Mycobacterial culture has a high sensitivity and is the test of choice for the microbiological diagnosis of tuberculosis and nontuberculous mycobacterial infections. However, the results of this culture require at least 2–3 weeks to obtain positivity. Staining is rapid and can be used as a complementary study, although its sensitivity is low. Gene amplification tests have an intermediate sensitivity and obtain results in 1–2 days. These last tests are indicated in cases with moderate or high clinical suspicion. In HIV patients with severe immunodeficiency (<200 CD4), lipoarabinomannan antigen detection in urine may be useful.
The identification of isolates from positive cultures is essential to evaluate the clinical significance of the culture results and consider the therapeutic options available. At present, there is a wide range of identification techniques available, which provide results within just 1–4 days.
The future of diagnostic techniques in tuberculosis and nontuberculous mycobacterial infections lies in greater development of gene amplification techniques and promoting the search for biomarkers which enable a new approach to the diagnosis of these infections.
El cultivo de micobacterias es la prueba de referencia, y la más sensible, en el diagnóstico microbiológico de la tuberculosis y de las infecciones por micobacterias no tuberculosas. Sin embargo, requiere al menos 2-3 semanas para ser positivo. La tinción, rápida, aunque poco sensible, actúa como un complemento. Las pruebas de amplificación genética tienen una sensitivity intermedia y obtienen resultados en 1-2 días. Estas últimas están indicadas cuando el grado de suspicion es moderado o alto. En pacientes infectados por el VIH con inmunodepresión severa (<200 CD4) puede ser útil la detección de antígeno lipoarabinomanano en orina.
La identificación de los aislamientos de los cultivos positivos es imprescindible para evaluar la significación clínica y la orientación terapéutica. Actualmente se dispone de un abanico de técnicas de identificación que facilitan resultados en periodos de solo 1-4 días.
El futuro del diagnóstico pasa por un mayor desarrollo de las técnicas de amplificación genética y por potenciar la búsqueda de biomarcadores que permitan un nuevo enfoque del diagnóstico de estas infecciones.
There are currently approximately 170 species of the genus Mycobacterium.1 The most important one is Mycobacterium tuberculosis, the tuberculosis (TB) agent, which causes 1.6 million deaths per year.2 The last report of cases in Spain indicates that the incidence of TB in 2014 was 10.8/100,000in.3 Non tuberculous mycobacterial infections (NTM) have increased in recent years, particularly in patients with chronic pulmonary diseases. Frequency of isolation presents a non-homogeneous geographical distribution in the different countries, related to environmental conditions where the NTM4develop. In our environment there are around 35–40% of clinical mycobacterial isolations. In this study the diagnostic microbiological methods of TB and NTM are reviewed. Commentary is made on the basic tests for direct diagnosis and those used in the identification of positive cultures (Tables 1 and 2).
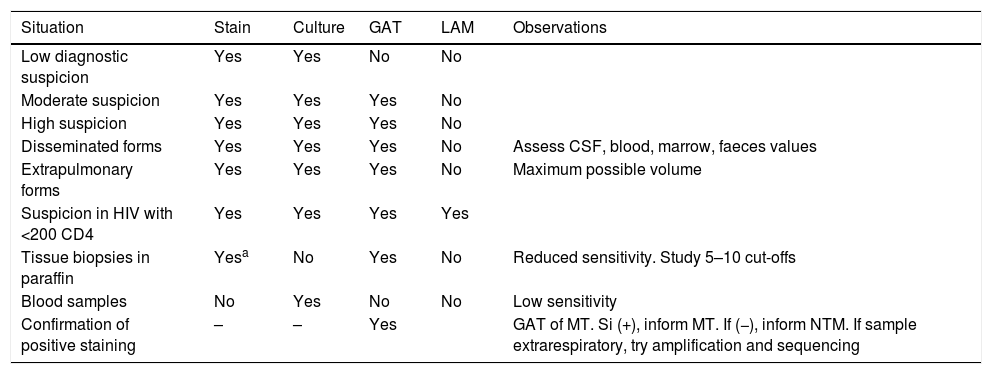
The use of direct diagnostic tests in clinical samples.
| Situation | Stain | Culture | GAT | LAM | Observations |
|---|---|---|---|---|---|
| Low diagnostic suspicion | Yes | Yes | No | No | |
| Moderate suspicion | Yes | Yes | Yes | No | |
| High suspicion | Yes | Yes | Yes | No | |
| Disseminated forms | Yes | Yes | Yes | No | Assess CSF, blood, marrow, faeces values |
| Extrapulmonary forms | Yes | Yes | Yes | No | Maximum possible volume |
| Suspicion in HIV with <200 CD4 | Yes | Yes | Yes | Yes | |
| Tissue biopsies in paraffin | Yesa | No | Yes | No | Reduced sensitivity. Study 5–10 cut-offs |
| Blood samples | No | Yes | No | No | Low sensitivity |
| Confirmation of positive staining | – | – | Yes | GAT of MT. Si (+), inform MT. If (−), inform NTM. If sample extrarespiratory, try amplification and sequencing |
GAT: genetic amplification techniques; LAM: lipoarabinomannan in urine; CSF: cerebrospinal fluid; NTM: non tuberculous mycobacteria; MT: Mycobacterium tuberculosis; HIV: human immunodeficiency virus.
