HIV pre-exposure prophylaxis (PrEP) consists of administering antiretroviral drugs to seronegative individuals with high risk practices. The aim of the study was to describe the characteristics of recent seroconverted HIV patients in order to determine the profile of the appropriate candidates for PrEP.
MethodsA descriptive study of all patients diagnosed with HIV infection in 2014, and who had achieved a documented negative serology over the previous 12 months. A specific form was completed to determine the sociodemographic, behavioural, and clinical features, with complementary tests being performed for other sexually transmitted infections.
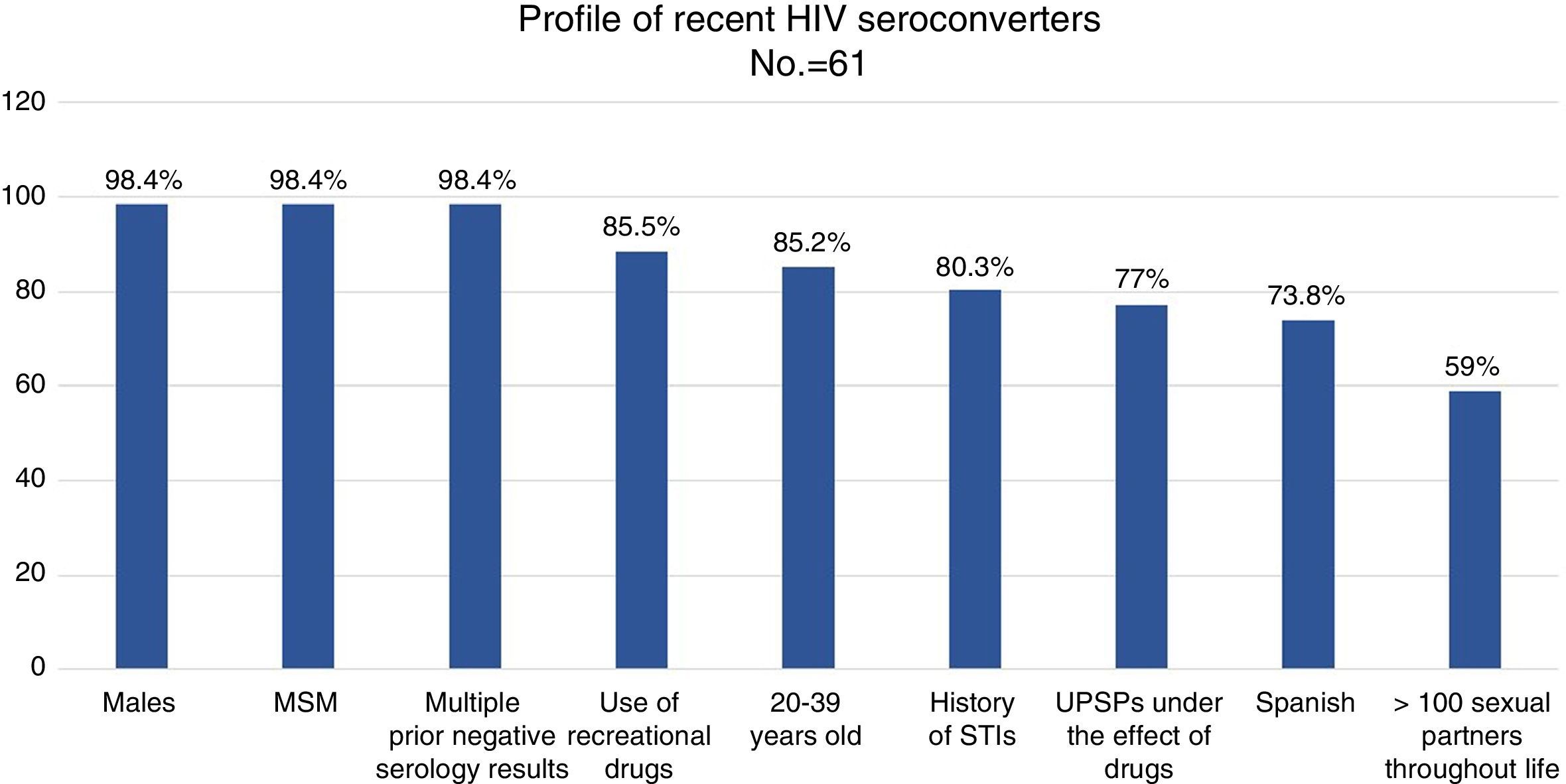
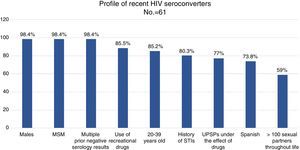
ResultsAlmost all (98.4%) of the 61 recent seroconverted were men who have sex with men, and aged between 20 and 39 years (88.5%). They also had a background of sexually transmitted infections (80.3%), performed multiple and unprotected sexual practices (82.7%), and under the effect of recreational drugs (87%).
