There are few published studies on predictors of response to treatment with sofosbuvir and simeprevir in HCV patients.
ObjectiveThe objective of the study was to analyse possible predictors of response to simeprevir (SMV) and sofosbuvir (SOF) in patients infected with hepatitis C genotypes 1 or 4.
Patients and methodsProspective observational cohort study in 12 hospitals. The primary efficacy endpoint was SVR rate 12 weeks after end of treatment (SVR12).
Results204 patients (62.3% male, mean age 55 years) were included: 186 (91.2%) genotype 1 (60.3% 1b 25% 1a) and 18 (8.8%) genotype 4. 132 (64.7%) cirrhotic (87.9% Child A), 33 (16.2%) F3, 31 (15.2%) F2, 8 (3.9%) F0–1. 80.8% MELD<10. 93 (45.6%) naive. Ribavirin was added in 68 (33.3%). Mean baseline viral load 2,151,549IU/ml (SD: 2,391,840). Treatment duration 12 weeks in 93.1%. 4 discontinued therapy: suicide, psychotic attack, hyperbilirubinaemia and liver cancer recurrence. 190 (93.1%) achieved SVR12. There were no differences in SVR12 depending on the genotype, treatment duration, ribavirin use, prior therapy, viral load (VL) or baseline platelets. In univariate analysis, undetectable VL at 4 weeks (p=0.042), absence of cirrhosis (p=0.021), baseline albumin ≥4g/dl (p=0.001) and MELD<10 (p<0.0001) were associated with higher SVR12. In multivariate analysis, only baseline MELD score <10 patients had higher SVR12 (p<0.001).
ConclusionsThe combination of simeprevir and sofosbuvir in patients infected with genotype 1 and 4 hepatitis C is highly effective. It is a safe therapy, especially in patients without ribavirin. This combination was more effective in patients with a MELD score below 10.
Hay pocos estudios publicados acerca de los factores predictivos de respuesta al tratamiento de la hepatitis C con sofosbuvir y simeprevir.
ObjetivoConocer qué factores influyen en la respuesta a simeprevir (SIM) y sofosbuvir (SOF) en pacientes infectados por los genotipos 1 o 4 de la hepatitis C.
Pacientes y métodosEstudio prospectivo observacional de cohortes en 12 hospitales. La efectividad se evaluó con respuesta virológica sostenida (RVS12).
ResultadosSe incluyeron 204 pacientes (62,3% varones, edad media 55 años). Ciento ochenta y seis (91,2%) genotipo 1 (60,3% 1 b, 25% 1 a) y 18 (8,8%) genotipo 4. Ciento treinta y dos (64,7%) cirróticos (87,9% Child A), 33 (16,2%) F3, 31 (15,2%) F2, 8 (3,9%) F0-1. Un 80,8% MELD<10. Noventa y tres (45,6%) naive. Se asoció ribavirina en 68 (33,3%). Carga viral basal media 2.151.549 UI/ML (DE: 2.391.840). Duración tratamiento 12 semanas en 93,1%. Cuatro suspendieron tratamiento: suicidio, brote psicótico, hiperbilirrubinemia y recurrencia hepatocarcinoma. Ciento noventa (93,1%) alcanzaron RVS12. No hubo diferencias RVS12 en función del genotipo, duración tratamiento, empleo de ribavirina, tratamiento previo, CV y plaquetas basales. En análisis univariante, negatividad carga viral a las 4 semanas (p=0,042), ausencia de cirrosis (p=0,021), albúmina basal ≥4g/dl (p: 0,001) y MELD<10 (p<0,0001) se asociaron con mayor RVS12. En estudio multivariante solo hubo relación significativa entre puntuación MELD basal <10 y mayor RVS12 (p<0,001).
ConclusionesLa combinación de simeprevir y sofosbuvir es muy eficaz en pacientes infectados por los genotipos 1 y 4 de la hepatitis C. Es un tratamiento seguro, especialmente en pacientes sin ribavirina. Esta combinación es más efectiva en pacientes con puntuación MELD inferior a 10.
