Burkholderia cepacia (B. cepacia) complex is composed of 20 phylogenetically closely related bacterial species. Some species have emerged as opportunistic pathogens in immunocompromised patients and are responsible for nosocomial outbreaks. The B. cepacia complex is a recognised respiratory pathogen in patients with cystic fibrosis. Burkholderia cenocepacia and Burkholderia multivorans (B. multivorans) are the most prevalent species in the world, according to the literature. However, research groups in Argentina have described a particular local epidemiology, with prevalence of Burkholderia contaminans (B. contaminans).
MethodsA total of 68 isolates of B. cepacia complex recovered of 46 cystic fibrosis patients attended at 14 hospitals distributed in 9 provinces of the country were studied. Identification was carried out by conventional phenotypic methods and was confirmed by recA gene sequencing. Sequences were analysed using the BLASTN program and comparing with B. cepacia complex type strains sequences deposited in GenBank. Antibiotic susceptibility tests were performed on isolates of the most prevalent species according to CLSI M45 guidelines.
ResultsThe prevalent specie was B. contaminans (49%, n=33) followed by B. cenocepacia (25%; n=17). The remaining species were Burkholderia seminalis (B. seminalis) (7%, n=5), B. cepacia (7%, n=5), B. multivorans (6%, n=4), Burkholderia vietnamensis (5%, n=3) and Burkholderia pyrrocinia (1%; n=1).
The 46% of B. contaminans isolates were resistant to SXT and 76% sensitive to MIN, MEM and CAZ. The isolates of B. cenocepacia were 100% resistant to SXT and MIN and 47% to CAZ and MEM. B. seminalis showed high levels of resistance to TMS (80%), CAZ (60%) and MIN (60%), and 60% of the isolates showed intermediate sensitivity to MEM.
ConclusionPrevious reports have described the prevalence of B. contaminans isolation from cystic fibrosis patients in Argentina, Spain and Portugal, and a case of two patients with cystic fibrosis in Ireland has recently been reported. Due to the high frequency with which B. contaminans is isolated in our country, it is necessary to promote the investigation of possible sources of infection and to understand the factors and mechanisms involved in the apparent greater transmissibility of this species. Different antimicrobial resistance profiles were detected between the species.
En la actualidad el complejo Burkholderia cepacia (B. cepacia) está compuesto por 20 especies filogenéticamente muy relacionadas. Algunas especies han emergido como patógenos oportunistas en pacientes inmunocomprometidos y son responsables de brotes intrahospitalarios. El complejo B. cepacia es un reconocido patógeno respiratorio en pacientes con fibrosis quística. B. cenocepacia y Burkholderia multivorans (B. multivorans) son las especies prevalentes en el mundo, según la literatura. Sin embargo, grupos de investigación en Argentina han descripto una epidemiología local particular, con prevalencia de la especie Burkholderia contaminans (B. contaminans).
MétodosSe estudiaron 68 aislamientos del complejo B. cepacia aislados de 46 pacientes con fibrosis quística de 14 hospitales distribuidos en 9 provincias del país. La identificación se llevó a cabo por métodos fenotípicos convencionales y fue confirmada por secuenciación parcial del gen recA. Los alineamientos de las secuencias se realizaron mediante el programa BLAST y fueron comparadas con las secuencias de las cepas tipo de cada una de las especies del complejo B. cepacia. Se determinó el perfil de sensibilidad a 4 agentes antimicrobianos para los aislamientos de las especies más prevalentes, según lo recomendado por la norma CLSI M45.
ResultadosLa especie prevalente resultó B. contaminans (49%, n = 33) seguida por B. cenocepacia (25%; n = 17). El resto de las especies identificadas fueron: Burkholderia seminalis (B. seminalis) (7%; n = 5), B. cepacia (7%; n = 5), B. multivorans (6%; n = 4), Burkholderia vietnamensis (5%, n = 3) y Burkholderia pyrrocinia (1%; n = 1).