ConclusionsThe evaluation of the risk factors for HIV infection in seronegative patients should enable the appropriate candidates for PrEP to be identified.
La profilaxis preexposición (PrEP) al VIH consiste en administrar fármacos antirretrovirales a personas negativas para el VIH con prácticas de riesgo. El objetivo del estudio fue describir las características de los serconvertores recientes al VIH para conocer el perfil de los candidatos a quienes proponer PrEP.
MétodosEstudio descriptivo de todos los pacientes diagnosticados de VIH durante 2014, con serología negativa documentada en los 12 meses previos. Se pasó un cuestionario estructurado para conocer características sociodemográficas, conductuales y clínicas, y se realizó despistaje de otras ITS.
ResultadosEl 98,4% de los 61 seroconvertores recientes eran hombres que tenían sexo con hombres, de 20 a 39 años (88,5%), con antecedentes de ITS (80,3%) y múltiples parejas con las que mantenían sexo sin preservativo (82,7%), bajo el efecto de drogas recreativas (87%).
ConclusionesEvaluar el riesgo para el VIH de los pacientes seronegativos permite identificar a los candidatos idóneos a quienes proponer la PrEP.
Pre-exposure prophylaxis (PrEP) for HIV is a new preventive tool that consists of administering antiretroviral drugs to people negative for HIV (seronegative) with significant risk behaviours, in order to reduce the likelihood of acquiring the infection.
Several randomised placebo-controlled clinical trials have confirmed that daily oral PrEP is safe and effective.1 Tenofovir disoproxil fumarate (TDF) or TDF plus emtricitabine (FTC), in a daily oral regimen, significantly reduces HIV incidence in all transmission categories: men who have sex with men (MSM), transsexual people, heterosexual (HTX) men and women, serodiscordant HTX couples and injecting drug users (IDUs).2–4
The FDA approved the indication for PrEP with TDF/FTC in 2012.5 The Centers for Disease Control and Prevention (CDC) have recommended the daily use of one tablet coformulated with 300mg of TDF and 200mg of FTC, due to its rapid diffusion and high concentration in the rectal and genital tract.6,7 The PROUD study,8 conducted at 13 sexually transmitted infection (STI) clinics in England, found the same preventive efficacy with daily PrEP that the IPERGAY study9 found with a pre- and post-coital “on-demand” regimen. The effectiveness of PrEP is closely correlated with the degree of adherence to PrEP.2,9
The WHO recommends offering daily oral PrEP to people who have a “substantial” risk of acquiring the infection and who belong to population groups with an HIV incidence higher than 3 per 100 people per year, together with other preventive measures such as promotion of the use of a condom, screening for other STIs, access to early diagnosis and universal antiretroviral therapy (ART). In these population groups, it is estimated that PrEP is cost-effective compared to ART throughout life.10
Follow-up of patients not infected with HIV with risk factors for acquiring the infection enables people who should be offered PrEP to be identified.
The objective of our study was to determine the socio-demographic characteristics, behavioural habits and clinical indicators of recent seroconverters (SCVs) to determine the profile of candidates for PrEP.
