Mother-to-child transmission (MTCT) is relevant in the global epidemiology of human-immunodeficiency virus (HIV), as it represents the main route of infection in children. The study objectives were to determine the rate of HIV-MTCT and its epidemiological trend between the Spanish-born and immigrant population in Catalonia in the period 2000–2014.
MethodsA prospective observational study of mother–child pairs exposed to HIV, treated in 12 hospitals in Catalonia in the period 2000–2014. HIV-MTCT rate was estimated using a Bayesian logistic regression model. R and WinBUGS statistical software were used.
ResultsThe analysis included 909 pregnant women, 1009 pregnancies, and 1032 children. Data on maternal origin was obtained in 79.4% of women, of whom 32.7% were immigrants, with 53.0% of these from sub-Saharan Africa. The overall HIV-MTCT rate was 1.4% (14/1023; 95% CI; 0.8–2.3). The risk of MTCT-HIV was 10-fold lower in women with good virological control (P=0.01), which was achieved by two-thirds of them. The proportion of immigrants was significantly higher in the period 2008–2014 (P<.0001), for the HIV-diagnosis (P<0.0001), and antiretroviral administration (P=0.02) during pregnancy, and for undetectable viral load next to delivery (P<0.001). There were no differences in the rate of MTCT-HIV among Spanish-born and immigrant women (P=0.6).
ConclusionsThere is a gradual increase in HIV pregnant immigrants in Catalonia. Although most immigrant women were diagnosed during pregnancy, the rate of MTCT-HIV was no different from the Spanish-born women.
La transmisión vertical (TV) es relevante en la epidemiología global del virus de la inmunodeficiencia humana (VIH), representando la principal vía de infección en la edad pediátrica. Los objetivos del estudio fueron determinar la tasa de TV del VIH y su tendencia epidemiológica entre la población autóctona e inmigrante en Catalunya entre 2000-2014.
MétodosEstudio observacional prospectivo de parejas madre-hijo expuestas al VIH atendidas en 12 hospitales de Catalunya en 2000-2014. Se estimó la tasa de TV del VIH aplicando un modelo bayesiano de regresión logística. Se utilizó el software estadístico R y WinBUGS.
ResultadosSe analizaron 909 gestantes, 1.009 embarazos y 1.032 niños; datos de origen materno en el 79,4% de las mujeres, el 32,7% inmigrantes y de estas el 53,0% de África subsahariana. La tasa de TV del VIH fue del 1,4% (14/1.023; IC95% 0,8-2,3). El riesgo de TV del VIH fue 10 veces menor en mujeres con buen control virológico (p=0,01), al que llegaron 2 tercios de ellas. No hubo diferencias en la tasa de TV del VIH entre mujeres autóctonas e inmigrantes (p=0,6). La proporción de mujeres inmigrantes fue significativamente mayor en el período 2008-2014 (p<0,0001), en relación con el diagnóstico de la infección por VIH (p<0,0001) y la administración de antirretrovirales (p=0,02) durante el embarazo, y con la viraemia indetectable próxima al parto (p<0,001).
ConclusionesExiste un aumento progresivo de gestantes inmigrantes con VIH en Catalunya. Aun siendo la mayoría diagnosticadas durante el embarazo, la tasa de TV del VIH no fue diferente a la hallada en las mujeres autóctonas.
Mother-to-child transmission (MTCT) of human immunodeficiency virus (HIV) during pregnancy, delivery or breastfeeding represents the most common route of infection in children and is a determining factor in the global epidemiology of HIV and AIDS among the paediatric population.1 Since the start of the HIV/AIDS pandemic, we have seen enormous progress in relation to the prevention of HIV MTCT, including access of infected pregnant women to antiretroviral therapy (ART), with subsequent control of viral replication, the main factor that has prevented more than 900,000 new infections in children since 2009.1 Furthermore, the introduction of ART has drastically reduced HIV-related morbidity and mortality and has generated a better quality of life and prognosis for people living with HIV.2
In Spain, 3366 new cases of HIV infection were reported in 2014; the estimated rate of new HIV diagnoses in 2014 was 9.34/100,000 inhabitants. 85% of new diagnoses were men, the median age was 35 years and 32.5% of new diagnoses involved people originally from other countries. Among women, heterosexual transmission accounted for 80.3% of new diagnoses.3
Growing economic inequality between different countries has determined new migration flows that have increased over the last 20 years. Spain is one of the countries receiving this population, which accounted for 9.6% of the population registered at the end of 2014, representing an absolute figure of 4,447,852 people.4 HIV infection in immigrants shows different characteristics to those of the native population. Most of the studies conducted indicate that HIV in immigrants is often diagnosed at a late stage, when the immunological classification and staging of HIV is advanced and the patient already has infectious or tumour complications of AIDS.5,6 Since 2010, new diagnoses of HIV infection in non-native individuals have accounted for approximately one-third of all new diagnoses in all 17 autonomous communities of Spain.3 It is important to note that more than 50% of new diagnoses in women and 43.4% of sexually-transmitted infections occur in immigrants, mainly of Latin American and sub-Saharan origin.3
In Catalonia, a constant increase has been observed in the proportion of new diagnoses of HIV infection among the migrant population, representing 41% of all cases reported in 2014 compared to just 24.6% in 2001, and 53% of the people affected were originally from Latin American and Caribbean countries.7 The number of new cases of HIV among men who have sex with men is still on the increase and is especially high among immigrants (3.7/100 people per year).7
The objectives of our study were to estimate the rate of HIV MTCT and its evolution over time among the native and immigrant population in Catalonia between 2000 and 2014, and to identify possible determinants of transmission in these populations.