El 46% de los aislamientos de B. contaminans fueron resistentes a SXT y el 76% sensible a MIN, MEM y CAZ. Los aislamientos de B. cenocepacia fueron 100% resistentes a SXT y MIN, y el 47% a CAZ y MEM. En B. seminalis se observa un alto nivel de resistencia a TMS (80%), CAZ (60%) y MIN (60%), y un 60% de los aislamientos mostraron sensibilidad intermedia a MEM.
ConclusiónLos únicos países que han documentado la prevalencia de B. contaminans en infecciones respiratorias de pacientes fibroquísticos por complejo B. cepacia son Argentina, España y Portugal, y recientemente se reportó un caso de 2 pacientes con fibrosis quística en Irlanda. Debido a la alta frecuencia con que B. contaminans es aislada en nuestro país, es necesario promover la investigación de las posibles fuentes de infección y comprender los factores y mecanismos implicados en la aparente mayor transmisibilidad de esta especie. Se observaron distintos patrones de sensibilidad entre las especies estudiadas.
The bacteria that comprise the Burkholderia cepacia (B. cepacia) complex are opportunistic pathogens capable of causing diseases in plants, humans and animals.1 In humans, chronic and often severe infections may occur. Patients with cystic fibrosis, patients with chronic granulomatous disease and immunocompromised patients are particularly sensitive to such infections.2 In the B. cepacia complex, the species Burkholderia multivorans (B. multivorans) and Burkholderia cenocepacia (B. cenocepacia) are most commonly found in these patients throughout the world, although the prevalence reported for each species varies by country and health centre being studied.3,4
In Argentina, the first B. cepacia complex isolates recovered from patients with cystic fibrosis were documented in the 1990s. At that time, in a sporadic fashion and with a very low prevalence (<0.1%), Gram-negative microorganisms were detected which could only be assigned to the genus Burkholderia spp. Detection of these microorganisms has started to increase in the last 15 years with values of 0.2–3.6%. In early 2004, B. cepacia complex outbreaks began to be reported at hospital care centres. An increase in the proportion of patients with cystic fibrosis was observed (19–36%). Isolates belonging to B. cepacia complex were recovered from these patients. Today, with the use of strict procedures for management to prevent infections, the incidence of Burkholderia spp. in these patients has been reduced to approximately 10%.4
B. cenocepacia, which is highly virulent and transmissible, is considered the most severe pathogen in the B. cepacia complex. It and B. multivorans account for the majority (80%) of infections in patients with cystic fibrosis around the world, although its prevalence varies by country in question.4 The species Burkholderia contaminans (B. contaminans), formally reported in 2009,5 has a low prevalence in patients with cystic fibrosis around the world, with marked exceptions in Argentina,4,6,7 Spain8,9 and Portugal,10 as well as Ireland, where a case was recently reported.11 The frequency with which B. contaminans is isolated in Argentina and Spain could result from patient-to-patient transmission, as well as exposure to sources of infection such as the environment and pharmaceutical products.12
Given that B. cepacia complex species are intrinsically resistant to the majority of clinically available antimicrobial agents, such as aminoglycosides, quinolones, polymyxins and β-lactam antibiotics, this type of infection is extremely difficult to treat.13
This study sought to identify B. cepacia complex species isolated from respiratory samples taken from patients with cystic fibrosis and sent to the reference laboratory during the period 2011–2015 and to determine the pattern of sensitivity to antimicrobial agents of the prevalent species.
Materials and methodsBacterial isolatesA total of 68 isolates presumptively identified as B. cepacia complex were sent to the Argentinian National Reference Laboratory Special Bacteriology Department of the Argentinian National Institute of Infectious Diseases – ANLIS “Dr. Carlos G. Malbrán” (Argentinian National Laboratories and Health Institutes Administration) during the period 2011–2015. These isolates came from 14 hospitals in 9 provinces of Argentina. The isolates were preserved as part of the Culture Collection of the Special Bacteriology Department, belonging to the Latin American Federation of Culture Collections (FELACC).