Material and methodsIn this study, people with documented negative serology in the 12 months prior to being diagnosed with HIV were considered recent SCVs. At an STI clinic in Madrid in 2014, 307 patients were diagnosed with HIV infection; of them, 61 (19.9%) were recent SCVs. A descriptive study was conducted of the socio-demographic, behavioural and clinical characteristics of these 61 recent SCVs. All of them were prospectively given a structured and validated epidemiological questionnaire in order to determine their socio-demographic information, sexual practices, frequency of condom use, history of STIs throughout their sex life, prior negative serology results for HIV, blood donations and toxic habits. The complementary tests performed included screening for other STIs: syphilis (ELISA and TPPA), gonorrhoea (Gram staining, culture in Thayer-Martin medium and PCR), infection with Chlamydia trachomatis and genital herpes (PCR), and hepatitis C virus (ELISA).
The statistical analysis was performed using SPSS PASW Statistics 18.0.
Results98.4% of the recent SCVs had more than one negative test performed throughout their life: 50% had 2–5, 46.7% had 6–15 and 3.3% had more than 15. 23% of the patients (n=14) had donated blood. 9.8% (n=6) of the recent SCVs had received post-exposure prophylaxis (PPE) at some point.
98.4% (n=60) were men. 85.2% were 20–39 years old, and 11.5% were 40–49 years old (range: 17–59 years old).
73.8% (n=45) were Spanish, 19.7% (n=12) were Latin American, 3 were European and one was American. 100% of the patients reported that they had been infected in Spain.
98.4% (n=60) were MSM, 3 were sex workers and one was an HTX woman. None was an IDU. 59% of the patients reported more than 100 sex partners in the course of their lives, and 36.1% of them (n=13) reported more than 500 sex partners in the course of their lives.
89.3% had anal sex without a condom with stable partners, and 76% had anal sex without a condom with occasional partners. Just one patient used a condom in oral sex. 9.8% (n=6) of the SCVs practised anal fisting.
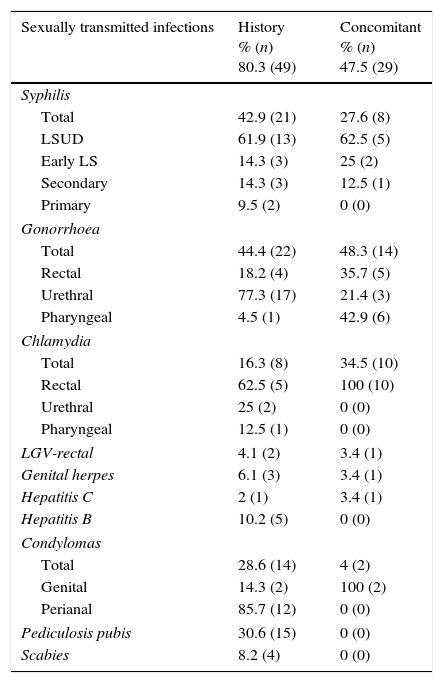
80.3% (n=49) had a history of STIs and 47.5% (n=29) had concomitant STIs when they were diagnosed with HIV (Table 1).
History of sexually transmitted infections and sexually transmitted infections concomitant with HIV diagnosis in recent seroconverters.
| Sexually transmitted infections | History % (n) 80.3 (49) | Concomitant % (n) 47.5 (29) |
|---|---|---|
| Syphilis | ||
| Total | 42.9 (21) | 27.6 (8) |
| LSUD | 61.9 (13) | 62.5 (5) |
| Early LS | 14.3 (3) | 25 (2) |
| Secondary | 14.3 (3) | 12.5 (1) |
| Primary | 9.5 (2) | 0 (0) |
| Gonorrhoea | ||
| Total | 44.4 (22) | 48.3 (14) |
| Rectal | 18.2 (4) | 35.7 (5) |
| Urethral | 77.3 (17) | 21.4 (3) |
| Pharyngeal | 4.5 (1) | 42.9 (6) |
| Chlamydia | ||
| Total | 16.3 (8) | 34.5 (10) |
| Rectal | 62.5 (5) | 100 (10) |
| Urethral | 25 (2) | 0 (0) |
| Pharyngeal | 12.5 (1) | 0 (0) |
| LGV-rectal | 4.1 (2) | 3.4 (1) |
| Genital herpes | 6.1 (3) | 3.4 (1) |
| Hepatitis C | 2 (1) | 3.4 (1) |
| Hepatitis B | 10.2 (5) | 0 (0) |
| Condylomas | ||
| Total | 28.6 (14) | 4 (2) |
| Genital | 14.3 (2) | 100 (2) |
| Perianal | 85.7 (12) | 0 (0) |
| Pediculosis pubis | 30.6 (15) | 0 (0) |
| Scabies | 8.2 (4) | 0 (0) |
LSUD, latent syphilis of unknown duration.