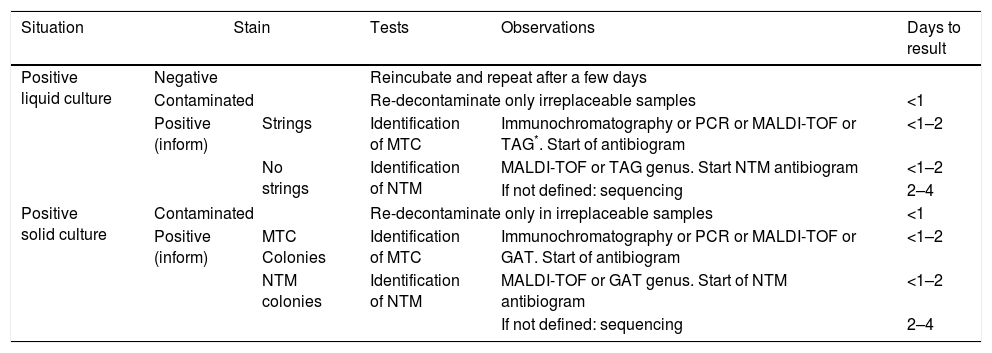
Algorithm of positive culture identification test usage.
| Situation | Stain | Tests | Observations | Days to result | |
|---|---|---|---|---|---|
| Positive liquid culture | Negative | Reincubate and repeat after a few days | |||
| Contaminated | Re-decontaminate only irreplaceable samples | <1 | |||
| Positive (inform) | Strings | Identification of MTC | Immunochromatography or PCR or MALDI-TOF or TAG*. Start of antibiogram | <1–2 | |
| No strings | Identification of NTM | MALDI-TOF or TAG genus. Start NTM antibiogram | <1–2 | ||
| If not defined: sequencing | 2–4 | ||||
| Positive solid culture | Contaminated | Re-decontaminate only in irreplaceable samples | <1 | ||
| Positive (inform) | MTC Colonies | Identification of MTC | Immunochromatography or PCR or MALDI-TOF or GAT. Start of antibiogram | <1–2 | |
| NTM colonies | Identification of NTM | MALDI-TOF or GAT genus. Start of NTM antibiogram | <1–2 | ||
| If not defined: sequencing | 2–4 | ||||
NTM: non tuberculous mycobacteria; MTC: Mycobacterium tuberculosis complex; PCR: polymerase chain reaction; GAT: genetic amplification techniques.
Lastly there is mention of some of the techniques which may be used to acquire future diagnostic relevance.
Direct diagnosis in clinical samplesSamplesThese should be representative of location and symptoms.5,6 The most common location in TB and NTM infections is the lung. In expectorating patients 2–3 series samples of early morning sputum is sufficient. When there is no expectoration, samples may be obtained with a bronchoscopy, through bronchial suction or bronchoalveolar lavage. In children or in situations without access to bronchoscopy, gastric suction may be used as an alternative. Biological fluids, such as cerebral spinal fluid, articular or pleural fluid, will be indicted in their clinical presentations, when it is not possible to obtain a relevant biopsy. With kidney disease series samples of early morning urine is used. In cases of tissue or organ compromise, the samples are obtained by puncture or preferably biopsy. If there is suspicion of disseminated infection, blood, bone marrow, and faeces cultures may be useful, In general, with the exception of advanced immunodepression, infections by mycobacteria are not highly bacteremic.
Achieving optimum sensitivity5 should take into consideration the following basic questions:
- 1.
Obtainment of sterile recipients: bacterial contamination reduces sensitivity.
- 2.
Appropriate minimum volume: 5ml in respiratory samples without diluting or 7–10ml in diluted samples. At least 1ml in cerebral spinal fluid; 5–10ml in other biological fluids. Biopsy instead of puncture.
- 3.
Transport: as a general rule, the samples should be sent to the laboratory within 2h of obtaining them. The biopsies and tissues should be kept damp with sterile distilled water. It is highly important to avoid formalin used in anatomopathological analysis since this is lethal for all microorganisms. Paraffinised samples may be used for detecting gene expression although the process of deparaffinlization and digestion of the tissue notably reduces sensitivity. The acid pH of gastric juices is harmful to the mycobacteria, and rapid transportation or the use of neutralising antibodies is therefore decisive.
- 4.
The quality of the samples, i.e. that they come from the infectious site, indicted by the presence of inflammatory cells.
- 5.
The petition of multiple tests atomises the samples and reduces their sensitivity. It is essential to have a prior differential diagnosis and be in contact with the laboratory to decide which are the most appropriate test to perform in each case.