MethodsA prospective observational study conducted from data collected in the NENEXP cohort from 1 January 2000 to 31 December 2014. All participating sites were authorised by the respective ethics committees. The NENEXP cohort includes mother–child pairs exposed to HIV and treated at 12 hospitals in Catalonia who meet the following criteria: 1. mother diagnosed with HIV infection before, during or a maximum of 72 hours after delivery; 2. to have agreed to participate in the study by signing the informed consent form.
Data were collected systematically from an agreed database by the study investigators. The following data were collected: invariable data for the mother: date of birth, date of HIV infection diagnosis, country of origin, probable route of infection and clinical stage of HIV infection, AIDS classification, and hepatitis B virus (HBV) and hepatitis C virus (HCV) co-infection; pregnancy data: number of pregnancies, number of deliveries, multiple birth, date of last period, HIV viral load (VL) and CD4+ lymphocyte count closest to the delivery date, mother's toxic habits during pregnancy and mother's ART; delivery data: date of delivery, type of delivery (vaginal, caesarean during labour or elective caesarean), gestational age, and administration of zidovudine (ZDV), lamivudine, nevirapine or other intrapartum antiretroviral drugs; and invariable data for the child: date of birth, gender, weight, length and head circumference at birth, preterm (term, moderately preterm or extremely preterm), antiretroviral prophylaxis administered, final HIV infection status (HIV infected, HIV uninfected, indeterminate HIV infection status) and final vital status of the child. The HIV MTCT infection definitions used were based on Spanish Recommendations.8