Hepatitis C virus (HCV) infection is one of the leading causes of liver disease in the world.1–5 Combinations of oral direct-acting antivirals against specific proteins of the hepatitis C virus genome are associated with heightened efficacy, more convenient dosage, a shorter duration of treatment and few side effects.6–10 This is the reason why it is currently the treatment of choice for these patients.1,11,12
The combination of simeprevir (SMV) and sofosbuvir (SOF) is useful for treating patients infected with HCV genotypes 1 and 4. SMV corresponds to the second generation of NS3-4A protease inhibitors, active against genotypes 1 and 4.13 It is well tolerated, but it has a low barrier to resistance. SOF is a nucleotide analogue prodrug that acts as an RNA-dependent NS5B polymerase inhibitor. It is a very potent, pan-genotypic antiviral with excellent tolerability and safety profile and a high barrier to resistance.14
The combination of SOF and SMV was initially used in the COSMOS study,15 with SVR12 rates higher than 90% and good tolerance. Phase III studies have confirmed their efficacy and safety in patients with and without cirrhosis of the liver.16–18
The objective of the study was to analyse effectiveness and safety, and predictive response factors to therapy with SMV and SOF in our setting.
Patients and methodsA prospective, observational cohort study which included patients who started treatment for HCV with SOF and SMV was conducted between 1 December 2014 and 1 December 2015 in 12 hospitals in the Autonomous Community of Castilla La Mancha. This combination of oral direct-acting antivirals was the first available in the Autonomous Community, and it was subsequently prioritised over other options due to its cost.
All adult patients with chronic HCV genotypes 1 or 4 treated with SOF 400mg and SMV 150mg daily for 12 or 24 weeks, with the addition or not of ribavirin (RBV) (1000–1200mg/day, if <75kg or ≥75kg, respectively) were included at the discretion of their physicians.
Follow-up was carried out at the baseline visit and at weeks 4, 8, 12 and 24 of treatment and at 12 weeks post-treatment. Five hundred variables were analysed, which included age, gender, BMI, comorbidities, toxic habits, transplant and previous treatment. The severity of liver disease was evaluated with fibrosis indexes (APRI, FORNS and FIB-4), elastography, MELD and Child–Pugh. The cut-off values for elastography were as follows: F0–1 up to 6.9kPa; F2 between 7 and 9.4kPa; F3 between 9.5 and 12.4kPa; F4 from 12.5kPa.
The duration of therapy and whether or not it was associated with RBV was recorded. During the follow-up visits, haemogram, biochemistry, coagulation and HCV viral load tests were carried out.
To assess therapeutic efficacy, the infection was considered cured if there was no HCV viral load detected at 12 weeks after the end of treatment (SVR12). Virologic response was also analysed at the end of treatment, understood as the presence of a negative viral load at the end of treatment. Deaths were considered to be treatment failure. Viral load was quantified by means of COBAS TaqMan HCV assay (version 2.0; Roche), with a lower limit of quantitation of 15IU/ml and a lower limit of detection of 10IU/ml.
To assess the safety of treatment, adverse effects and treatment adherence were recorded at weeks 4, 8, 12 and 24. Adverse effects were classified as mild (they did not affect daily life), moderate (they did affect daily life or required admission to hospital, but were not life threatening), and severe (they were life threatening). A haemoglobin count of under 12g/dl was considered anaemia. If the count was between 10 and 12g/dl it was considered mild, between 8 and 10g/dl, moderate, and under 8g/dl, severe.
The SPSS version 22 software program was used to conduct the statistical analysis by calculating associations between related samples and descriptive statistics. The quantitative variables were measured with mean, median and range. For the univariate study, the Chi-squared test was used for the categorical variables and the Student's T test for the quantitative variables. Statistical significance was set at p<0.05. Logistic regression was carried out for those variables that had a p value of at least <0.1 in the univariate study.
The SVR12 overall and in different subgroups of patients (RBV association, previous treatment, cirrhosis, genotype, platelets higher than 100,000/mm3 or not, treatment duration, albumin <4 or ≥4mg/dl, and MELD <9 or ≥10) were calculated. Subsequently, a backward stepwise logistic regression analysis was carried out with the Ward statistic to find out the predictor variables for SVR. The model was considered significant with a p value of <0.001.
The study was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines. It was approved by the Central Ethics Committee. The patients gave their consent to participate in the study.
