To describe the distribution of Streptococcus pneumoniae serotypes isolated in cerebrospinal fluid (CSF) between 2007–2018 in the Community of Madrid (CM) and to identify those with higher meningeal tropism.
MethodsStrains isolated from invasive pneumococcal disease were sent to the Regional Laboratory of Public Health by Microbiology laboratories of public and private hospitals of the CM. The frequency of serotypes from CSF was compared with that observed in other samples.
ResultsA total of 6115 strains were processed and 5% (n = 304) were isolated from CSF. Seven serotypes (11A, 19F, 23B, 10A, 24F, 23A and 35F) showed a frequency significantly higher in CSF than in other sterile samples. Serotypes 24F, 11A and 23B showed high penicillin-resistance.
ConclusionThe frequency and resistance of certain pneumococcal serotypes with high meningeal tropism could compromise the treatment of central nervous system infections.
Se describe la distribución de serotipos de Streptococcus pneumoniae aislados en líquido cefaloraquídeo (LCR) entre los años 2007–2018 en la Comunidad de Madrid (CM) identificando aquellos con mayor tropismo meníngeo.
MétodosSe estudiaron las cepas de episodios de enfermedad neumocócica invasora enviadas al Laboratorio Regional de Salud Pública por los servicios de Microbiología de hospitales públicos y privados de la CM. La frecuencia de serotipos procedentes de LCR se comparó con la observada en otras muestras.
ResultadosSe procesaron 6.115 cepas. El 5% (n = 304) se aislaron en LCR. Siete serotipos (11A, 19F, 23B, 10A, 24F, 23A y 35F) mostraron una frecuencia en LCR significativamente mayor que en otras muestras estériles. Los serotipos 24F, 11A y 23B mostraron alta resistencia a la penicilina.
ConclusiónLa frecuencia y resistencia de determinados serotipos de neumococo con elevado tropismo meníngeo podría comprometer el tratamiento de las infecciones del sistema nervioso central.
Meningitis is a serious form of presentation of invasive pneumococcal disease (IPD).1 Isolation of Streptococcus pneumoniae in samples of cerebrospinal fluid (CSF) is the most specific microbiological method for the diagnosis of this infection.2 The treatment of pneumococcal meningitis generally includes penicillin or third-generation cephalosporins (cefotaxime or ceftriaxone) sometimes combined with vancomycin.3 The polysaccharide capsule of Streptococcus pneumoniae is considered essential for causing meningitis and the capsule type appears to have a direct effect on the severity of the clinical course of the disease.4 The objectives of this study are to report the distribution of serotypes in strains isolated in CSF samples from 2007 to 2018 in the Community of Madrid (CM) and to identify those with higher meningeal tropism.
Material and methodsStrains from episodes of IPD sent by microbiology departments at public and private hospitals in the CM to the Regional Public Health Laboratory during the study period were studied. Isolates were serotyped with a latex agglutination test (Pneumotest-Latex) and a standard Quellung reaction test5 using type and factor antisera (Statens Serum Institut, Copenhagen, Denmark). Sensitivity to antibiotics (penicillin, cefotaxime, and vancomycin) was determined with the Etest® (bioMerieux, France) gradient diffusion method according to the criteria for interpretation of the European Committee for Antimicrobial Susceptibility Testing (EUCAST)6 (minimum inhibitory concentration of penicillin ≤ 0.06 mg/l sensitive and >0.06 mg/l resistant [cut-off point for meningitis], cefotaxime ≤ 0.5 mg/l sensitive and >2 mg/l resistant and vancomycin ≤ 2 mg/l sensitive and >2 mg/l resistant). Frequencies of serotypes from CSF samples were compared to those observed in samples from other locations using the odds ratio (OR), with a 95% confidence interval. Changes over time (four-year periods of 2007−2010, 2011−2014 and 2015−2018) were analyzed by calculating the chi-squared test for linear trend.
