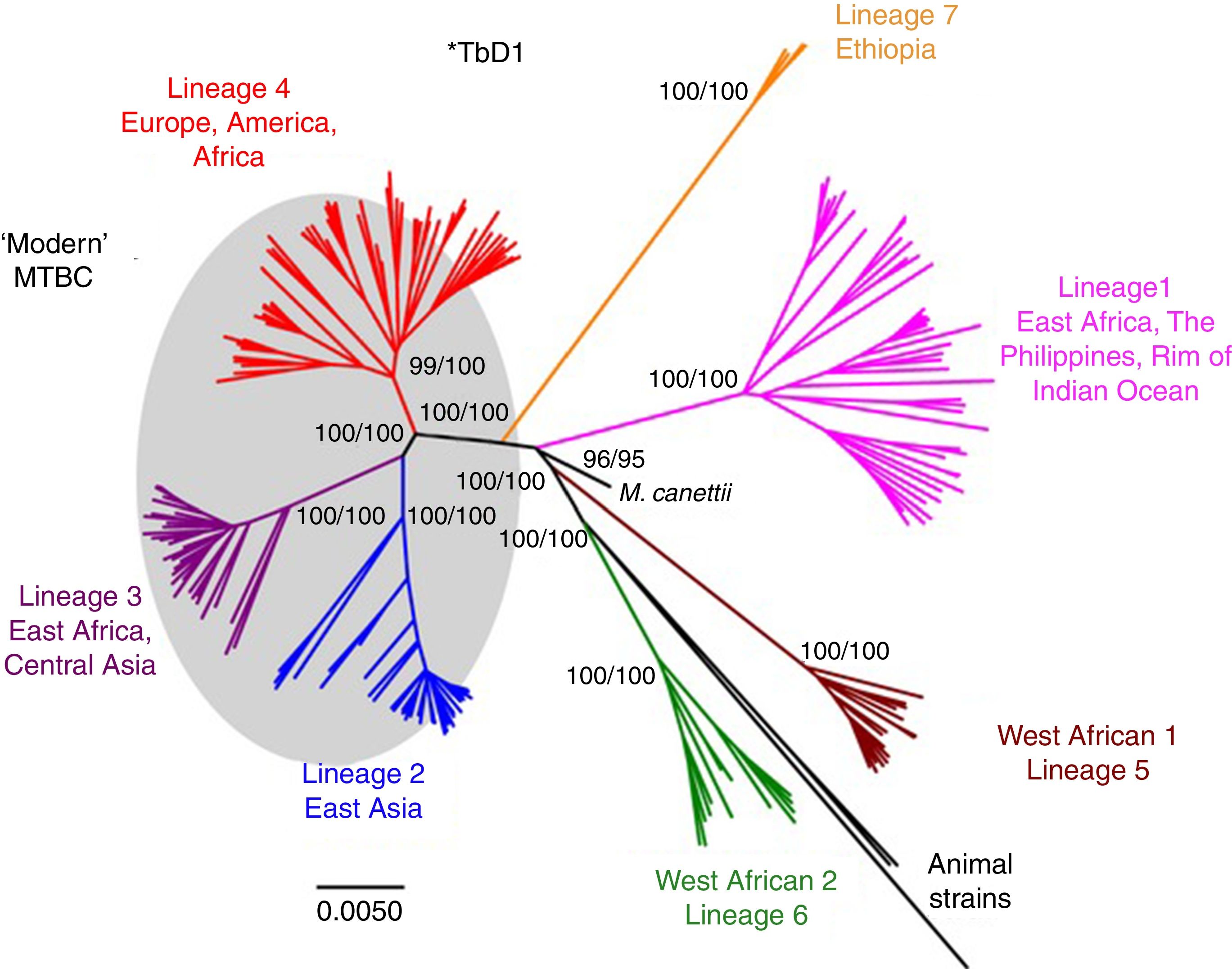
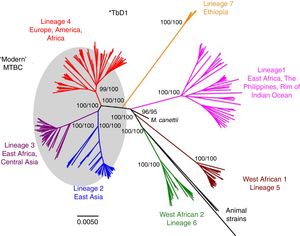
Worldwide, there are different lineages of M. tuberculosis (lineage 1–lineage 7) (Fig. 3). Lineage 4 is the most widespread, probably due to intrinsic factors and patterns of human migration throughout history.16 It has been postulated that exploration and the Spanish and Portuguese conquests underlie the global spread of lineage 4, especially its sub-lineage L4.3/LAM. Each lineage may have several sub-lineages that predominate in different geographical areas and determine its pathogenic characteristics.16 It has been indicated that at least one genotype of M. tuberculosis (Beijing strain, lineage 2) may be more aggressive and more prone to acquiring resistance.17–20 Migration movements are blurring the boundaries of the geographic distribution of lineages and are painting a motley picture which makes it difficult to predict the potential impact on global patterns of transmissibility and pathogenicity. Moreover, resistance to first-line anti-tuberculosis drugs tends to be greater in patients from countries with a high incidence of TB. Patients with multi-drug-resistant (MDR) TB, i.e. TB resistant to isoniazid and rifampin, are concentrated in China, India, former Soviet Union countries and southern Africa. Until recently, it was believed that MDR TB paid the price of a decreased fitness by acquiring resistance mutations and that its transmissibility was decreased. However, in many countries, MDR TB is primarily acquired through contact with a bacilliferous patient and its rate of transmissibility is similar to that of multi-drug-sensitive TB.21,22
Lineages of TB. Taken from Brites and Gagneux.58
All this underscores the fact that eradicating TB in countries with a low incidence means working to control TB in migrant source countries, as well as implementing specific programmes to control TB in immigrants in host countries. If a massive influx of immigrants is not accompanied by a comprehensive healthcare programme that includes a standardised screening strategy for imported diseases as well as health promotion and access measures, rates of TB and resistance to first-line drugs may rise in the coming years.23 On an economic level, investment by host countries in the national programmes of immigrant source countries has been shown to economically benefit host countries.24