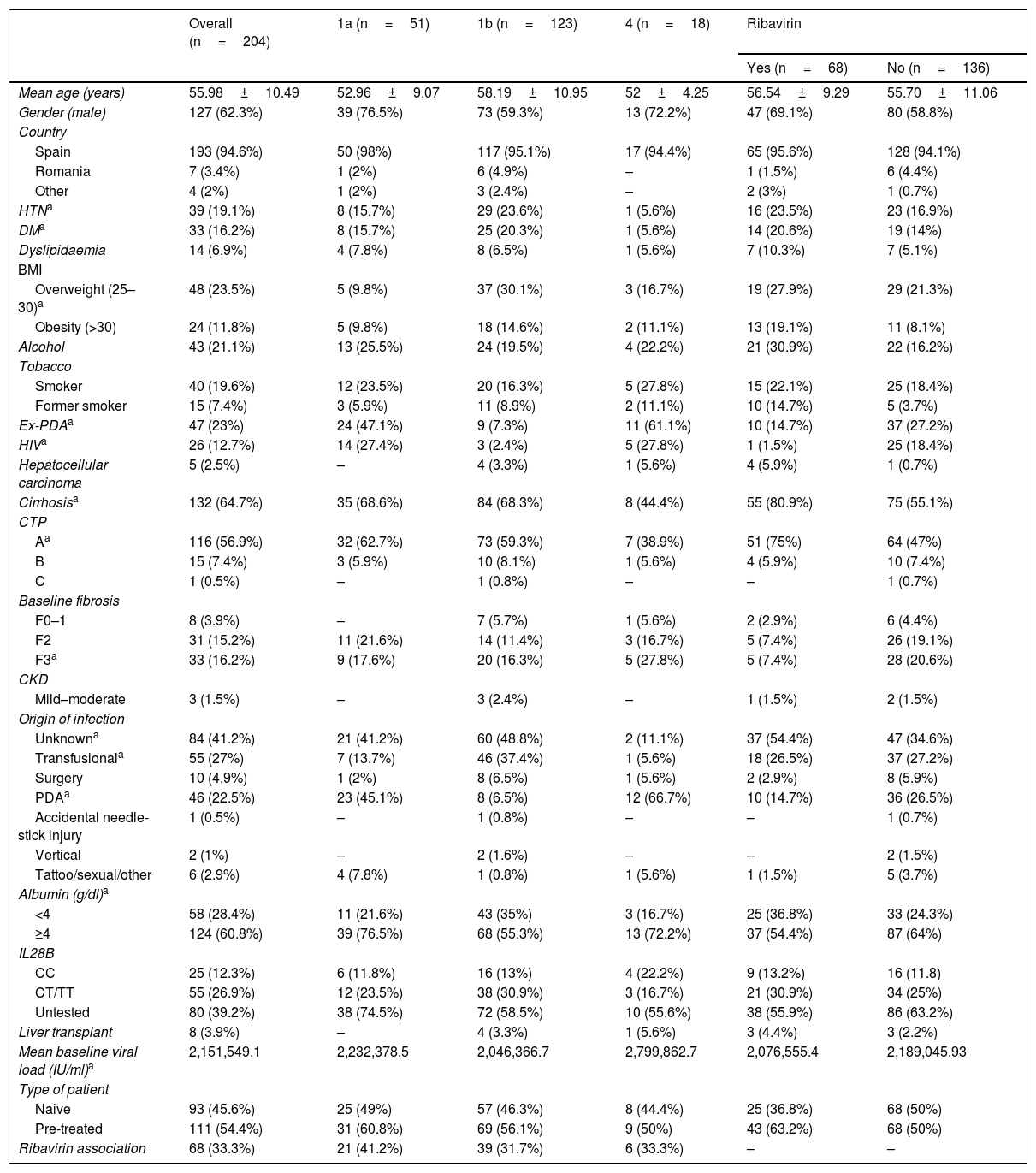
ResultsPatient demographic characteristicsTwo-hundred and four patients treated with SMV and SOF were included in the study, with associated RBV in 68 (33.3%) cases. Table 1 summarises the clinical and demographic characteristics of the patients.
Patient overall demographic and clinical characteristics, by subtype and by association with ribavirin.