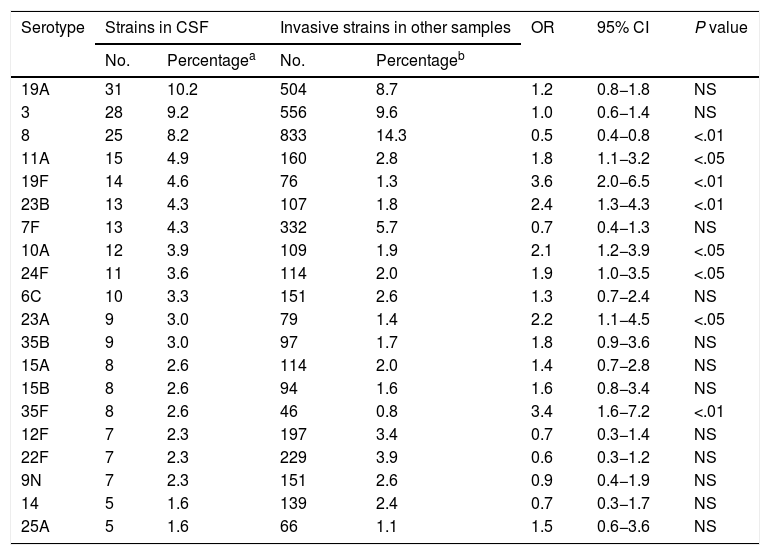
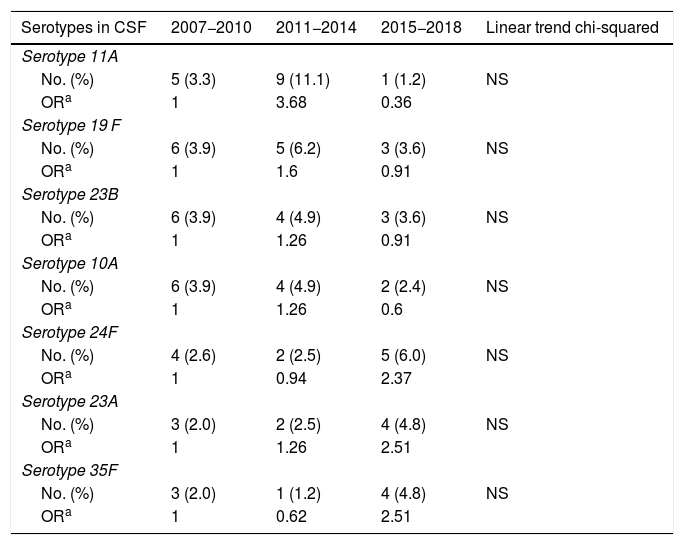
ResultsA total of 6115 invasive pneumococcal strains were processed. Among these strains, 5% (n = 304) were isolated in CSF samples. Of these, 94.7% were identified at the serotype level (totaling 41 different serotypes), 3.9% were identified at the serogroup level (totaling 6 different serogroups), and 1.3% were not serotyped. In total, 20 different serotypes were isolated in 80.4% of CSF samples; of these, serotypes 19F, 23B, 35F, 11A, 24F, 10A and 23A showed a higher frequency of appearance in CSF than in other locations (P < .05) (Table 1). Variation in the frequency of isolation of these 7 serotypes over time did not attain statistical significance for any of them (Table 2); however, all strains isolated in CSF decreased to a statistically significant extent (P < .01) from 140 (5.7%; OR: 1) in 2007−2010 to 81 (5.2%; OR: 0.91) in 2011−2014 and then to 83 (4.0%; OR: 0.68) in 2015−2018. Taken together, of the total of 304 strains isolated in CSF, 102 (33.6%) were resistant to penicillin, 43 (14.1%) showed intermediate sensitivity to cefotaxime and 2 (0.7%) were resistant to cefotaxime. All strains were sensitive to vancomycin. Regarding the sensitivity to antimicrobial agents of the different serotypes, 9 (60.0%) of the 15 strains of serotype 11A isolated in CSF were resistant to penicillin and 7 (46.7%) showed intermediate sensitivity to cefotaxime; 5 (35.7%) of the 14 strains of serotype 19F were resistant to penicillin and (7.1%) showed intermediate sensitivity to cefotaxime; 7 (53.8%) of the 13 strains of serotype 23B were resistant to penicillin and all were sensitive to cefotaxime; the 12 strains of serotype 10A proved sensitive to both penicillin and cefotaxime; 7 (63.6%) of the 11 strains of serotype 24F were resistant to penicillin and (9.1%) presented intermediate sensitivity to cefotaxime; one (11.1%) of the 9 strains of serotype 23A were resistant to penicillin and all were sensitive to cefotaxime; and the 8 strains of serotype 35F were sensitive to both antibiotics.