Risk of developing tuberculosis in the host country (reactivation vs acute infection)The risk of an immigrant having TB is closely related to host factors, pathogen factors and the interaction between them. Initially, the risk of acquiring a TB infection will be determined by the rate of TB in the community. This in turn will depend on individual factors, living and working conditions, contamination, infrastructure, and healthcare programmes implemented to control TB. Moreover, host factors (concomitant diseases, malnutrition, smoking, etc.) and pathogen factors (virulence, initial infection, etc.) will ultimately modulate the risk of infection or reactivation of a recent or past infection.25
TB in immigrants is primarily due to reactivation of a latent tuberculosis infection (LTI) acquired prior to the migration process. However, there is also the possibility of the patient arriving with active TB or the infection occurring in the host country due to deficient living and working conditions.12,26 Some studies in Spain have found that 22%–28% of cases of TB diagnosed in the immigrant population belonged to clusters, thus pointing to transmission in the host country.27,28 Indeed, some studies have indicated that recent infections in the host country significantly contribute to the disease burden in the immigrant population.29
The diagnosis of TB in immigrants generally occurs within 3 years of their arrival in the host country.30,31 The risk of having TB in immigrants seems to be equivalent to that of the population of the host country 10 years after arrival32; however, the risk may persist for a longer period of time.33 Immigrants who travel often to their countries of origin have a higher risk of having TB due to an acute infection during their travels, especially when these exceed 3 months.34
Although LTI reactivation constitutes the primary mechanism by which TB is acquired in the immigrant population, the dynamics of TB in our society have many subtleties to bear in mind, including the possibility of acute infections in the host country and the risk of infection during sojourns in countries of origin.
Healthcare access and barriers for the immigrant populationManaging social determinants and risk factors associated with TB means enacting policies to decrease poverty, increase food safety, improve living conditions, stop smoking and alcohol use, and prevent development of diabetes mellitus. All this falls under universal healthcare coverage, understood as the provision of comprehensive and high-quality healthcare services for all with no need for a great deal of economic expenditure.4 A framework for action that fails to encompass this big-picture view of the problem will not succeed in eradicating TB.