The high percentage of lipids from the wall of mycobacteria is responsible for the difficulty of the colouring agents penetrating, and their subsequent departure. This property, known as acid-alcohol resistance, is what causes a lack of good staining with Gram staining and that specific stains exist. These are divided into 2 types, as observed with an optic microscope (OM) or florescence. The most well known of the former is the Ziehl-Neelsen staining, which colours the mycobacteria red and the remaining structures blue. The latter emphasise the florescent coloured mycobacteria on a dark background. The most used is the autamine rhodamine stain.6
The specificity of the stain is high, with few distinguishable exceptions from its morphology (Nocardia, Rhodococcus, Tsukamurella, Cryptosporidium, Cyclospora and Isospora), since the majority of the microorganisms do not stain red or fluorescent.6
Stain sensitivity is conditioned by factors such as the extent of the disease, the sample volume, concentration of mycobacteria and above all, the sample type. The limit of sensitivity would be between 50,000 and 100,000 mycobacteria/ml. overall sensitivity may range between 20% and 80% of processed samples, depending on their anatomical type and origin.5 In respiratory samples it is higher, particularly in carious lesions, and in tissues, such as enlarged lymph nodes. In our setting it was situated between 50% and 80%. Sensitivity is very poor in biological fluids due to the low concentration of mycobacteria. The quality of staining is unsuitable in blood samples or very haematic samples and this practice is not recommended. The stain from the concentrate after decontamination of the sample for the culture is 11–26% more sensitive than that directly made from the sample.5 Florescent samples are equally accepted, and are 10% more sensitive than those based on MO,5 although in general it is recommended that the positive signs be confirmed with MO6 stain or a gene amplification technique. Stains may be closely observed, at least 300 fields in MO and 30 for florescent staining, prior to issuing a negativity report. Positivity is reported as semi quantified of 1+ to 4+.6 The result, mainly in pulmonary forms, provides information on the spread of the condition, its infectivity and isolation period, in the case of TB. Assessment of the therapeutic response is also useful.
NTM are increasingly commonplace. However, when direct staining tests positive, in general it is not possible to clearly differentiate between them and complex Mycobacterium tuberculosis (MTC).
Culture methodsThe culture is the most sensitive diagnostic method. It has been estimated that its detection limit lies between 10 and 100bacteria/ml.5 Its main drawback is the long incubation period, from several days up to 6 weeks before a final negativity report may be issued. This is due to the speed of mycobacteria division, which is between 18 and 24h, 40 times slower than the majority of bacteria. For this same reason, in samples where flora or other bacteria are present, such as those of respiratory origin, they should be previously eliminated through a chemical decontamination process with the preservation of the mycobacteria. There are several decontamination methods. The most well known is based on the use of NaOH and N-acetyl-l-cysteine (Kubica method). It is advisable not to use it in commonly sterile samples, as it is not totally innocuous for mycobacteria. Furthermore, a proportion of cultures will be contaminated despite decontamination. A percentage between 3% and 5% is considered acceptable, although it depends on the type of samples of each laboratory. Samples which contain Pseudomonas aeruginosa, capsulated bacteria, fungi and yeasts, among others, will be more likely to contaminate the cultures. Decontamination methods aimed at this type of sample exist.6
The majority of mycobacteria species are usually nutritionally demanding, and therefore use specific culture media. However, the majority of them may grow in common enriched media, such as blood agar, chocolate agar or those used in blood cultures although more slowly than in the specific ones. At present, for primary isolation from clinical samples the combined use of a liquid culture and a solid culture is recommended. Solid media may be based in coagulated egg or agar. The most well known of both types are Löwenstein-Jensen and Middlebrook 7H11, respectively. Other methods which contain eggs are Trudeau, Coletsos, ATS or Petragnani. Middlebrook 7H10 would be the other medium based on agar. In several geographical areas selective media are used which include antibiotic (7H11S o 7H10S) for primary isolation. There are numerous liquid media, including Middlebrook 7H9, Dubos, Youmans or Proskauer-Beck. The former is most currently used. The majority of liquid media used in diagnosis are semi-automated, based on the use of incubators which are capable of detecting growth by O2 consumption or production of CO2, through a system of continuous monitoring of bottles or tubes that contain the culture medium and the sample. They usually use Midlebrook 7H9 as the base medium, with enriched supplements and antibiotic solutions to neutralise microorganisms which have resisted decontamination. Three methods are the most common: VersaTREK® (Thermo Fisher Scientific, Oakwood Village, OH, US), BacT ALERT 3D® (BioMerieux, Marcy l’Etoile, France) and MGIT960® (Becton Dickinson, Sparks, MD, US). The first contains several cellulose sponges in the liquid medium, which also means that a solid culture surface area for growth is possible. The incubator has pressure sensors which are capable of detecting growth from the consumption of O2. BacT ALERT 3D® has sensors which record the colour changes resulting from the production of CO2 in an indicator content at the bottom of each culture bottle. MGIT960® uses tubes which contain a base of silicone which includes an indicator that emits florescence to changes in the consumption of O2 in the tube. The cultures for the majority of mycobacteria are incubated at 35–37°C. The species which cause skin and soft tissue diseases, such as Mycobacterium ulcerans, Mycobacterium marinum, Mycobacterium haemophilum and Mycobacterium chelonae, grow better at 25°C and 30°C. In contrast, Mycobacterium xenopi grows better at 42°C and Mycobacterium thermoresistibile at 52°C. Some species need specific supplements to grow in cultures. The clearest examples of these are M. haemophilum, which requires haemin, haemoglobin or ferric ammonium citrate solution or Mycobacterium genavense. The latter grows very slowly, between 3 and 6 months, generally alone in liquid media with acidified pH, like that used for the pyrazinamide antibiogram in the MGIT960® system or in solid media supplemented by mycobacteria J, activated carbon or blood.