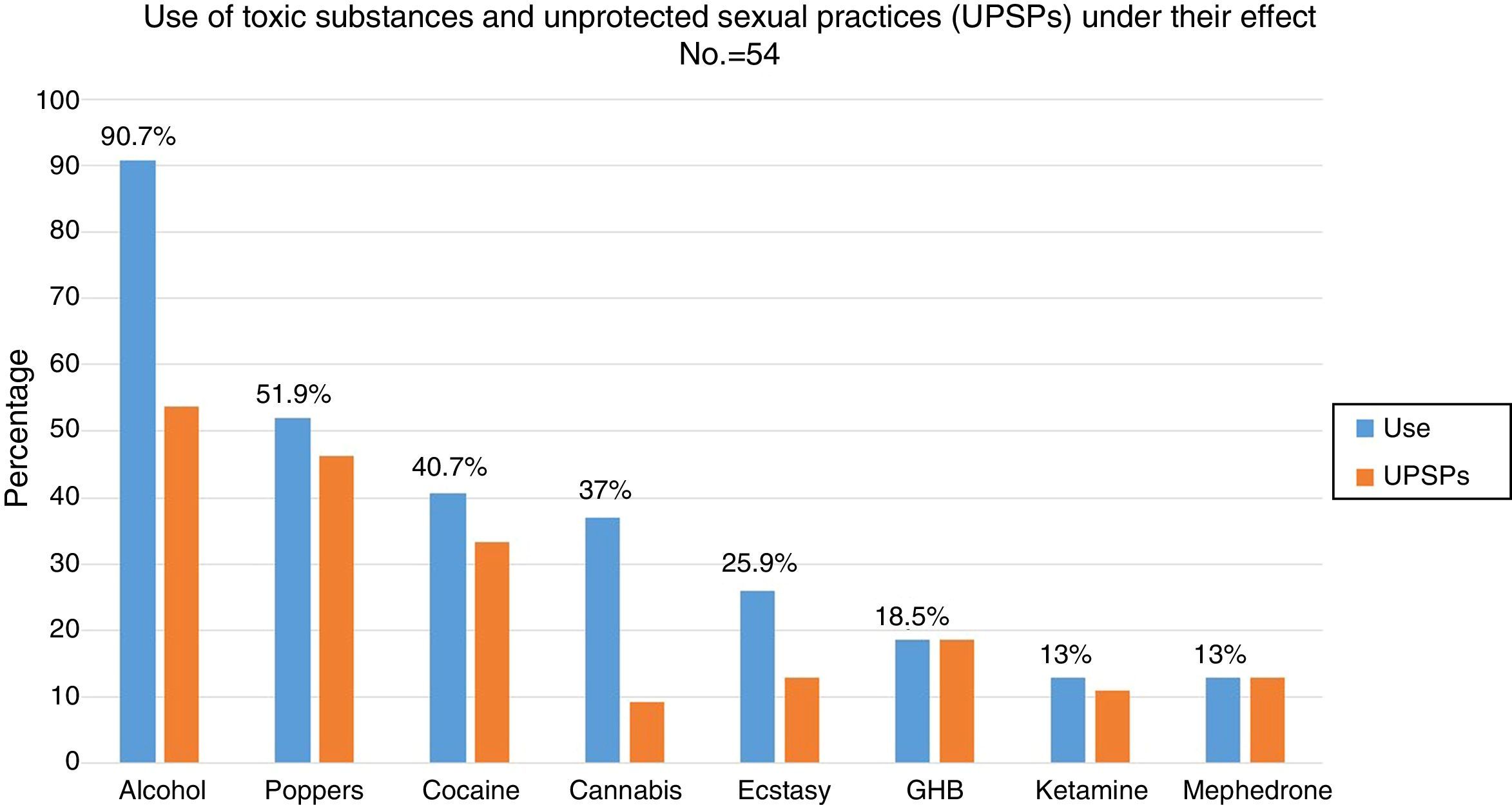
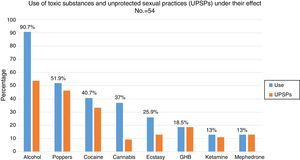
88.5% (n=54) had used recreational drugs in the last year; of them, 87% (n=47) had engaged in unprotected sexual practices under the effect of these substances. The most commonly used drugs, from most common to least common, were: alcohol “in excess” (90.7%), poppers (51.9%), cocaine (40.7%), cannabis (37%), ecstasy (25.9%), gamma-hydroxybutyric acid (GHB) (18.5%), ketamine (13%) and mephedrone (13%). The drugs with the strongest association with engaging in unprotected sexual practices were: mephedrone (100%), GHB (100%), poppers (96.3%), ketamine (85.7%), cocaine (77.3%), alcohol “in excess” (65.9%) and ecstasy (53.8%) (Fig. 1). Among the recreational drug users, 38.8% shared snorting utensils.