In general, immigrants are healthy when they arrive from their country of origin (“effect of the healthy immigrant”)35 and their health needs overlap with those of the native population. However, several studies have found that, as time goes by, inequality in social welfare and healthcare sets in due to immigrants’ low socio-economic status, insufficient adaptation to social welfare and healthcare services, cultural and language barriers, and, in some cases, legal restrictions inherent to healthcare and social welfare systems.36
In general, difficulty accessing healthcare systems in host countries causes delayed diagnosis of TB in an individual37,38 and secondarily causes an increase in transmission among that individual's contacts. A study published in Spain in 2014 analysed a total of 20 studies that evaluated immigrant access to healthcare services in that country.39 In general, due to the universal nature of Spanish healthcare until 2012, no major differences were observed in the use of primary care between the immigrant population and the native population. Differences revealed themselves by geographic area (those from Eastern Europe and sub-Saharan Africa used fewer healthcare resources) and with respect to specialised medical care (the immigrant population had less access).40 Greater use of emergency services on the part of this population was also seen.39
The immigrant population perceives TB as a serious but curable disease, yet harbours many fears related to the possibility of infecting other people, being stigmatised or suffering possible consequences in their social relationships and occupational status. Once treatment has started, family support and the doctor–patient relationship are key to ensuring good adherence. The concept of LTI is difficult for the immigrant population to understand, although in general immigrants are quite amenable to screening. Patient-centred TB programmes aimed at early diagnosis of active TB and LTI in people at risk of reactivation would go a long way towards decreasing TB rates in the immigrant population. To achieve this aim, these programmes must be developed with consideration for the barriers that immigrants encounter and the professionals who implement these programmes must be aware of this.41 Information campaigns that inform immigrants about TB and the healthcare system have been shown to reduce diagnostic delays and stigma.42
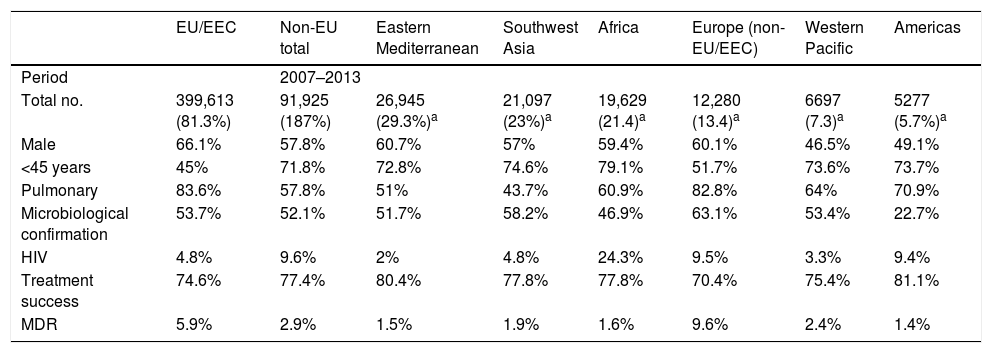
Characteristics of tuberculosis in the immigrant populationThroughout the European Union, immigrant patients with TB tend to be HIV-negative men between the ages of 25 and 44 with pulmonary impairment who have received no prior treatment. The percentage of cases with a microbiologically-confirmed diagnosis, as well as an available antibiogram, is higher than in the native population. Resolution rates have been found to be above those for TB in native patients.10 Rates of resistance to anti-tuberculosis drugs are usually higher in the immigrant population; however, as with other characteristics, they will be determined by the countries of origin and characteristics of the immigrant population. Therefore, being familiar with the characteristics of the immigrant population is essential for dealing with cases of TB in this population, since these emulate the characteristics present in their countries of origin.26 More information is available in Table 3.
Characteristics of the immigrant population with TB in Europe.