Maternal, pregnancy, delivery and neonatal variables were described using frequencies, ratios, means, standard deviations and quartiles, depending on the type of variable. Also, the link between the mother's country of origin and qualitative variables was explored using the Chi-square test (or Fisher's exact test) while the link between the mother's country of origin and quantitative variables was explored using Student's t-test.
The rate of HIV MTCT (number of children infected/total number of children excluding children with indeterminate status) was estimated with a 95% confidence interval, and a Bayesian logistic regression model was used for analysis purposes, contemplating VL, intrapartum ZDV and the mother's country of origin. To prevent the loss of unknown data on the adjustment variables, these were generated with the same model using the multiple imputation technique. Logistic regression was used to impute VL based on whether ART had been received and the type of prophylaxis and to generate missing data on the mother's country of origin based on year of birth. The intrapartum ZDV variable was imputed by considering the distribution of all intrapartum ZDV data.
The analysis was performed using the R statistical software and WinBUGS 1.4.3 package (MRC Biostatistics Unit, Cambridge, UK).
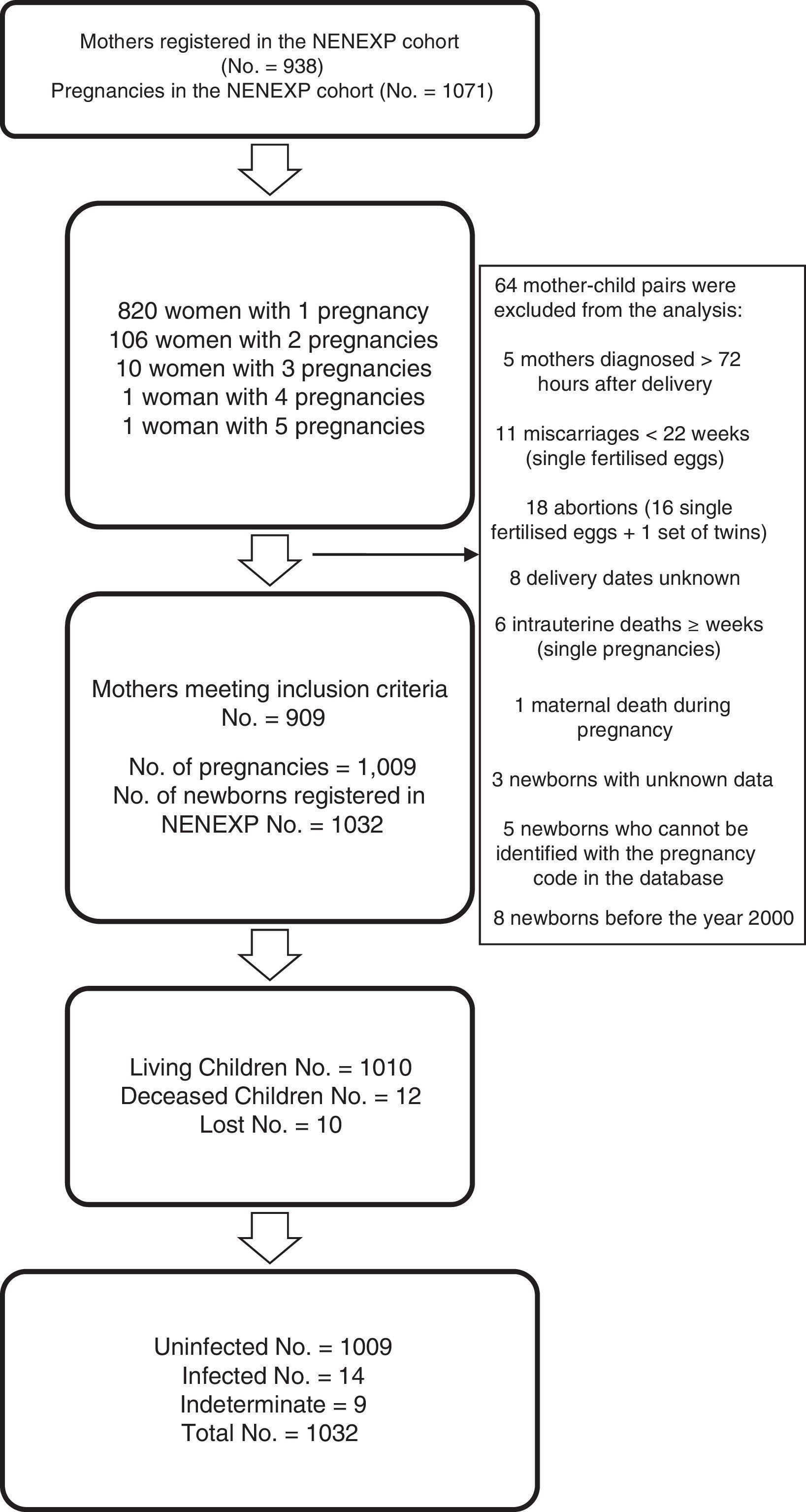
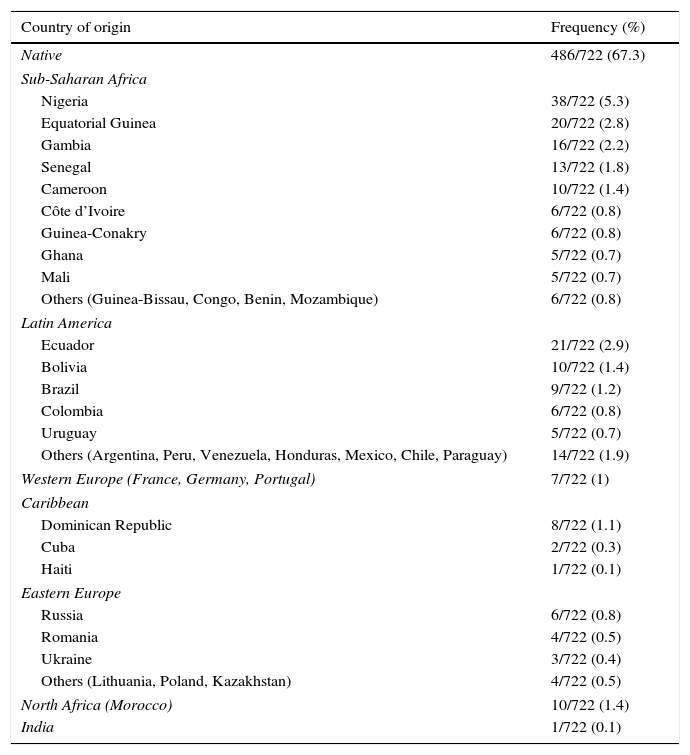
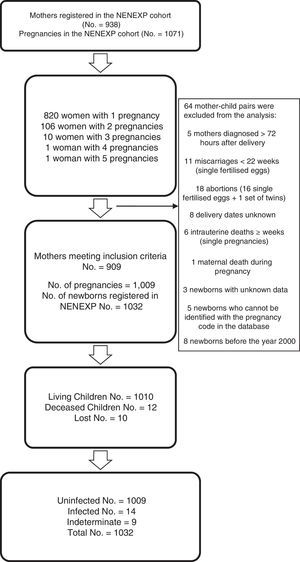
ResultsData were collected for 938 women with HIV infection and 1071 pregnancies; 64 mother–child pairs were excluded (Fig. 1). For the purpose of this analysis, 909 pregnant women, 1009 pregnancies and 1032 children were finally included. Data on the mother's country of origin were available for 79.4% of the women (Table 1); 32.7% were immigrants and most of these were from sub-Saharan Africa (53.0%).