Phenotypic identificationThe isolates were recovered from sputum samples from 47 patients with cystic fibrosis taken during episodes of respiratory exacerbation or clinical decline. There were 13 patients from whom multiple isolates were sent throughout the period studied, 2011–2015. Of these patients, 7 had chronic infection. That is to say, they had positive cultures for more than a year with the same B. cepacia complex species.
Biochemical characterisation of isolates was performed as reported by Henry et al.,14 together with the use of API® 20NE miniaturised commercial galleries (bioMérieux, Marcy l’Etoile, France) according to the manufacturer's instructions.
Genotypic identificationThe alkaline lysis method of DNA extraction was used as reported by Storms et al.15 The recA gene was amplified according to the protocol reported by Mahenthiralingam et al.16 The amplification products were purified using the AccuPrep® PCR Purification Kit (Bioneer, Bioneer Corporation, Korea) according to the manufacturer's recommendations. The purified products were used immediately or stored at −20°C until they were used. For amplicon sequencing, the BCR3 primer16 was used and the method of cycle sequencing with the BigDye Terminator Cycle Sequencing kit (Applied Biosystems, United States) and the ABI PRISM® 3.0 Genetic Analyzer sequencer (Applied Biosystems, United States) were employed. The sequences were prepared at the Neuroviral Disease Division of the Virology Department of the Argentinian National Institute of Infectious Diseases—ANLIS Dr. Carlos G. Malbrán.
The nucleotide sequences were analysed and corrected with the program Chromas 2.4.3 (Technelysium Pty. Ltd., Helensvale, Australia) and compared to the sequences available in GenBank. The acceptance criterion for species assignment was >99% compared to the sequences of the strains deposited in the database of the NCBI reported by Kong et al.17
Antibiotic susceptibilityThe most commonly identified species—B. contaminans, B. cenocepacia and Burkholderia seminalis (B. seminalis)—were tested by the disc diffusion method for their sensitivity to the following antibiotics: ceftazidime (CAZ), minocycline (MIN), meropenem (MEM) and sulfamethoxazole/trimethoprim (SXT). The isolates were classified as susceptible, intermediate or resistant, according to the interpretation criteria specified by the CLSI M45 standard.18
ResultsAll isolates were biochemically indistinguishable. Results were obtained by partially sequencing the recA gene and comparing it to the online database. The prevalent species was B. contaminans (49%, n=33) followed by B. cenocepacia (25%; n=17). All other species identified had a uniform distribution: B. seminalis (7%; n=5), B. cepacia (7%; n=5), B. multivorans (6%; n=4), Burkholderia vietnamensis (5%, n=3) and Burkholderia pyrrocinia (1%; n=1). All isolates identified by the molecular method as B. contaminans exhibited a greenish pigment and positive haemolysis.
The species associated with the 7 patients with cystic fibrosis with chronic infection were B. cenocepacia (one patient), B. multivorans (2 patients), B. contaminans (3 patients) and B. seminalis (one patient).
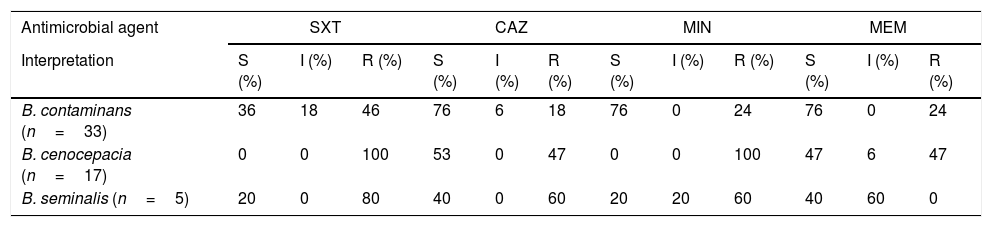
Concerning sensitivity, 46% (n=15) of isolates of B. contaminans were resistant to SXT, and the majority were sensitive (76%; n=25) to MIN, MEM and CAZ. Resistance to SXT and MIN was observed in all B. cenocepacia isolates, whereas 47% (n=8) were resistant to CAZ and MEM. B. seminalis showed a high level of resistance to TMS (80%; n=4), CAZ (60%; n=3) and MIN (60%; n=3), and 60% (n=3) of isolates showed intermediate sensitivity to MEM. Table 1 lists all results.