| EU/EEC | Non-EU total | Eastern Mediterranean | Southwest Asia | Africa | Europe (non-EU/EEC) | Western Pacific | Americas | |
|---|---|---|---|---|---|---|---|---|
| Period | 2007–2013 | |||||||
| Total no. | 399,613 (81.3%) | 91,925 (187%) | 26,945 (29.3%)a | 21,097 (23%)a | 19,629 (21.4)a | 12,280 (13.4)a | 6697 (7.3)a | 5277 (5.7%)a |
| Male | 66.1% | 57.8% | 60.7% | 57% | 59.4% | 60.1% | 46.5% | 49.1% |
| <45 years | 45% | 71.8% | 72.8% | 74.6% | 79.1% | 51.7% | 73.6% | 73.7% |
| Pulmonary | 83.6% | 57.8% | 51% | 43.7% | 60.9% | 82.8% | 64% | 70.9% |
| Microbiological confirmation | 53.7% | 52.1% | 51.7% | 58.2% | 46.9% | 63.1% | 53.4% | 22.7% |
| HIV | 4.8% | 9.6% | 2% | 4.8% | 24.3% | 9.5% | 3.3% | 9.4% |
| Treatment success | 74.6% | 77.4% | 80.4% | 77.8% | 77.8% | 70.4% | 75.4% | 81.1% |
| MDR | 5.9% | 2.9% | 1.5% | 1.9% | 1.6% | 9.6% | 2.4% | 1.4% |
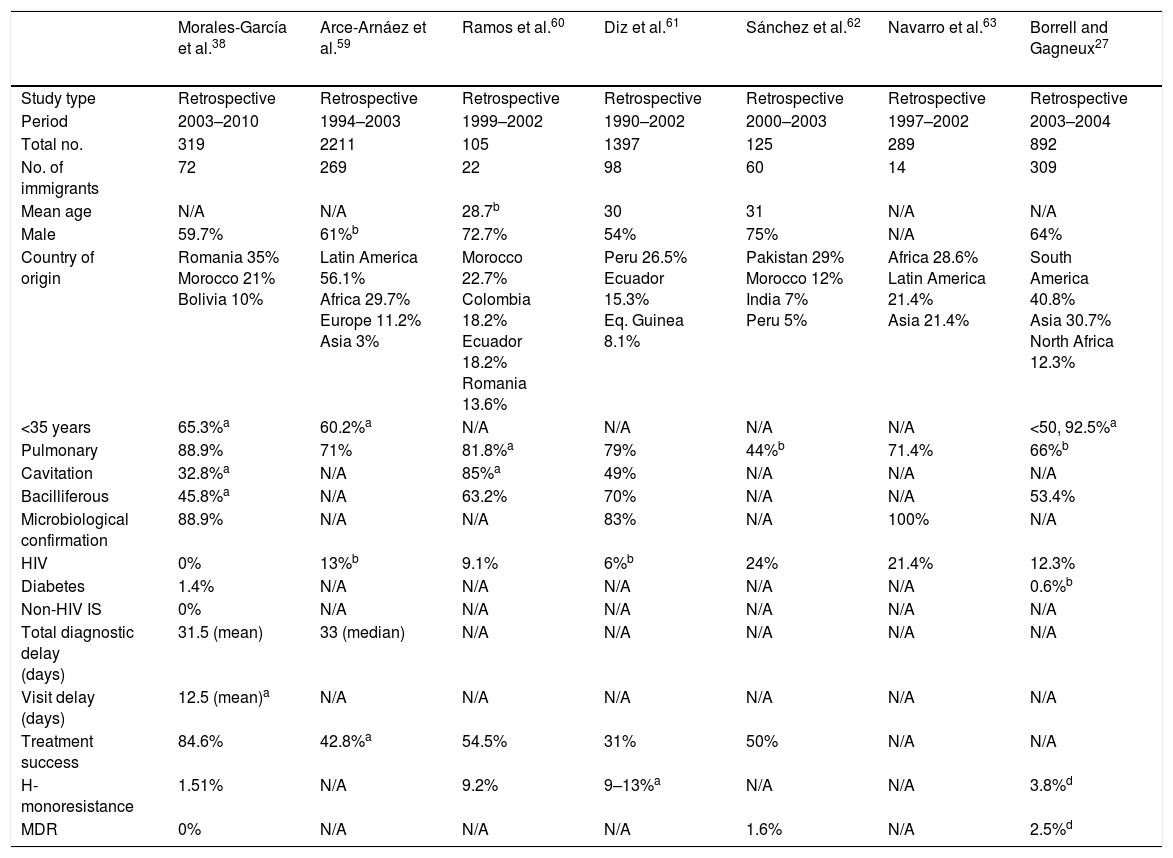
The form of presentation is not consistent throughout all studies; however, it seems that immigrant patients are diagnosed with extra-pulmonary forms more often than native patients. Of course, the pulmonary form is predominant in both populations.38,43 These variations may probably be explained by host factors and mycobacteria-related factors (mycobacteria sub-lineage and virulence factors). On a radiological level, immigrant patients have a higher rate of cavitated lesions and consequently a higher rate of bacilliferous TB.9,38,43Table 4 shows a selection of the most important Spanish studies comparing the characteristics of native and immigrant populations.