Serotypes of Streptococcus pneumoniae most commonly isolated in CSF. Community of Madrid, 2007–2018.
| Serotype | Strains in CSF | Invasive strains in other samples | OR | 95% CI | P value | ||
|---|---|---|---|---|---|---|---|
| No. | Percentagea | No. | Percentageb | ||||
| 19A | 31 | 10.2 | 504 | 8.7 | 1.2 | 0.8−1.8 | NS |
| 3 | 28 | 9.2 | 556 | 9.6 | 1.0 | 0.6−1.4 | NS |
| 8 | 25 | 8.2 | 833 | 14.3 | 0.5 | 0.4−0.8 | <.01 |
| 11A | 15 | 4.9 | 160 | 2.8 | 1.8 | 1.1−3.2 | <.05 |
| 19F | 14 | 4.6 | 76 | 1.3 | 3.6 | 2.0−6.5 | <.01 |
| 23B | 13 | 4.3 | 107 | 1.8 | 2.4 | 1.3−4.3 | <.01 |
| 7F | 13 | 4.3 | 332 | 5.7 | 0.7 | 0.4−1.3 | NS |
| 10A | 12 | 3.9 | 109 | 1.9 | 2.1 | 1.2−3.9 | <.05 |
| 24F | 11 | 3.6 | 114 | 2.0 | 1.9 | 1.0−3.5 | <.05 |
| 6C | 10 | 3.3 | 151 | 2.6 | 1.3 | 0.7−2.4 | NS |
| 23A | 9 | 3.0 | 79 | 1.4 | 2.2 | 1.1−4.5 | <.05 |
| 35B | 9 | 3.0 | 97 | 1.7 | 1.8 | 0.9−3.6 | NS |
| 15A | 8 | 2.6 | 114 | 2.0 | 1.4 | 0.7−2.8 | NS |
| 15B | 8 | 2.6 | 94 | 1.6 | 1.6 | 0.8−3.4 | NS |
| 35F | 8 | 2.6 | 46 | 0.8 | 3.4 | 1.6−7.2 | <.01 |
| 12F | 7 | 2.3 | 197 | 3.4 | 0.7 | 0.3−1.4 | NS |
| 22F | 7 | 2.3 | 229 | 3.9 | 0.6 | 0.3−1.2 | NS |
| 9N | 7 | 2.3 | 151 | 2.6 | 0.9 | 0.4−1.9 | NS |
| 14 | 5 | 1.6 | 139 | 2.4 | 0.7 | 0.3−1.7 | NS |
| 25A | 5 | 1.6 | 66 | 1.1 | 1.5 | 0.6−3.6 | NS |
95% CI: 95% confidence interval; CSF: cerebrospinal fluid; NS: not significant; OR: odds ratio.
Changes over time in the main serotypes of Streptococcus pneumoniae isolated in CSF at a higher frequency than in other usually sterile samples during the periods 2007–2010, 2011–2014 and 2015–2018.
| Serotypes in CSF | 2007−2010 | 2011−2014 | 2015−2018 | Linear trend chi-squared |
|---|---|---|---|---|
| Serotype 11A | ||||
| No. (%) | 5 (3.3) | 9 (11.1) | 1 (1.2) | NS |
| ORa | 1 | 3.68 | 0.36 | |
| Serotype 19 F | ||||
| No. (%) | 6 (3.9) | 5 (6.2) | 3 (3.6) | NS |
| ORa | 1 | 1.6 | 0.91 | |
| Serotype 23B | ||||
| No. (%) | 6 (3.9) | 4 (4.9) | 3 (3.6) | NS |
| ORa | 1 | 1.26 | 0.91 | |
| Serotype 10A | ||||
| No. (%) | 6 (3.9) | 4 (4.9) | 2 (2.4) | NS |
| ORa | 1 | 1.26 | 0.6 | |
| Serotype 24F | ||||
| No. (%) | 4 (2.6) | 2 (2.5) | 5 (6.0) | NS |
| ORa | 1 | 0.94 | 2.37 | |
| Serotype 23A | ||||
| No. (%) | 3 (2.0) | 2 (2.5) | 4 (4.8) | NS |
| ORa | 1 | 1.26 | 2.51 | |
| Serotype 35F | ||||
| No. (%) | 3 (2.0) | 1 (1.2) | 4 (4.8) | NS |
| ORa | 1 | 0.62 | 2.51 | |
CSF: cerebrospinal fluid; NS: not significant; OR: odds ratio.