Distribution of mothers according to country of origin.
| Country of origin | Frequency (%) |
|---|---|
| Native | 486/722 (67.3) |
| Sub-Saharan Africa | |
| Nigeria | 38/722 (5.3) |
| Equatorial Guinea | 20/722 (2.8) |
| Gambia | 16/722 (2.2) |
| Senegal | 13/722 (1.8) |
| Cameroon | 10/722 (1.4) |
| Côte d’Ivoire | 6/722 (0.8) |
| Guinea-Conakry | 6/722 (0.8) |
| Ghana | 5/722 (0.7) |
| Mali | 5/722 (0.7) |
| Others (Guinea-Bissau, Congo, Benin, Mozambique) | 6/722 (0.8) |
| Latin America | |
| Ecuador | 21/722 (2.9) |
| Bolivia | 10/722 (1.4) |
| Brazil | 9/722 (1.2) |
| Colombia | 6/722 (0.8) |
| Uruguay | 5/722 (0.7) |
| Others (Argentina, Peru, Venezuela, Honduras, Mexico, Chile, Paraguay) | 14/722 (1.9) |
| Western Europe (France, Germany, Portugal) | 7/722 (1) |
| Caribbean | |
| Dominican Republic | 8/722 (1.1) |
| Cuba | 2/722 (0.3) |
| Haiti | 1/722 (0.1) |
| Eastern Europe | |
| Russia | 6/722 (0.8) |
| Romania | 4/722 (0.5) |
| Ukraine | 3/722 (0.4) |
| Others (Lithuania, Poland, Kazakhstan) | 4/722 (0.5) |
| North Africa (Morocco) | 10/722 (1.4) |
| India | 1/722 (0.1) |
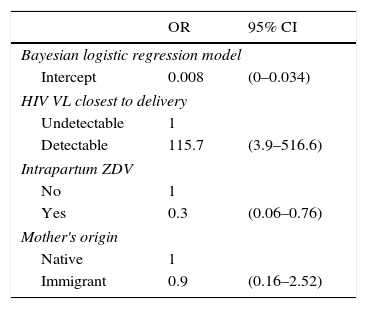
The rate of HIV MTCT was 1.4% (14/1023; 95% confidence interval: 0.8–2.3) (Table 2), which was not associated with the mother's country of origin, although it was associated with detectable maternal VL and no administration of intrapartum ZDV (Table 3). No differences were observed between time periods (1.1% in 2008–2014 vs 1.5% in 2000–2007; p=0.7). Over the last 4 years of follow-up, no cases of HIV MTCT were reported in the NENEXP cohort.
Bayesian multivariate logistic regression model for the rate of HIV MTCT.
| OR | 95% CI | |
|---|---|---|
| Bayesian logistic regression model | ||
| Intercept | 0.008 | (0–0.034) |
| HIV VL closest to delivery | ||
| Undetectable | 1 | |
| Detectable | 115.7 | (3.9–516.6) |
| Intrapartum ZDV | ||
| No | 1 | |
| Yes | 0.3 | (0.06–0.76) |
| Mother's origin | ||
| Native | 1 | |
| Immigrant | 0.9 | (0.16–2.52) |
95% CI: 95% confidence interval.