Spanish studies comparing immigrant and native populations.
| Morales-García et al.38 | Arce-Arnáez et al.59 | Ramos et al.60 | Diz et al.61 | Sánchez et al.62 | Navarro et al.63 | Borrell and Gagneux27 | |
|---|---|---|---|---|---|---|---|
| Study type | Retrospective | Retrospective | Retrospective | Retrospective | Retrospective | Retrospective | Retrospective |
| Period | 2003–2010 | 1994–2003 | 1999–2002 | 1990–2002 | 2000–2003 | 1997–2002 | 2003–2004 |
| Total no. | 319 | 2211 | 105 | 1397 | 125 | 289 | 892 |
| No. of immigrants | 72 | 269 | 22 | 98 | 60 | 14 | 309 |
| Mean age | N/A | N/A | 28.7b | 30 | 31 | N/A | N/A |
| Male | 59.7% | 61%b | 72.7% | 54% | 75% | N/A | 64% |
| Country of origin | Romania 35% Morocco 21% Bolivia 10% | Latin America 56.1% Africa 29.7% Europe 11.2% Asia 3% | Morocco 22.7% Colombia 18.2% Ecuador 18.2% Romania 13.6% | Peru 26.5% Ecuador 15.3% Eq. Guinea 8.1% | Pakistan 29% Morocco 12% India 7% Peru 5% | Africa 28.6% Latin America 21.4% Asia 21.4% | South America 40.8% Asia 30.7% North Africa 12.3% |
| <35 years | 65.3%a | 60.2%a | N/A | N/A | N/A | N/A | <50, 92.5%a |
| Pulmonary | 88.9% | 71% | 81.8%a | 79% | 44%b | 71.4% | 66%b |
| Cavitation | 32.8%a | N/A | 85%a | 49% | N/A | N/A | N/A |
| Bacilliferous | 45.8%a | N/A | 63.2% | 70% | N/A | N/A | 53.4% |
| Microbiological confirmation | 88.9% | N/A | N/A | 83% | N/A | 100% | N/A |
| HIV | 0% | 13%b | 9.1% | 6%b | 24% | 21.4% | 12.3% |
| Diabetes | 1.4% | N/A | N/A | N/A | N/A | N/A | 0.6%b |
| Non-HIV IS | 0% | N/A | N/A | N/A | N/A | N/A | N/A |
| Total diagnostic delay (days) | 31.5 (mean) | 33 (median) | N/A | N/A | N/A | N/A | N/A |
| Visit delay (days) | 12.5 (mean)a | N/A | N/A | N/A | N/A | N/A | N/A |
| Treatment success | 84.6% | 42.8%a | 54.5% | 31% | 50% | N/A | N/A |
| H-monoresistance | 1.51% | N/A | 9.2% | 9–13%a | N/A | N/A | 3.8%d |
| MDR | 0% | N/A | N/A | N/A | 1.6% | N/A | 2.5%d |
| Peñaranda et al.64 | Tirado Balaguer et al.65 | Santiyán et al.66 | Millares Lozano et al.67 | García-García et al.43 | Martínez-Lirola et al.29 | Sanz Barbero and Blasco Hernández68 | |
|---|---|---|---|---|---|---|---|
| Study type | Retrospective | Retrospective | Retrospective | Retrospective | Prospective | Retrospective | Cross-sectional |
| Period | 1999–2003 | 1995–2003 | 2003–2012 | 2005 | 2006–2007 | 2003–2006 | 2013 |
| Total no. | 408 | 644 | 1667 | 104 | 1490 | 394 | 296 |
| No. of immigrants | 89 | 84 | 620 | 22 | 442 | 211 | 296 |
| Mean age | 31 | N/A | 32.29b | 30b | N/A | 30b | 30 |
| Male | 65% | N/A | 63.2% | 61.9% | 60.2% | N/A | 66.2% |
| Country of origin | Morocco 20% Ecuador 16% Nigeria 5% Senegal Romania 5% India 5% | Romania 36.9% Morocco 23.8% Colombia 7.1% | Bolivia 18.39% Romania 14.52% Ecuador 12.42% Pakistan 6.93% | Latin America 27.8% North Africa 27.8% Sub-Saharan Africa 27.8% | Romania 13.9% Bolivia 13.5% Morocco 11.5% Pakistan 9.5% | Morocco 39.8% Romania 15.6% Senegal 7.6% Mali 6.6% | Ecuador 27.4% Morocco 19.3% Romania 9.8% |
| <35 years | N/A | N/A | N/A | N/A | <50, 93.5%a | N/A | N/A |
| Pulmonary | 67% | N/A | N/A | 71.4%b | 79.2%b | N/A | N/A |
| Cavitation | N/A | N/A | N/A | 36.8% | N/A | N/A | N/A |
| Bacilliferous | N/A | N/A | 39.5%a | N/A | 60% | N/A | N/A |
| Microbiological confirmation | 100% | 100% | N/A | N/A | 77.2% | 100% (inclusion criterion) | N/A |
| HIV | 23.6% | N/A | 12.35%b | 33.3% | 4.3% | N/A | 8.3% |
| Diabetes | N/A | N/A | 2.3%b | N/A | N/A | N/A | 3.3% |
| Non-HIV IS | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Total diagnostic delay (days) | N/A | N/A | N/A | 45.5 | 42 (21–91) | N/A | N/A |
| Visit delay (days) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Treatment success | 85.5% | N/A | N/A | N/A | 83.5%b | N/A | N/A |