The cultures are monitored continuously in liquid culture equipment, detecting potential positivity when they are produced. The solid cultures are inspected 1–2times/week for the first 3 weeks and once during the following 3 weeks. The sensitivity of the culture media is linked to the bacterial load of the sample, both linked to the disease evolution time period in each patient and in clinical location. When level of suspicion is high or moderately high, having 2–3 series of samples will increase sensitivity. In our medium, in samples of respiratory origin, this may range between 70% and 85%, just like in solid tissue such as enlarged lymph nodes. In locations with biological fluids, performance is poorer, at best no more than between 35% and 50%. The best performance is observed with the use of automated liquid cultures.7 With regard to the detection period of positive results, the majority become positive between the first and third week. The mean is 14 days. For samples with positive bacilloscopy, the positivity drops to 13 days for MTC isolation and 11 days in NTM. When the bacilloscopy is negative, this takes place between 17 and 16 days for MTC and NTM respectively. The solid cultures are positivised between the second and fourth week. Overall, almost 100% of solid and liquid cultures are positivised during the first 4 weeks. In follow-up samples, after initiating treatment, this percentage would be reached after 5 weeks. Furthermore, recovery in liquid media is considerably superior to that of solids, especially for isolation of NTM.
Methods based on the detection of genetic or antigen materialGenetic methods applied to the direct sample in diagnosis of Mycobacterium tuberculosis complex (MTC)Tests for molecular detection in clinical samples are based on amplification and subsequent detection of nucleic acids. These are used increasingly to diagnose TB due to the speed with which results are obtained and to the sensitivity when compared with staining. A huge quantity of techniques have been developed based on the amplification of specific sequences of MTC, mainly 16S ARNr, IS6110 or the gene rpoB, and many of them have been marked. They have been included in several recent publications.8–10 In this review some of them will be commented upon. It is important to consider that the sensitivity of amplification techniques is lower than that of the culture in those samples with negative stain, and therefore they cannot be used to rule out the disease. To increase the sensitivity of these tests and achieve similar results to the culture, the inhibitors found in the sample must be efficaciously eliminated to detect ≤10–100mycobacteria/ml, the lower limit of culture detection,11 and also for their application in the routine the consistency and systematisation of the technique is important, so that they are minimally handled.12 For this reason, the use of commercial techniques rather than traditional ones are more appropriate. In 2010, the Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, US) appeared as a totally automated method, with minimum handling prior to the introduction of the sample in the cartridge where the whole reaction was to take place. The technique consists in a nested PCR at real time of the rpoB gene, which codes for the sub-unit of the polymerase RNA. It uses 5 genetic probes of molecular beacon type, each one marked with a different fluorophore. It totally covers the area of 81 base pairs, between codons 426 and 452 of the rpoB gene (507 and 533 referred to the nomenclature of the gene in Escherichia coli), determinant of rifampicin resistance. In this way it simultaneously reports the presence of MTC and the most frequent mutations in the rpoB gene, predicting resistance to rifampicin which in the majority of cases is connected to multi-resistance. According to a recent review by Cochrane,13 its sensitivity is 89%, with that of 68% for samples with bacilloscopy negativity, and specificity is 98%. Its rapidity and extreme simplicity makes it highly attractive, having been promoted by the WHO as a tool for rapidly diagnosing both TB and multi-resistant strains in areas with high rates.14 (http://www.who.int/tb/publications/xpert-mtb-rif-assay-diagnosis-policy-update). In developed countries the greatest drawback is price, although in a very recent study it is reported that its use in all patients could be cost-effective, by improving differential diagnosis.15 The low sensitivity of this system in paucibacillary samples has driven the same company to develop an Xpert® MTB/RIF Ultra system which collects a larger sample volume than the previous model and adds 2 new targets which are found in a greater number in the MTC (IS6110 y IS1081) genome. Both changes have successfully increased sensitivity, which has led the WHO to propose its use, particularly in cases of patients infected by HIV and paediatric patients.16 However, there is a need for studies to be conducted which will endorse the specificity results of this new system.
Other methods available on the market in our environment for the direct detection are those produced by HAIN Lifescience: Fluorotype® MTB y GenoQuick® MTB (HAIN Lifescience, Nehren, Germany). Fluorotype® MTB is a semi-automated method which has recently been marketed, based on real time PCR. Initial assessment17 indicates a specificity of 98.9%, with sensitivity of 100% for samples with microscope positivity and 56.3% for microscope negativity. GenoQuick® MTB applies a GenoQuick® technology for MTC diagnosis directly from a pulmonary and extrapulmonary sample. This may be carried out in small laboratories since only a thermo-cycler specific to the system is required. In 2–3h it completes the extraction and amplification of the nucleic acids and denaturalises to hybridise with specific gold-marked probes, using a lateral flow suspension system, and combined in a specific site on the hybridisation test strip.18
Commercial tests have also been developed for the detection of resistance mutations and are also useful in the diagnosis of TB due to the fact that in their most recent versions improvements have been made to the sensitivity which means they may be applied on the clinical sample. FluoroType® MTBDRplus (HAIN Lifescience, Nehren, Germany), TB Resistance modules (AID Diagnostika, Strassberg, Germany)19 and AnyplexTM plus MTB/NTM/MDR-TB (Seegene, Seoul, Korea)20 are several of those available.