Results for sensitivity obtained from the 55 isolates studied.
| Antimicrobial agent | SXT | CAZ | MIN | MEM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interpretation | S (%) | I (%) | R (%) | S (%) | I (%) | R (%) | S (%) | I (%) | R (%) | S (%) | I (%) | R (%) |
| B. contaminans (n=33) | 36 | 18 | 46 | 76 | 6 | 18 | 76 | 0 | 24 | 76 | 0 | 24 |
| B. cenocepacia (n=17) | 0 | 0 | 100 | 53 | 0 | 47 | 0 | 0 | 100 | 47 | 6 | 47 |
| B. seminalis (n=5) | 20 | 0 | 80 | 40 | 0 | 60 | 20 | 20 | 60 | 40 | 60 | 0 |
CAZ: ceftazidime; I: intermediate; MEM: meropenem; MIN: minocycline; N: number of isolates; R: resistant; S: sensitive; STX: sulfamethoxazole/trimethoprim.
Proper isolation and identification of pathogens in patients with cystic fibrosis are critical steps towards disease management. They have a significant impact on antimicrobial treatment, patient care, prevention of new infections and management of care units, as well as improvement of patient quality of life.
The seriousness of disease due to infection with B. cepacia complex in patients with cystic fibrosis is substantially higher than with other bacteria, and the variability of the prognoses for these patients is striking, since whereas some experience a fulminant decline in lung function, others who are BCC carriers experience no obvious adverse effects for long periods of time.
Given that B. cenocepacia and B. multivorans are the prevalent species throughout the world, the majority of studies dedicated to B. cepacia complex pathogenesis are focused on these 2 species. Therefore, information on the impact of the other species is needed. It should be noted that the literature19 shows that all species of B. cepacia complex cause clinical worsening, from chronic or transient infections to cepacia syndrome.
Unlike that reported in other countries, particularly Argentina, Spain and Portugal, B. contaminans has a higher prevalence in patients with cystic fibrosis. Recently, a case involving 2 brothers with this disease was reported in Ireland. Infections with B. contaminans in patients with cystic fibrosis are usually transient in nature and therefore cause colonisation of the respiratory tract more than true infection. However, Nunvar et al.20 documented a case of a patient whose death was associated with septicaemia resulting from B. contaminans.
Due to the high frequency with which B. contaminans is isolated in Argentina, it is necessary to promote research on potential sources of infection and above all to understand the factors and mechanisms involved in the apparently greater transmissibility of this species or environmental distribution.
Sequencing of the recA gene remains the gold-standard method for identification on a species level in the B. cepacia complex. The use of well-characterised sequences of standard or reference strains is important for determining percentages of similarity. However, the reporting of new species probably necessitates the incorporation of new genes and techniques enabling differentiation among them.
The results for the antibiotic sensitivity of B. cenocepacia and B. seminalis showed resistance to more than 2 antibiotics. This confirmed the trait of multi-drug-resistance characterising the B. cepacia complex. B. contaminans, the prevalent species in the population with cystic fibrosis in Argentina, showed a pattern that maintained high levels of sensitivity to CAZ, mycocyclin and MEM. No changes were observed in patterns of sensitivity in isolates from patients with documented long-term chronic infection.
Conflicts of interestThe authors declare that they have no conflicts of interest.
The authors would like to thank Laboratorios Britania S.A. Argentina for providing discs for antimicrobial sensitivity studies.
Please cite this article as: Cipolla L, Rocca F, Martinez C, Aguerre L, Barrios R, Prieto M. Prevalencia de especies del complejo Burkholderia cepacia en pacientes con fibrosis quística en Argentina durante el período 2011-2015. Enferm Infecc Microbiol Clin. 2018;36:431–434.