| H-monoresistance | N/A | 10.1% | N/A | 8.3% | 8.6%a | 9.3% | N/A |
| MDR | 0% | 0% | 0% | 2.7%a | 1% | N/A |
| Ospina et al.30 | Soler Rangel et al.69 | Basterrechea et al.70 | Navascués Ortega et al.71 | Ordobás Gavín et al.72 | Ruiz López et al.73 | |
|---|---|---|---|---|---|---|
| Study type | Retrospective | Retrospective | Retrospective | Retrospective | Retrospective | Retrospective |
| Period | 1991–2013 | 1997–2006 | 2003–2007 | 2000–2007 | 1996–2004 | 1999–2004 |
| Total no. | 3284 | 832 | 903 | 457 | 10,268 | 158 |
| No. of immigrants | 3284 | 311 | 94 | 120 | 2067 | 66 |
| Mean age | N/A | 32.37b | 31b | N/A | N/A | 29.9b |
| Male | 65.7% | 59.8%b | 63% | N/A | N/A | N/A |
| Country of origin | Latin America 37.3% Indian sub-continent 26.6% | Ecuador 25% Morocco 17% Romania 12% Peru 11% | Morocco 26.6% Ecuador 10.6% Pakistan 9.6% | Latin America 36.6% North Africa 28.3% Western Europe 14.2% Sub-Saharan Africa 8.3% | N/A | N/A |
| <35 years | 64%c | N/A | 77.6% | N/A | N/A | N/A |
| Pulmonary | 58.1% | 100% | 68.1% | N/A | 73.79% | N/A |
| Cavitation | 32.3% | 78.8%b | N/A | N/A | N/A | N/A |
| Bacilliferous | 48.2% | 34% | N/A | N/A | N/A | |
| Microbiological confirmation | N/A | 91.7% | 72.3% | 100% | N/A | N/A |
| HIV | 10% | 6.4%b | 5.3% | N/A | N/A | N/A |
| Diabetes | 2.8% | 3.5%b | N/A | N/A | N/A | N/A |
| Non-HIV IS | N/A | 0.3% | 4.3%e | N/A | N/A | N/A |
| Total diagnostic delay (days) | N/A | N/A | 68 | N/A | N/A | N/A |
| Visit delay (days) | N/A | N/A | N/A | N/A | N/A | N/A |
| Treatment success | 84% | 74% | 79.8%b | N/A | N/A | N/A |
| H-monoresistance | 9.2% | 8.3% | N/A | 11.6% | N/A | 10.2% |
| MDR | 2.1% | 4% | 0% | 2.5% | N/A | 2.1% |
Causes of delayed diagnosis vary by patient origin, but are primarily lumped under outside reasons related to the healthcare system, or individual reasons such as fear of being reported to the authorities, losing their job, being kicked out of their homes or enduring the stigma associated with the disease. In general, native patients have a higher number of risk factors for developing active disease, such as HIV infection, parenteral drug use, smoking, alcoholism, diabetes, immunosuppression, chronic kidney failure and immunosenescence. These also underlie the greater mortality in the native population.9,38,43 In addition, contacts of immigrants with TB have higher rates of latent infections and active disease than contacts of native individuals with TB.38
In general, immigrants with TB wait longer after developing symptoms to seek medical assistance. However, once they enter the healthcare system, they are diagnosed earlier than native patients, probably due to a stronger diagnostic suspicion on the part of the healthcare staff. This divide is not as sharp in cases of patients with bacilliferous pulmonary TB.38 In general, immigrants are diagnosed in the emergency department at a higher rate than the native population.43
The same diagnostic techniques are used in the two groups. The introduction of molecular techniques has helped to reduce the length of diagnostic delays, particularly in patients without bacilliferous TB and with HIV infection. This aids in starting treatment early and decreasing transmission.44 With regard to drug resistance, studies conducted in Spain have yielded mixed results. Monoresistance to isoniazid ranges from 1.5% to 10.2% (Table 4). Fortunately, diagnoses of MDR TB remain very few and far between.43 It is likely that rates of resistance to anti-tuberculosis drugs in the immigrant population correspond to rates of resistance reported by countries of origin. Therefore, resistance may be inferred through familiarity with the local immigrant population.