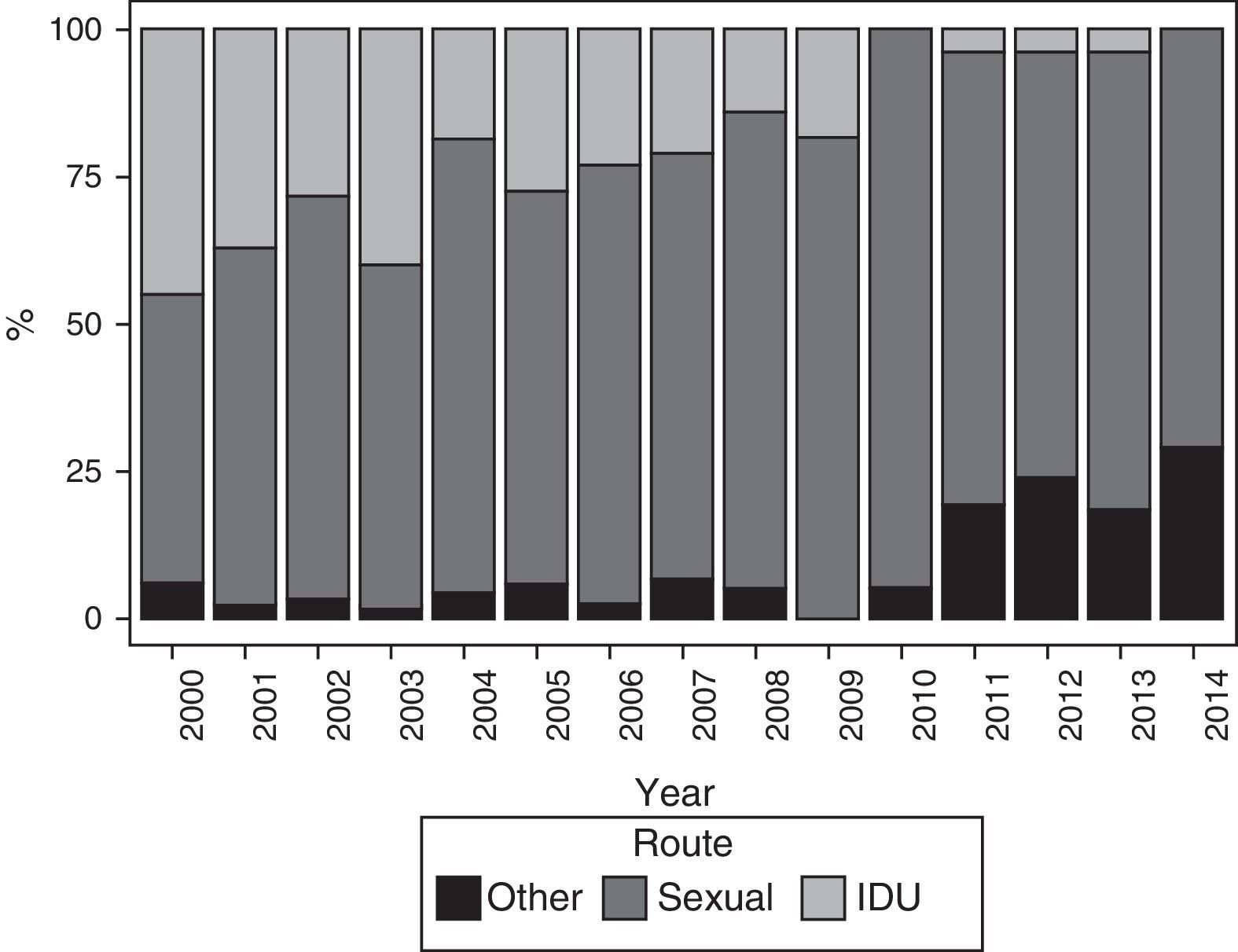
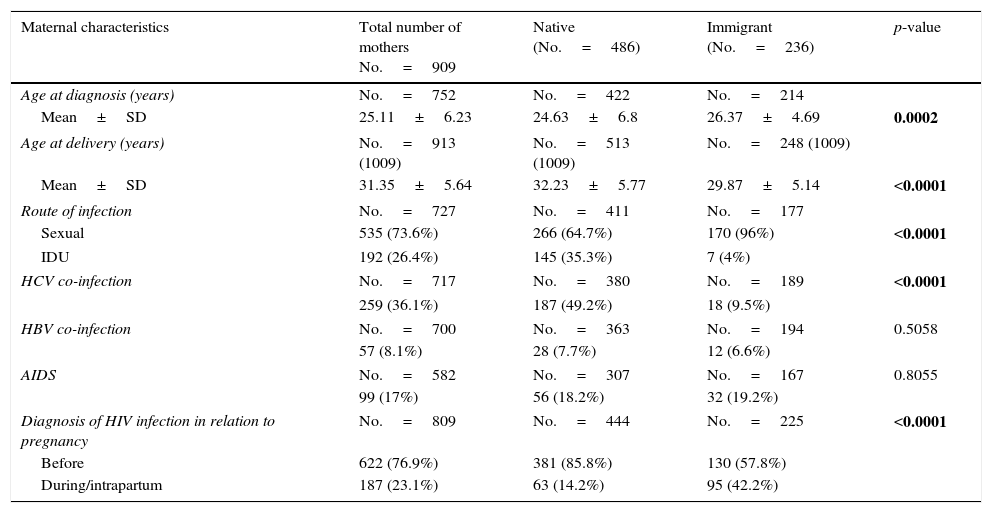
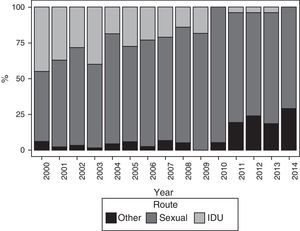
The main route of HIV infection was sexual (68.9%), with a significant increase observed over the years (p<0.0001) (Fig. 2). 36.1% of women were co-infected with HCV and 76.9% were diagnosed with HIV infection before their first pregnancy (Table 4).