| Overall (n=204) | 1a (n=51) | 1b (n=123) | 4 (n=18) | Ribavirin | ||
|---|---|---|---|---|---|---|
| Yes (n=68) | No (n=136) | |||||
| Mean age (years) | 55.98±10.49 | 52.96±9.07 | 58.19±10.95 | 52±4.25 | 56.54±9.29 | 55.70±11.06 |
| Gender (male) | 127 (62.3%) | 39 (76.5%) | 73 (59.3%) | 13 (72.2%) | 47 (69.1%) | 80 (58.8%) |
| Country | ||||||
| Spain | 193 (94.6%) | 50 (98%) | 117 (95.1%) | 17 (94.4%) | 65 (95.6%) | 128 (94.1%) |
| Romania | 7 (3.4%) | 1 (2%) | 6 (4.9%) | – | 1 (1.5%) | 6 (4.4%) |
| Other | 4 (2%) | 1 (2%) | 3 (2.4%) | – | 2 (3%) | 1 (0.7%) |
| HTNa | 39 (19.1%) | 8 (15.7%) | 29 (23.6%) | 1 (5.6%) | 16 (23.5%) | 23 (16.9%) |
| DMa | 33 (16.2%) | 8 (15.7%) | 25 (20.3%) | 1 (5.6%) | 14 (20.6%) | 19 (14%) |
| Dyslipidaemia | 14 (6.9%) | 4 (7.8%) | 8 (6.5%) | 1 (5.6%) | 7 (10.3%) | 7 (5.1%) |
| BMI | ||||||
| Overweight (25–30)a | 48 (23.5%) | 5 (9.8%) | 37 (30.1%) | 3 (16.7%) | 19 (27.9%) | 29 (21.3%) |
| Obesity (>30) | 24 (11.8%) | 5 (9.8%) | 18 (14.6%) | 2 (11.1%) | 13 (19.1%) | 11 (8.1%) |
| Alcohol | 43 (21.1%) | 13 (25.5%) | 24 (19.5%) | 4 (22.2%) | 21 (30.9%) | 22 (16.2%) |
| Tobacco | ||||||
| Smoker | 40 (19.6%) | 12 (23.5%) | 20 (16.3%) | 5 (27.8%) | 15 (22.1%) | 25 (18.4%) |
| Former smoker | 15 (7.4%) | 3 (5.9%) | 11 (8.9%) | 2 (11.1%) | 10 (14.7%) | 5 (3.7%) |
| Ex-PDAa | 47 (23%) | 24 (47.1%) | 9 (7.3%) | 11 (61.1%) | 10 (14.7%) | 37 (27.2%) |
| HIVa | 26 (12.7%) | 14 (27.4%) | 3 (2.4%) | 5 (27.8%) | 1 (1.5%) | 25 (18.4%) |
| Hepatocellular carcinoma | 5 (2.5%) | – | 4 (3.3%) | 1 (5.6%) | 4 (5.9%) | 1 (0.7%) |
| Cirrhosisa | 132 (64.7%) | 35 (68.6%) | 84 (68.3%) | 8 (44.4%) | 55 (80.9%) | 75 (55.1%) |
| CTP | ||||||
| Aa | 116 (56.9%) | 32 (62.7%) | 73 (59.3%) | 7 (38.9%) | 51 (75%) | 64 (47%) |
| B | 15 (7.4%) | 3 (5.9%) | 10 (8.1%) | 1 (5.6%) | 4 (5.9%) | 10 (7.4%) |
| C | 1 (0.5%) | – | 1 (0.8%) | – | – | 1 (0.7%) |
| Baseline fibrosis | ||||||
| F0–1 | 8 (3.9%) | – | 7 (5.7%) | 1 (5.6%) | 2 (2.9%) | 6 (4.4%) |
| F2 | 31 (15.2%) | 11 (21.6%) | 14 (11.4%) | 3 (16.7%) | 5 (7.4%) | 26 (19.1%) |
| F3a | 33 (16.2%) | 9 (17.6%) | 20 (16.3%) | 5 (27.8%) | 5 (7.4%) | 28 (20.6%) |
| CKD | ||||||
| Mild–moderate | 3 (1.5%) | – | 3 (2.4%) | – | 1 (1.5%) | 2 (1.5%) |
| Origin of infection | ||||||
| Unknowna | 84 (41.2%) | 21 (41.2%) | 60 (48.8%) | 2 (11.1%) | 37 (54.4%) | 47 (34.6%) |
| Transfusionala | 55 (27%) | 7 (13.7%) | 46 (37.4%) | 1 (5.6%) | 18 (26.5%) | 37 (27.2%) |
| Surgery | 10 (4.9%) | 1 (2%) | 8 (6.5%) | 1 (5.6%) | 2 (2.9%) | 8 (5.9%) |
| PDAa | 46 (22.5%) | 23 (45.1%) | 8 (6.5%) | 12 (66.7%) | 10 (14.7%) | 36 (26.5%) |
| Accidental needle-stick injury | 1 (0.5%) | – | 1 (0.8%) | – | – | 1 (0.7%) |
| Vertical | 2 (1%) | – | 2 (1.6%) | – | – | 2 (1.5%) |
| Tattoo/sexual/other | 6 (2.9%) | 4 (7.8%) | 1 (0.8%) | 1 (5.6%) | 1 (1.5%) | 5 (3.7%) |
| Albumin (g/dl)a | ||||||
| <4 | 58 (28.4%) | 11 (21.6%) | 43 (35%) | 3 (16.7%) | 25 (36.8%) | 33 (24.3%) |
| ≥4 | 124 (60.8%) | 39 (76.5%) | 68 (55.3%) | 13 (72.2%) | 37 (54.4%) | 87 (64%) |
| IL28B | ||||||
| CC | 25 (12.3%) | 6 (11.8%) | 16 (13%) | 4 (22.2%) | 9 (13.2%) | 16 (11.8) |