At present, little information is available on the meningeal tropism of Streptococcus pneumoniae, and the role of the serotype-determining polysaccharide capsule is debated. Although elements other than the capsule (neuraminidase A) appear to promote the crossing of the blood–brain barrier,7 it has been postulated that transit through the vascular endothelium by transcellular migration8 and meningitis severity are related to capsule thickness.4 The prevalence of pneumococcal serotypes varies according to geographic location and vaccination policy. In 2006, the seven-valent conjugate vaccine (VCN7) was added to the child immunization schedule of the Community of Madrid (CM); in 2010, this vaccine was replaced with the 13-valent conjugate vaccine (VCN13). It was removed from the schedule in 2012 and re-added in 2015. From 2012 to 2015, it continued to be administered at the individual recommendation of pediatricians.9 In this regard, the distribution of pneumococcal serotypes in CSF in the CM differs in certain ways from that of other places. In Brazil, where the 10-valent conjugate vaccine (VCN10) has been in use since 2010, the serotypes most commonly isolated in CSF from 1996 to 2012 were 14, 3, 6B, 19F, 23F, 18C, 4, 6A, 10A, and 8.10 In Iran, which does not have systematic pneumococcal vaccination, the main serotypes in CSF were 18C, 14, 19A, 6A, 7F, 4, 3, 9V, 8, 23F, and 5.11 In the Netherlands, which has vaccine coverages of 40% for VCN7, 53% for VCN10 and 68% for VCN13, the serotypes most commonly found in CSF were 3, 7F, 23F, 14, 6B, and 19F.12 In France in 2012, serotypes not included in the VCN13 vaccine accounted for 67.6% of cases of meningitis in children; the main ones were 12F, 24F, 22F, and 15B/C.13 Certain serotypes frequently detected among cases of IPD in the CM throughout the period, such as 19A (which decreased from 2008–2010 to 2013–2015) and 3 (which also decreased, though to a lesser extent),9 also show a high frequency of isolation in CSF. Serotype 8, very common in cases of IPD in the CM,9 was the third most commonly identified serotype in CSF, yet it was significantly isolated most often in other clinical samples from invasive infections. Moreover, serotypes 11A, 19F, 23B, 10A, 24F, 23A, and 35F, heretofore uncommon in cases of IPD in the CM, show clear meningeal tropism. It should be noted that none, except for 19F, is covered by the currently available conjugate vaccines, though two of them, 10A and 11A, are currently included in the already patented 20-valent conjugate vaccine (VCN20)14 and, although there is a risk of serotype replacement,15 according to our results, for the moment, an increase in these non-vaccine serotypes has not occurred in cases of meningitis. The sensitivity to antimicrobial agents of the serotypes that show more central nervous system tropism varied by serotype. Notably, serotypes 24F, 11A, 23B, and to a lesser extent 19F showed high resistance to penicillin (cut-off point for meningitis). In conclusion, the greater frequency and resistance of these pneumococcal serotypes with high meningeal tropism could compromise the treatment of central nervous system infections.
Conflicts of interestJ.C. Sanz has attended conferences funded by Pfizer. All other authors declare that they have no conflicts of interest.
Please cite this article as: Sanz JC, de Miguel S, Ordobás M, García Comas L. Serotipos de Streptococcus pneumoniae con tropismo meníngeo en casos de enfermedad neumocócica invasora. Comunidad de Madrid, 2007–2018. Enferm Infecc Microbiol Clin. 2020;38:371–374.