Descriptive analysis of maternal and gestational variables in general and by the mother's country of origin.
| Maternal characteristics | Total number of mothers No.=909 | Native (No.=486) | Immigrant (No.=236) | p-value |
|---|---|---|---|---|
| Age at diagnosis (years) | No.=752 | No.=422 | No.=214 | |
| Mean±SD | 25.11±6.23 | 24.63±6.8 | 26.37±4.69 | 0.0002 |
| Age at delivery (years) | No.=913 (1009) | No.=513 (1009) | No.=248 (1009) | |
| Mean±SD | 31.35±5.64 | 32.23±5.77 | 29.87±5.14 | <0.0001 |
| Route of infection | No.=727 | No.=411 | No.=177 | |
| Sexual | 535 (73.6%) | 266 (64.7%) | 170 (96%) | <0.0001 |
| IDU | 192 (26.4%) | 145 (35.3%) | 7 (4%) | |
| HCV co-infection | No.=717 | No.=380 | No.=189 | <0.0001 |
| 259 (36.1%) | 187 (49.2%) | 18 (9.5%) | ||
| HBV co-infection | No.=700 | No.=363 | No.=194 | 0.5058 |
| 57 (8.1%) | 28 (7.7%) | 12 (6.6%) | ||
| AIDS | No.=582 | No.=307 | No.=167 | 0.8055 |
| 99 (17%) | 56 (18.2%) | 32 (19.2%) | ||
| Diagnosis of HIV infection in relation to pregnancy | No.=809 | No.=444 | No.=225 | <0.0001 |
| Before | 622 (76.9%) | 381 (85.8%) | 130 (57.8%) | |
| During/intrapartum | 187 (23.1%) | 63 (14.2%) | 95 (42.2%) | |
| Pregnancy characteristics | Total number of mothers No.=1009 | Native (No.=547) | Immigrant (No.=263) | p-value |
|---|---|---|---|---|
| Gestational age at birth | No.=976 | No.=536 | No.=250 | |
| mean±SD | 37.36±2.2 | 37.25±2.13 | 37.63±2.2 | 0.0209 |
| Preterm birth | No.=976 | No.=536 | No.=250 | 0.0177 |
| 201 (20.6%) | 123 (22.9%) | 39 (15.6%) | ||
| Maternal toxic habits | No.=1003 | No.=546 | No.=260 | <0.0001 |
| 174 (17.3%) | 133 (24.4%) | 10 (3.8%) | ||
| Immunological status (according to CD4 lymphocyte count closest to delivery) | No.=555 | No.=299 | No.=153 | 0.83 |
| Stage 1 | 351 (63.2%) | 187 (62.5%) | 93 (60.8%) | |
| Stage 2 | 169 (30.5%) | 92 (30.8%) | 51 (33.3%) | |
| Stage 3 | 35 (6.3%) | 20 (6.7%) | 9 (5.9%) | |
| HIV VL closest to delivery | No.=723 | No.=398 | No.=198 | 0.0004 |
| Detectable | 264 (36.5%) | 168 (42.2%) | 54 (27.3%) | |
| ART | No.=1009 | No.=547 | No.=263 | 0.02 |
| Yes | 777 (77%) | 377 (68.9%) | 202 (76.8%) | |
| Type of ART | No.=416 | No.=224 | 0.45 | |
| Combined ART (with protease inhibitors and/or NNRTIs) | 633 (81.5%) | 346 (83.2%) | 181 (80.8%) | |
| Other combinations (monotherapy/dual therapy) | 144 (18.5%) | 70 (16.8%) | 43 (19.2%) | |
ART: antiretroviral therapy, HBV: hepatitis B virus, HCV: hepatitis C virus, HIV VL: human immunodeficiency virus viral load, IDU: injection drug users, NNRTIs: non-nucleoside reverse transcriptase inhibitors.
In bold: significant p-values for association of the variable analysis based on the mother's country of origin.
Good virological control at the time of delivery was achieved in approximately two-thirds of pregnant women (63.5%) and was associated with the 2008–2014 period (81.7% vs 52% in 2000–2007; p<0.0001), with mothers infected as a result of sexual activity compared to injection drug users (IDUs) (67% vs 53.4%; p=0.002), and with women not co-infected with HCV (71.2% vs 53.9%; p<0.0001). Mothers with a detectable VL had a 10-fold greater risk of infecting their children (p=0.01).
91.3% (921/1009) of the pregnant women received antiretroviral drugs during pregnancy and the most commonly used ART regimen was based on protease inhibitors (388/921; 42.1%). There were no differences between the type of ART used and preterm birth (p=0.57). 69.5% of the pregnant women received ART for 6 weeks or more during pregnancy, achieving better virological control at the time of delivery than those receiving ART<6 weeks or no ART (69.4% vs 45.6%; p<0.0001). Mothers who received ART had a 93% lower risk of MTCT (p<0.0001).