In order to increase sensitivity in diagnosis recent literature reports different proposals which may possibly be introduced in the future. One of these is the XtracTB12 system. It was designed for inclusion in an automated platform in a central laboratory. It combines several DNA targets with several lavages to reduce the presence of the qPCR inhibitors with posterior amplification of 2 specific MTC targets, IS6110 and senX3-regX3, to raise test sensitivity and specificity and minimise the probability of false negatives. The first tests show sensitivity of 5 genomic copies/mL in sputum which rival the culture sensitivity. This system does not detect resistant genes, but the technology could be used for this.
Lastly, although trends lie in the use of commercial automated or semi-automated tests, the WHO21 has recommended the use of the loop-mediated isothermal amplification or LAMP technique in its rapid diagnostic strategy of TB. It consists in isothermal amplification which uses 6 primers, linked to 8 different targets. It requires minimum technology and manual reading with an UV lamp, and it is therefore cost-effective and potentially accessible to many countries. Recent WHO21 data state that it has a specificity of 98%, sensitivity of 96.6% for microscopy positive samples and 42.2% for negative samples.
Genetic methods applied to the direct sample in diagnosis of nontuberculous mycobacteria (NTM)Molecular diagnosis of the NTM directly in the clinical sample has been the focus of less attention than the diagnosis of TB, probably due to incidence and epidemiological consideration, although in recent years there is increasing interest in it. The diversity of species is a hindrance to specific focus, and the majority of techniques in the literature are based on traditional methods and generally aimed at gene diagnosis, particularly when amplifying the gene 16S rRNA.One approximation exercised in some laboratories is to sequence the amplification product to try to diagnose the species. Several commercial tests are applicable to NTM diagnosis. Anyplex plus MTB/NTM MDR-TB combines the identification of MTC and of Mycobacterium sp. in a single amplification in real time. Another would be the GenoType® (GT) NTM-DR (HAIN Lifescience, Nehren, Germany) test which is able to detect mutations of resistance to macrolides and aminoglycosides whilst simultaneously identifying several species of NTM frequently isolated in clinical samples, such as M. chelonae, M. avium, M. intracellulare and M. chimaera, and the 3 sub-species of M. abscessus (sub. abscessus, sub. bolletii, sub. massiliense). This test is used mainly in the characterisation of cases of clinical isolation. No contrasted experience exists as of yet with regard to its use directly on clinical samples.
Lipoarabinomannan antigen detection in urineDirect detection in urine of the lipoarabinomannan wall antigen (LAM) in urine is an innovative technique. LAM is a specific thermo-stable glycolipid released by the mycobacteria. It is filtered by the kidney and is present in the urine of patients with active TB. It was initially analysed in serum, but the formation of immune complexes hindered its detection. The Determine TB LAM Ag® (Alere Inc., Waltham, MA, U.S.A.) test is based on immunochromatography and uses specific capture antibodies against the LAM antigen. The antigen present in urine is captured by an antibody conjugate and colloidal-gold conjugate forming an immunocomplex, which in turn is combined to anti-LAM antibodies attached to a nitrocellulose membrane. Detection is visible thanks to the colloidal-gold particles with which the complex is marked. The sensitivity of this test ranges between 38% and 50.7% for TB and specificity ranges between 87.8% and 89%, under the microscope, with the culture in a solid and/or liquid medium. In severely immunodepressed HIV patients, CD4<200, sensitivity increases considerably up to 62%.5 It has several advantages which make it highly useful in certain circumstances: urine is an easier sample to collect than sputum, it may be less variable in quality and is safer to handle. It requires no infrastructure, may be collected by the patient's bedside, without equipment, at a cost below 3$. Several studies have recently been published comparing Determine TB LAM Ag® and Xpert MTB/RIF® in countries with a high rate of tuberculosis and HIV.22,23 The LAM test is more sensitive in patients with low levels of CD4, and may show reduction of 4% in morality after 8 weeks in patients who were diagnosed on admittance to hospital using this test.23 With these results, its use in hospitals is recommended where the patients are admitted with severe immunodepression and without the ability to expectorate. Apart from sensitivity, another limitation acknowledged by the manufacturer is that it cannot differentiate between MTC and other mycobacteria.
Usage strategy of direct diagnosis testsThe clinical characteristics of TB and NTM infections form part of a suspected diagnosis in a large number of infectious sub-acute or chronic conditions, many of which then have a different final diagnosis. Rational use of tests is therefore advisable, in accordance with the degree of clinical suspicion (Table 1). The culture must always be made in all samples where it is of interest to rule out mycobacteria. It is also advisable to process 2–3 series samples, especially in the lungs. Staining is the fastest and cheapest test, but the least sensitive. It has a very low sensitivity in biological fluids and its indication in these samples should probably be re-assessed.
Tests based on genetic amplification are only indicated if there is a high or fairly high degree of suspicion and almost always in TB. In lymph nodes samples in children or in immunodepressed patients amplification of MTC will be indicated and that of the genus Mycobacterium. In skin samples, the most common aetiology will be M. marinum or another NTM infetion, and the amplification of the gene, with or without MTC amplification will therefore be indicated. In patients with chronic pulmonary disease and symptoms of exacerbation, when the use of amplification techniques is being suggested, they should mostly be directed at the NTM.