The choice of anti-tuberculosis treatment is made with consideration for individual patient characteristics such as pregnancy status, concomitant medication, prior anti-tuberculosis treatments, comorbidities and patterns of resistance. Until an antibiogram is available, at least 4 drugs believed to be active against the patient's strain of M. tuberculosis will be used.45 There seem to be no differences in terms of treatment toxicity. Treatment success, which includes completed treatment and microbiological resolution, is similar in the two groups. However, immigrant patients stop treatment more often than the native population, and native patients have higher mortality rates in the course of treatment due to their greater comorbidity rates and older age.38,43,46 Stopping treatment in the immigrant population in Spain has been linked to being male, not living with any family members and moving to other communities. To these must be added other known variables such as prior anti-tuberculosis treatment, poor understanding of the disease and parenteral drug use.47,48 To maintain a good success rate, it is necessary to have a programme centred on patient needs that includes interventions in all sociocultural matters. It is also necessary to have economic resources to fund strategies such as directly observed treatment and community health agents.43,49
At present, a wide range of drugs and combinations is available to treat LTI.50 The choice of treatment for LTI in immigrants and native patients must be made with a view to the pattern of resistance of the index case if known; otherwise, any regimen having been shown to reduce reactivation of the disease shall be used.
Proposals for decreasing tuberculosis in the immigrant populationEfforts to control TB, especially in countries with a low incidence such as Spain, should place a special emphasis on risk groups such as immigrants, since these groups may have limited access to means of TB prevention, diagnosis and treatment.51 The WHO and the International Organization for Migration have proposed a number of efforts to decrease the incidence of TB in the immigrant population. These efforts urge the development of national TB plans that are inclusive with intersectoral and cross-border immigration, policies and systems, and operational research.52
Clearly, to decrease the incidence of TB in the immigrant population, the host country should ensure that everybody who has TB or requires preventive measures be able to access diagnostic tests, treatment and preventive measures. This means developing political and administrative measures (universal access to healthcare, ease of obtaining a healthcare card, etc.), raising awareness among healthcare staff and educating this population in using the healthcare system.53
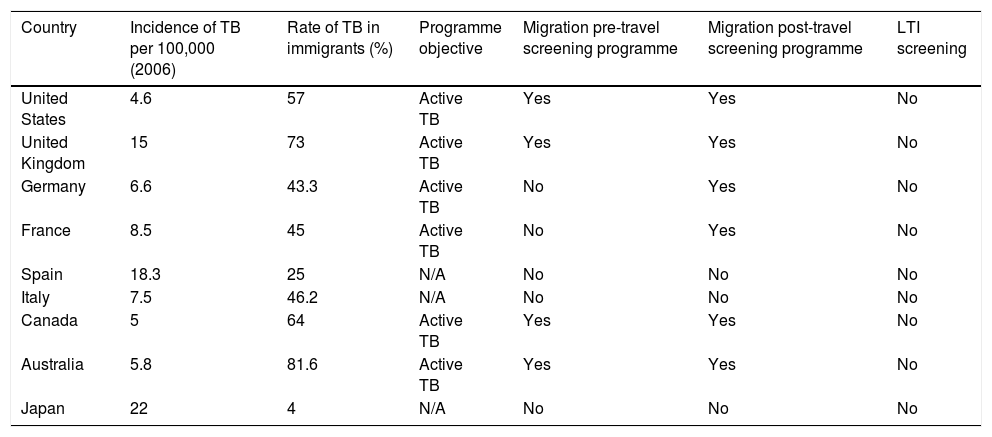
Screening of the immigrant population is a cornerstone of these programmes for TB control. In the area of prevention, screening of this population is essential to find both active cases and people with LTI. Although screening algorithms are highly variable, more and more data are emerging that indicate that the most cost-effective strategy would be to perform an IGRA test in people under 35 years of age from a country with a high incidence of TB.54 Interventions may be classified as pre-travel or on-arrival depending on when screening is performed. The objective of screening performed in the country of origin (pre-travel) is to diagnose active disease to prevent transmission in the host country. When the programme is carried out in the host country, it is often broadened to include testing for LTI55 (Table 5). From a practical perspective, with a view to proper implementation, it is essential to involve and train primary care practitioners, as primary care is where this population is most likely to come into contact with the healthcare system.