Delivery characteristics59.9% of the babies were born by elective caesarean section, although its indication decreased significantly in 2008–2014 (48.7% vs 65.8% in 2000–2007; p<0.0001). Women with controlled viraemia at the time of delivery had a higher probability of vaginal delivery (26.4% vs 17.1%; p=0.002). The type of delivery did not affect the probability of HIV MTCT. No intrapartum prophylaxis with ZDV was associated with a higher risk of MTCT (p=0.02). No cases of MTCT were observed following vaginal delivery in mothers with an undetectable VL.
Neonatal characteristics78.3% of the newborns went to term, 19.8% were moderately preterm and only 1.9% were extremely preterm, with a reduction in preterm deliveries observed in 2008–2014 compared to the first period of the cohort (16.4% vs 24.6%; p=0.003). Of the 1032 children included in the cohort, 12 (1.2%) died and 10 cases (0.9%) were lost to follow-up (Fig. 1).
Comparative analysisThe comparative analysis of the Spanish and immigrant pregnant women is shown in Table 4. The percentage of pregnancies of immigrant women was higher in 2008–2014 than in 2000–2007 (50.2% vs 22.8%, respectively; p<0.0001). HIV infection was primarily diagnosed before pregnancy in native women, with a significant difference in percentages compared to immigrant women (85.8% vs 57.8%; p<0.0001). Approximately one in every 4 native women consumed harmful substances during pregnancy (24.4% vs 3.8% in immigrant women; p<0.0001). No differences in immunological status were observed between the two groups. It was more common for immigrant women to receive ART for ≥6 weeks during pregnancy (76.8% vs 68.9%; p=0.02) and for their viraemia to be undetectable at the time of delivery (72.7% vs 57.8%; p<0.001). There were no differences regarding the type of ART (protease inhibitors vs NNRTIs) based on the mother's country of origin (p=0.14). Preterm birth was more common among native women (22.9% vs 15.6%; p=0.02) and there were no differences in use of intrapartum ZDV or the type of delivery between native and immigrant women. We also observed no differences in the rate of HIV MTCT among native and immigrant women (p=0.6) (Table 2).
DiscussionPregnant women are a particularly important population group for HIV screening because early diagnosis and adequate management practically eliminate the risk of HIV MTCT.
The results of our study reflect changes in the clinical epidemiology of HIV infection in Catalonia and the development of measures designed to prevent HIV MTCT in high-income countries over the last 15 years. A gradual increase in the percentage of pregnant immigrants can be observed over the course of the study period and the distribution per country of origin is similar to that observed by other authors,9–12 with women from sub-Saharan Africa clearly predominating.4
Since 1994, the Department of Health of Catalonia has recommended screening all pregnant women for HIV infection during the first trimester.13 In our study, 76.9% of women were diagnosed before pregnancy and there are significant differences between native and immigrant women. Immigrant women were diagnosed more frequently during pregnancy, which may be due to this being their first contact with the health system or the fact that they are younger, asymptomatic and with higher reproductive desire, as shown in a CoRis cohort study.14 Similar results had been reported in France, where it was highlighted that infected pregnant women from Africa in particular were diagnosed during pregnancy.15 Other studies involving cohorts from our country also showed differences in time of diagnosis (e.g. around 70% of pregnant immigrants in Madrid were diagnosed during pregnancy compared to 19.4% of native women).16 As a comparison, 85.8% of native women were diagnosed before pregnancy, often due to the presence of clinical manifestations associated with IDU infection or risky behaviour. Therefore, immigrant women should be a clear target of health promotion and prevention programmes during pregnancy.5 Unfortunately, we do not have any information on the date on which the women in the cohort arrived in Spain, which would probably explain their late diagnosis in some cases.
Our results show and highlight the importance of universal HIV screening during the first trimester as it allows diagnosis of a high percentage of pregnant immigrants and the start of ART in order to prevent transmission of the infection. Likewise, starting ART during the first trimester has been proven to be sufficient to reach the end of the pregnancy with complete control of viral replication (undetectable VL), which was more common in the group of immigrant women in our cohort. The risk of HIV MTCT was no different among pregnant native and immigrant women.
HCV co-infection affected 36.1% of women, which is clearly higher than the percentage affected in other cohorts of HIV-infected women,17,18 and it was associated with a history of IDU,17 which is much more common among native women in whom IDU transmission was more prevalent. In fact, regarding the route of HIV infection, the probability of HCV co-infection in women with sexually-transmitted HIV infection was still highly associated with women of native origin and the risk for immigrant women was significantly lower (p<0.0001).
No significant relationship was shown between the clinical stage of AIDS and the mother's country of origin; other Spanish cohorts have observed similar results.16 Most of the pregnant women (89.2%) received ART, although 36.7% of patients did not achieve an undetectable VL at the time of delivery. We have no data on ART adherence or resistance-associated viral mutations that explain these findings. Likewise, we may have thought that the more potent ART combinations developed over recent years would provide better viraemia control, although there were no differences between the two periods analysed.