Provided that they are available, using commercial methods is ideal, since test quality control and consistency is guaranteed. It is also advisable for there to be as little handling as possible. If suspicion is high, using the tests on series samples will increase sensitivity. An important application of genetic amplification will be to confirm or rule out the positive stain samples as MTC. It is a good idea to do this shortly after staining to maintain or cancel respiratory isolation and to plan treatment.
In extrapulmonary sites, it is important for the required sample volume to be available, after as exhaustive as possible differential diagnosis to reduce the range of tests to be performed and also bear in mind the diagnosis when biopsies or surgically preserved tissues are taken, with the preservation of a part of the samples being formalin-free.
Isolation identificationIsolation identification maybe carried out from several approximations (Table 2). A common strategy is to confirm with a stain that the growth corresponds to mycobacteria. For species identification methods based on antigen detection may be used, such as immmunochromatography, in gene amplification, with all of its possible variants or in the matrix assisted laser desorption ionisation time-of-flight mass spectrometry (MALDI-TOF). All of these methods offer greater precision and speed than identification based on biochemical tests, which have traditionally been the basis of identification but are currently only being used in specific situations. Current molecular methods have also led to a very minority use of the techniques based on high pressure liquid chromatography or gas chromatography, which in their day were an alternative to biochemical methods.
Morphology of colonies and stainsWhen cultures are positivised confirmation must be made that mycobacteria are growing. The liquid culture incubators indicate when the culture of a sample reaches the stipulated growth as a cut-off point to be considered positive. Visual inspection is a lead, since MTC and other but not all species of NTM, do not make contact in the medium, unlike those of rapid growth and conventional bacteria.
In solid cultures macroscopic growth of colonies is observed. Depending on the species, the morphology differs. MTC usually grows in flat, rugged colonies that look dry and may be white or light yellow. In the other species different morphologies may be observed, and some are plemophic. Approximately 10 species are pigmented, and may or may not be stimulated by light (photochromogenic or scotochomogenic). When the solid cultures are contaminated, the pH of the medium changes or they digest it in part and generate different colonies, making them clearly evident.
Ziehl-Neelsen staining of a solid or liquid medium confirms positivity, rules out contamination of the culture or mixed cultures, and false positives. The stain morphology adopted by the mycobacteria grown in the cultures, and especially in the liquid ones, offers a fairly precise distinction between whether it is MTC or NTM. The grouping of several hundred bacilli forming strings is highly indicative of MTC. For years it was considered a single template for MTC, but recently it has been observed in cultures of M. abscessus and it has also been described in other NTM24 species. Other species, such as M. avium complex, Mycobacterium kansasii or M. xenopi, usually have very characteristic morphologies in the stain.25 This initial approximation allows for a positivity report to be issued and for the appropriate tests to be indicated for identification of the isolated species.
Identification by immunochromatographyFor fast identification and differentiation between MTC and other mycobacteria several commercial tests have been developed which are based on the detection of the MPT64 antigen, which was detected in the culture filtrates of MTC.26 This simple, fast test with very little manipulation offers results in under 15min and without the need for any equipment, making costs very low. It uses a nitrocellulose membrane with the monoclonal antibody, anti-MPT64 of a mouse attached to it. The test must be performed from a positive culture. In the case of a liquid culture it is directly applied. From solid medium colonies must be re-suspended in an extraction cap provided by the manufacturer. When the bacterial suspension and antibodies come into contact, if the antigen has been excreted from the medium, a line of colour appears. Sensitivity and specificity are very close to 100%. However, this technique may present false negatives since several strains from the complex (as seen in several isolations of Mycobacterium bovis BCG) do not produce this protein due to polymorphisms of the gene which codes the antigen MPT64. The mpt64 (Rv1980c) gene is found in a differential region called RD2.27 The RD2 region was detected in strains of M. bovis BCG between 1927 and 1931, coinciding with the reports vaccine mitigation. It affects several BCG subtypes, including BCG Pasteur, BCG Danish and BCG Glaxo, whilst not affecting BCG Japan and Moreau, among others.28
Several commercial products exist which use this methodology as diagnosis (SD BIOLINE TB Ag MPT64 Rapid Test® kit, Alere Healthcare SLU, Hospitalet de Llobregat, Spain; Capilia TB®, TAUNS Laboratories Inc., Numazu, Shizouka, Japan; BD MGIT TBc Identification test®, Becton Dickinson Diagnostics, Sparks, MD, US).
Identification with PCR methodsThis is mostly aimed at MTC identification. It is based on the use of amplification methods, either commercial or traditional, with specific MTC gene targets such as IS6110. Potentially all methods designed for direct detection of MTC may be used as confirmatory identification tests.
Identification with Line Probe Assay methodsLine Probe Assay (LPA) methods are based on the specific amplification and subsequent hybridation in solid phase with immobilised genetic probes on nitrocellulose strips. Hybridation becomes evident using an enzymatic process. Different commercial tests exist, including INNO-LiPA® MYCOBACTERIA (Innogenetics, Ghent, Belgium) and GT Mycobacterium (HAIN Lifescience, Nehren, Germany). The former carries out amplification of the intergenic 16S-23S space, and then hybridises on a nitrocellulose strip which has22 probes to enable the identification of the MTC and another 16 NTM, of clinical relevance. The GT Mycobacterium test amplifies the 23S ARNr gene and there are 3 different formats available: the GT MTBC, which identifies the different species integrating the MTC; the GT CM format which identifies MTC and 13 species of NTM, and lastly the GT AS presentation, which identifies 16 further species, so that as a whole 32 species may be identified, including the most frequently isolated cases.