DiscussionAccording to the CDC guidelines, candidates for PrEP would be seronegative MSM and HTXs with an HIV-positive partner, a bacterial STI in the last 6 months, multiple sexual partners, systematic non-use of a condom and sex workers. IDUs and transsexual people with risk practices would also be candidates for PrEP.6 In our study, the profile of the recent SCVs matched the profile specified by the CDC as belonging to candidates for PrEP. Other markers in addition to these which could also help to identify candidates with a higher risk of acquiring HIV infection were taken into account. These other markers included number of prior negative serology results and sexual practices without a condom under the effect of recreational drugs.
A study published in 2015 estimated that, in the United States, PrEP would be indicated in approximately 1,232,000 adults: 24.7% of MSM (492,000 people), 18.5% of IDUs (115,000 people) and 0.4% of HTXs (468,000 people). The criteria used to arrive at this estimate among adult, seronegative and sexually active MSM were: systematic non-use of a condom, history of STIs in the last 12 months and sexual relations without a condom with a partner with unknown HIV serology.11 89.3% of the patients in our study had anal sex without a condom with their stable partner, and 76% had anal sex without a condom with occasional contacts. 80.3% had a history of an STI, which is an objective marker of risk for acquiring HIV, according to a study conducted at an STI clinic in New York.12
In the United States and Spain, the high percentage of MSM with an indication for PrEP is linked to the high incidence of new diagnoses of HIV in this group. The EPI-VIH group, a network of 20 HIV/STI diagnosis centres throughout Spain, conducted a prospective study on HIV prevalence that analysed 155,890 people and found that the prevalence among MSM, at the first visit, increased from 7.4% to 12.7% between 2000 and 2010. In Spain, the incidence of newly diagnosed HIV from 2009 to 2014 decreased among HTXs and IDUs; however, it modestly increased among Spanish MSM.13 At an STI clinic in Madrid, among 6437 MSM analysed, at the first visit, HIV prevalence increased from 12% in 2012 to 14.8% in 2014 (p<0.001).14
Given this epidemiological situation, it appears necessary to implement new additional preventive interventions such as PrEP. A British study published in January 2016 estimated that implementation of PrEP, together with early diagnosis and universal access to ART, would halve HIV incidence among MSM by 2020.15
Our study was conducted at an STI clinic in Madrid. It may not be possible to extrapolate the data to other healthcare systems or geographic regions.
MSM seronegative for HIV between 20 and 39 years of age, with several prior negative tests, with a history of STIs, with multiple sexual partners and who have sexual relations without a condom, often under the effect of alcohol and other recreational drugs, are ideal candidates for being offered PrEP as an additional preventive measure (Fig. 2). STI clinics may identify and follow up HIV-negative people with a high risk of acquiring HIV and instate personalised preventive counselling. Therefore, they are ideal systems for proposing and administering PrEP in order to prevent new infections.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Ayerdi-Aguirrebengoa O, Vera-García M, Puerta-López T, Raposo-Utrilla M, Rodríguez-Martín C, Del Romero-Guerrero J. ¿A quién proponer la profilaxis preexposición al virus de la inmunodeficiencia humana? Enferm Infecc Microbiol Clin. 2017;35:299–302.