Strategies for screening the immigrant population in developed countries with a low incidence of tuberculosisa
| Country | Incidence of TB per 100,000 (2006) | Rate of TB in immigrants (%) | Programme objective | Migration pre-travel screening programme | Migration post-travel screening programme | LTI screening |
|---|---|---|---|---|---|---|
| United States | 4.6 | 57 | Active TB | Yes | Yes | No |
| United Kingdom | 15 | 73 | Active TB | Yes | Yes | No |
| Germany | 6.6 | 43.3 | Active TB | No | Yes | No |
| France | 8.5 | 45 | Active TB | No | Yes | No |
| Spain | 18.3 | 25 | N/A | No | No | No |
| Italy | 7.5 | 46.2 | N/A | No | No | No |
| Canada | 5 | 64 | Active TB | Yes | Yes | No |
| Australia | 5.8 | 81.6 | Active TB | Yes | Yes | No |
| Japan | 22 | 4 | N/A | No | No | No |
There is significant variation within the programme itself depending on type of migrant, length of stay and profession as well as special situations (e.g. adoptions). The table above provides an overview of the situation.
ITL: latent tuberculosis infection; N/A: not applicable; TB: tuberculosis.
Adapted from Alvarez et al.55
The likelihood of an immigrant patient having MDR TB will vary depending on his or her geographic area of origin. Although the number of cases of MDR TB in Spain is low, this disease represents a growing problem that is generating concern.43 To address this problem, reference hospitals must have laboratories equipped with suitable diagnostic tools, including molecular biology techniques for early detection of resistance to anti-tuberculosis drugs. In addition, specialist practitioners with experience in treating and following up these patients are needed, since these patients need complex treatments and experience high rates of adverse effects.44
TB programmes should be patient-centred. This takes on greater importance in immigrant patients, since their social, cultural and religious realities may be very different from those of the native population. These sociocultural differences may tremendously alter the patient's perception of the disease and the healthcare system, with repercussions for disease prognosis and transmission.47 Hence the increasing emphasis on the importance of creating specialised units for managing patients with TB, which feature not only physicians, microbiologists and nurses but also professionals such as case managers (with experience in treatment adherence and contact study) and community health agents.
Finally, this type of population must be taken into account in TB research and figure among its priorities. Operational research is necessary to evaluate different strategies that ensure equitable healthcare access for all immigrants and to develop innovative tools for prioritising and identifying populations at risk.56,57
LimitationsThis article was not prepared according to the standards of a systematic review. As a result, it is subject to article selection bias. Although many surveillance systems in Europe and Spain are solid, it is impossible for a system to record all migrations and cases of TB. These limitations notwithstanding, the information provided herein constitutes a useful review on the topic of immigration and TB.
ConclusionTB in countries with a low incidence of TB is approaching eradication in the next few decades. When this objective will be achieved depends on the implementation of programmes that include the immigrant population among their priority objectives, as this population accounts for a significant percentage of active cases of TB diagnosed in countries with a low incidence of TB. Creating a patient-centred system that addresses social, cultural and economic considerations will help to accelerate the eradication of TB.
Conflicts of interestThere are no conflicts of interest.
Please cite this article as: Sánchez-Montalvá A, Salvador F, Molina-Morant D, Molina I. Tuberculosis e inmigración. Enferm Infecc Microbiol Clin. 2018;36:446–455.