Identification with MALDI-TOF mass spectrometryThis technique is fast being introduced into the microbiology laboratories, especially for the identification of bacterial isolations, although it is extended to other microorganisms such as fungi and parasites. It consists of the analysis of the proteic profile of microorganisms through a mass spectrometry and the comparison with a spectral library of previously identified profiles for different species. In recent years bacterial identification has been revolutionised and results may be achieved in minutes from positive cultures (Table 2). The samples to be analysed may be solid or liquid cultures. For analysis approximately 1μl of the sample is mixed with a matrix which aids ionisation after being bombarded by a laser beam, which provokes disruption and migration of the proteins throughout the length of the mass spectrometry tube, in keeping with its electric load and size, with a flying time being determined for each one of them and creating a profile for each sample. The equipment has library of profiles to compare with that of the sample. The degree of similarity is interpreted in a number or score. The software of the equipment indicates the interpretation intervals of the scores obtained, classifying the samples by a percentage or a category of identification reliability. At present, 2 MALDI-TOF mass spectrometry units are being marketed, the MALDI™ Biotyper (Bruker Daltonics GmbH, Bremen, Germany) and the VITEK® MS (bioMérieux, Marcy l’Etoile, France), although the first one appears to be enjoying greater profusion. Although the usage protocols may vary slightly, the results obtained seem to be equivalent in both. There are 2 essential aspects in the use of MALDI-TOF: correct extraction and the widest available library of templates. The complexity and resistance of the mycobacterial wall involves intense lysis with physical and chemical methods and a previous extraction of proteins, which are not necessary for standard bacteria. This preparation must be absolutely meticulous to achieve the best possible extraction. The spectral library is the second crucial aspect. With the exception of sequencing, there is no other method in the market that enables more species to be identified than MALDI-TOF. For example, the Biotyper unit by Bruker has recently developed a version (v4) which contains 880 templates, of which 450 are clinically isolated and correspond to 159 species, out of the approximate 170 species comprising the genus. This version co-exists with the previous one (v3), which was able to identify 149 species.29 The number of spectrums of each species will also determine the precision of identification. Several species are extremely difficult to differentiate because their spectrums are very similar. Such is the case of M. intracellulare and M. chimaera or the 3 subspecies of M. avium (sub. avium, sub. silvaticum, sub. paratuberculosis) or the 3 of M. abscessus, or between M. abscessus and M. chelonae, or between M. fortuitum and M. porcinum among others. Apart from the library, operative aspects such as culture incubation time, or manual reading of the laser shots on the sample, instead of automatically performing this, may increase identification efficacy. Some aspects require standardisation, including unifying extraction, imposing greater accuracy of profile interpretation criteria and cut-off points used and establishing the number of profiles of each species needed to consider identification highly reliable. A work group undertaking this standardisation currently exists in Europe.
MALDI-TOF is being regarded as a major method of mycobacteria identification, with a spectrum of identified species highly superior to any other non-sequencing technique. The laboratories which wish to implement it have to consider that it is a complex method and that a learning and handling process that may require several months will be necessary.
Identification by sequencingSequencing is considered to be the benchmark reference for mycobacteria identification. The most frequently used gene is the 16S rRNA, although hsp65, rpoB and 16S-23S internal transcriber spacer are also used and often a combination of 2 or more of them. Technical sequencing usage is widely known, consistent in an initial amplification with the gene initiators to be studied, followed by a second amplification of each of the DNA chain strands with nucleotides marked with fluorescent molecules. This second product, once purified, is analysed with the sequencer, which issues a result in the form of written sequence and electropherogram. The resulting template is introduced into a data base that contains a multitude of sequences to which it is compared. For acceptable identification the percentage of similarity with the sequences identified from the data base must be at least 99%, and ideally 99.5%. There are several data bases which belong to companies or institutions such as MicroSEQ® ID Analysis, RIDOM or EzTaxon, that contain templates which have been submitted to an identification control. Other data bases allow free access for the introduction of sequences with minimal requirements and without demonstrating identification. This offers no guarantee of control that the sequences introduced are what they say they are. However, the latter contain a very high number of sequences, several of them over 20 million and in practice they therefore offer considerable reliability. The most well known ones are EMBL and Genbank. Many laboratories use 16S rRNA gene sequencing with and hsp65 as support in identification of slow growing mycobacteria and rpoB for the fast growing ones.30 The relative diffusion of sequencing leads to different operational settings. If a sequencing unit is not owned, the amplification product may be sent to specialise external laboratories, at a low cost and with short result periods. Sequencing also has limitations in interpretation. Several species are difficult to differentiate, such as the components of MTC, which are indistinguishable, the distinction between M. intracellulare and M. chimaera, the sub-species of M. avium or those of M. abscessus.