Towards the end of the 1990s, elective caesarean section was established as another measure for preventing HIV MTCT.19,20 Between 1987 and 2003, this type of delivery increased from 32.2% to 58.2% (p<0.001) in the NENEXP cohort;10 however, it has been shown that improved viraemia control with maternal ART allows safe vaginal delivery in infected mothers and the number of vaginal births has increased significantly over recent years from 17.1% in 2000–2007 to 33.2% in 2008–2014 (p<0.0001). In our study, no cases of MTCT were observed following vaginal delivery in mothers with an undetectable VL. As has already been shown in other studies,21,22 vaginal birth would be the delivery route of choice in all women receiving ART and with complete control of viral replication at the end of pregnancy, unless a caesarean section is indicated for other reasons. However, caesarean section was a determining protective factor for reducing HIV MTCT in those women receiving suboptimal ART or not receiving ART at any point during their pregnancy (p<0.01).23
The incidence of preterm delivery in our study was significantly higher than the incidence published in the “Indicadors de salut perinatal a Catalunya. Any 2014. Informe complet”24 report for the population of women giving birth in Catalonia. In the general population, preterm delivery accounts for 6–10% of births and is the main cause of perinatal morbidity and mortality worldwide.25,26 In various studies, including meta-analyses and case-control series, a link has been observed between HIV infection and preterm delivery, with rates varying from 18% to 29%,27–31 as is the case in our study (21.7%). Several authors have reported a higher incidence of preterm delivery among women receiving ART, especially when such therapy included protease inhibitors,32–37 which we have not observed in our study.
An earlier publication on the NENEXP cohort (1987–2003 period) studied 1105 mother–child pairs and showed that the rate of HIV MTCT fell from 20.4% to 3.5% (p<0.001).10 This rate fell even further in the 2000–2014 period, reaching 1.4%, with no cases of HIV MTCT reported among the Catalonian NENEXP cohort over the last 4 years.
The sample size analysed in the NENEXP cohort is one of the study's strong points. Participation in the NENEXP cohort study accounted for 55.3% (25,951/46,952) of all deliveries in public hospitals in Catalonia in 2014.24
One limitation of the study is the fact that some public hospitals and all hospitals from the private sector were not included. We had to exclude 5.8% (62/1071) of all deliveries initially reported in the cohort. It is also worth mentioning that some variable data are missing, such as age at diagnosis (17.3% of missing data), route of maternal infection (20%), and especially maternal immunological status (43.8%) and VL (26.6%) closest to the time of delivery, which could potentially distort the analysis of risk factors associated with HIV MTCT.
Since the cohort had a 15-year follow-up period (2000–2014) and considering that a consensus has been reached in recent years on the suitability of repeating HIV testing in the third trimester,8 we cannot recommend such screening based on the results of our study. Nevertheless, it has been shown that HIV testing in the third trimester should be included in the screening of pregnant women to identify new infections during pregnancy.8
Although the objective of the study was not to analyse the follow-up of children born to the cohort's HIV-positive mothers, these data are available and we hope to reveal these results in the near future.
ConclusionsOur study shows a gradual increase in the immigration of pregnant women with HIV infection in Catalonia. These women are generally of sub-Saharan origin, are generally younger at the time of delivery, with low co-morbidity, and are generally diagnosed during pregnancy, although MTCT figures are similar to those reported for native women. Nevertheless, it has become evident that programmes for the early detection of HIV infection in the immigrant population are required. It is important to provide good antenatal care to this group by ensuring the implementation of pregnancy care protocols.
FundingNone.
Conflicts of interestNo conflicts of interest declared.
All members of the NENEXP research group.
Olga Calavia (Hospital Universitari Joan XXIII, Tarragona), Lourdes García (Hospital de Mataró, Consorci Sanitari del Maresme), Maite Coll (Hospital General de Granollers, Barcelona), Valentí Pineda (Consorci Sanitari Parc Taulí, Sabadell, Barcelona), Neus Rius (Hospital Universitari Sant Joan de Reus, Tarragona), Núria Rovira (Hospital General de Manresa, Barcelona), Joan Masip (Centre d’Estudis Epidemiològics sobre les ITS i SIDA de Catalunya-CEEISCAT).
The NENEXP cohort study began in Catalonia in 2000 and has been supported by the various FIPSE (Foundation for Innovation and Prospective Health in Spain) amendments: FIPSE 3081/99, FIPSE 36352/02 and 36535/05. It has contributed data on the history of HIV MTCT in Catalonia and the evolution of infection in pregnant women over recent years.
Please cite this article as: Soriano-Arandes A, Noguera-Julian A, López-Lacort M, Soler-Palacín P, Mur A, Méndez M, et al. El embarazo como una oportunidad de diagnóstico del virus de la inmunodeficiencia humana en mujeres inmigrantes en Catalunya. Enferm Infecc Microbiol Clin. 2018;36:9–15.