Identification usage strategyPositive isolations should always be identified on a species level or at least a complex level. The majority species in our environment is MTC, although NTM isolations are increasingly frequent. The fastest and most cost effective technique is the Ziehl-Neelsen isolation staining. It confirms that this is Mycobacterium spp. and in liquid cultures in many cases helps to distinguish if this is MTC from its string formation, or NTM. Suspicion of MTC maybe confirmed using an immunochromatography test and obtain identification the same day. Alternatively, any method based on PCR or MALDI-TOF may be used. In theory it is possible to have the result the same day, but routine conditions would probably delay this 1–2days. The differentiation of MTC species is not generally necessary due to the great majority of isolations corresponding to M. tuberculosis, with the others being very infrequent. It would be indicated in the following cases: (1) isolation resistant to pyrazinamide (M. bovis and M. bovis BCG are resistant to this drugs); (2) suspicion of M. bovis, from epidemiological data or of M. bovis BCG used in the treatment of urinary bladder tumour, and (3) suspicion of Mycobacterium africanum in West African patients.
If initial tests do not confirm MTC or the staining clearly points to NTM, the LPA techniques may be used. If a MALDI-TOF unit is available, it is currently an alternative to LPA techniques, although it may also be used when the latter do not successfully identify the isolations. Both techniques require hours of manual dedication. Sequencing is the technique of choice, usable when identification with the other techniques is not possible or when there are doubts regarding results. In these cases it will often be necessary to sequence 2 or more different genes. It is important that the laboratory issues partial reports which are helpful in clinical management. It is therefore initially important to know whether the case is MTC or not, from the epidemiological isolation conditions of the patient and from a different treatment. If this is NTM, it will be important to know whether it is slow or fast growing, since the pattern of empirical treatment would vary. Similarly, if the infection is significant and initial identification is M. abscessus, it is important to know whether the macrolides may be used (M. abscessus sub. massiliense and several strains of the other 2 sub-species). In this case, the use of the GenoType NTM DR LPA test will confirm or rule out the erm gene which codes an inducible methylase from the macrolides. Depending on the species and the techniques used, final identification of NTM may take between 2 and 7 days, although an initial approximation would be available in 1–2 days.
The future of diagnosisThe diagnosis of mycobacteria (TB and NTM) presents with 3 essential types of difficulties: firstly, poor sensitivity of tests in extrapulmonary sites; secondly, the slowness of the most sensitive test, the culture, which leads to inferred diagnosis and empirical treatments and thirdly, the difficulty of obtaining sufficient samples in several sites. The ideal test should be able to be performed on universal samples, such as blood or urine, should be rapid and sensitive.
Standard staining and culture tests have probably reached their limit and it would be difficult to upgrade their performance. Genetic amplification tests still have room for improvement, both in amplification performance and the achievement of a more effective extraction of nucleic acids. The usefulness of detecting DNA eliminated through the urinary tract observed in several previous studies should also be explored. Mass sequencing has a future in diagnosis. Its potential is huge, especially in the characterisation of mutations and other determinants of resistance. However, at present expansion is limited due to the sensitivity of amplification which is still sub-optimal. Notwithstanding, other points of focus are required, beyond the detection of the microorganism or its DNA: In recent years efforts have been made in the search for biomarkers aimed mainly at TB. This is yet an incipient route and has not yet been developed. Several details may be noted.31 For example, in relation to the biomarkers of the patient, in studies which analyse numerous metabolites in the blood, an increase in some of them has been observed in patients with active TB, compared with healthy subjects. Other studies show that there could be an increase in the production of interferon gamma in patients with latent TB about to evolve into active TB. With regard to microorganism markers, it has been indicated that the LAM antigen detected in urine could improve its sensitivity using visualisation methods of the result that are not enzymatic. A special mention deserves to be made to the detection of aromatic components. The analysis of exhaled air for TB diagnosis is an appealing, non invasive alternative where the patient feels no pain. It is known that certain patients may release several odours according to their status. Using chromatographic techniques a large number of volatile organic components have been identified in clinical samples which could serve as markers for monitoring different stages of diseases. The advances in odour-sensing technology and artificial intelligence are investigating this issue and artificial noses have been designed to identify M. tuberculosis cultures with almost 100% surety. After a pilot study carried out in Bangladesh with highly successful results,32 an electronic portable management nose was developed with a battery based on metal oxides with the possibility of industrial production at a low cost (Aenose®, eNose Company, Zutphen, Netherlands). A recent study used Anose® in patients with a positive culture compared with healthy subjects and showed a sensitivity and specificity of 91% and 93%, respectively, with acceptance among participants in the study and easy to use. Broader studies with more variables such as the assessment of different simultaneous diseases33 will probably be necessary. In this same mode, the results of gas sensors in arrays on quartz have also been presented, obtaining similar sensitivity and specificity rates.34
To conclude, the diagnostic future probably consists in combining the maximum performance of existing tests, improving amplification techniques and investing time and money into biomarker research. The ideal test should be able to simultaneously detect mutations of resistance to all drugs and be as automated as possible, at an affordable price, as well as being able to produce an easily obtainable sample.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Samper S, González-Martin J. Diagnóstico microbiológico de las infecciones causadas por el género Mycobacterium. Enferm Infecc Microbiol Clin. 2018;36:104–111.