| CT/TT | 55 (26.9%) | 12 (23.5%) | 38 (30.9%) | 3 (16.7%) | 21 (30.9%) | 34 (25%) |
| Untested | 80 (39.2%) | 38 (74.5%) | 72 (58.5%) | 10 (55.6%) | 38 (55.9%) | 86 (63.2%) |
| Liver transplant | 8 (3.9%) | – | 4 (3.3%) | 1 (5.6%) | 3 (4.4%) | 3 (2.2%) |
| Mean baseline viral load (IU/ml)a | 2,151,549.1 | 2,232,378.5 | 2,046,366.7 | 2,799,862.7 | 2,076,555.4 | 2,189,045.93 |
| Type of patient | ||||||
| Naive | 93 (45.6%) | 25 (49%) | 57 (46.3%) | 8 (44.4%) | 25 (36.8%) | 68 (50%) |
| Pre-treated | 111 (54.4%) | 31 (60.8%) | 69 (56.1%) | 9 (50%) | 43 (63.2%) | 68 (50%) |
| Ribavirin association | 68 (33.3%) | 21 (41.2%) | 39 (31.7%) | 6 (33.3%) | – | – |
albumin: baseline; BMI: body mass index; CKD: chronic kidney disease; CTP: Child-Turcotte-Pugh; DM: diabetes mellitus; HIV: human immunodeficiency virus; HTN: hypertension; PDA: parenteral drug addict; UK: United Kingdom; Uk: Ukraine.
62.3% (n=127) were male. The mean age at the start of treatment was 55 (median: 55; range 27–83). The majority were Spanish (94.6%). One hundred and eighty-six (91.2%) were infected with genotype 1, 123 (60.3%) with 1b and 51 (25%) with 1a. In 7 cases, genotype 1 could not be subtyped; in four there was a mixed 1a and 1b infection, and in another 1a and 4e. Eighteen patients (8.8%) were infected with genotype 4. 57.5% presented with a genotype of interleukin IL28B C/T, 31.2% CC and 11.3% TT.
Twenty-six (12.7%) had an HIV co-infection, nine (4.4%) were solid organ transplant recipients (8 of liver), and three had an HBV co-infection.
The cause of infection was unknown for 84 patients (41.2%). Transfusion was the most common (27%), followed by parenteral drug use (22.5%).
One hundred and thirty-two patients (64.7%) had cirrhosis. Of these, 116 were Child–Pugh grade A, 15 grade B and one grade C. Seventeen were or had been decompensated. The mean MELD score in patients with cirrhosis was 8.2 (median: 7; range: 6–15). Baseline albumin was <4 in 58 patients and ≥4 in 124.
Ninety-three (45.6%) were naïve and 111 (54.4%) had been treated previously. The mean baseline viral load was 2,151,549IU/ml (median: 1,304,979; SD: 23,918,490; range: 5,390–11,900,000IU/ml).
Treatment efficacyThe expected duration of treatment was 12 weeks in 190 (93.1%) patients and 24 weeks in 14 (6.9%). With the exception of one patient who committed suicide at 11 weeks of treatment and three who discontinued treatment at week 8 (1.5%), due to a psychotic episode, hyperbilirubinaemia and advanced hepatocellular carcinoma recurrence, the rest (200; 98%) successfully completed the expected duration.
The majority of patients (156/204; 76.5%) normalised transaminases. The overall virologic response at the time of conclusion of treatment (virologic response at the end of treatment) was 99.5% (203 of the 204 patients; just one patient did not clear the viral load during treatment; 95% CI: 98–100%). One hundred and ninety patients (93.1%; 95% CI: 80–93%) achieved SVR12. Aside from the patient who committed suicide, one woman died due to liver failure with SVR4; 11 patients had virologic relapse in the first 12 weeks after conclusion of treatment.
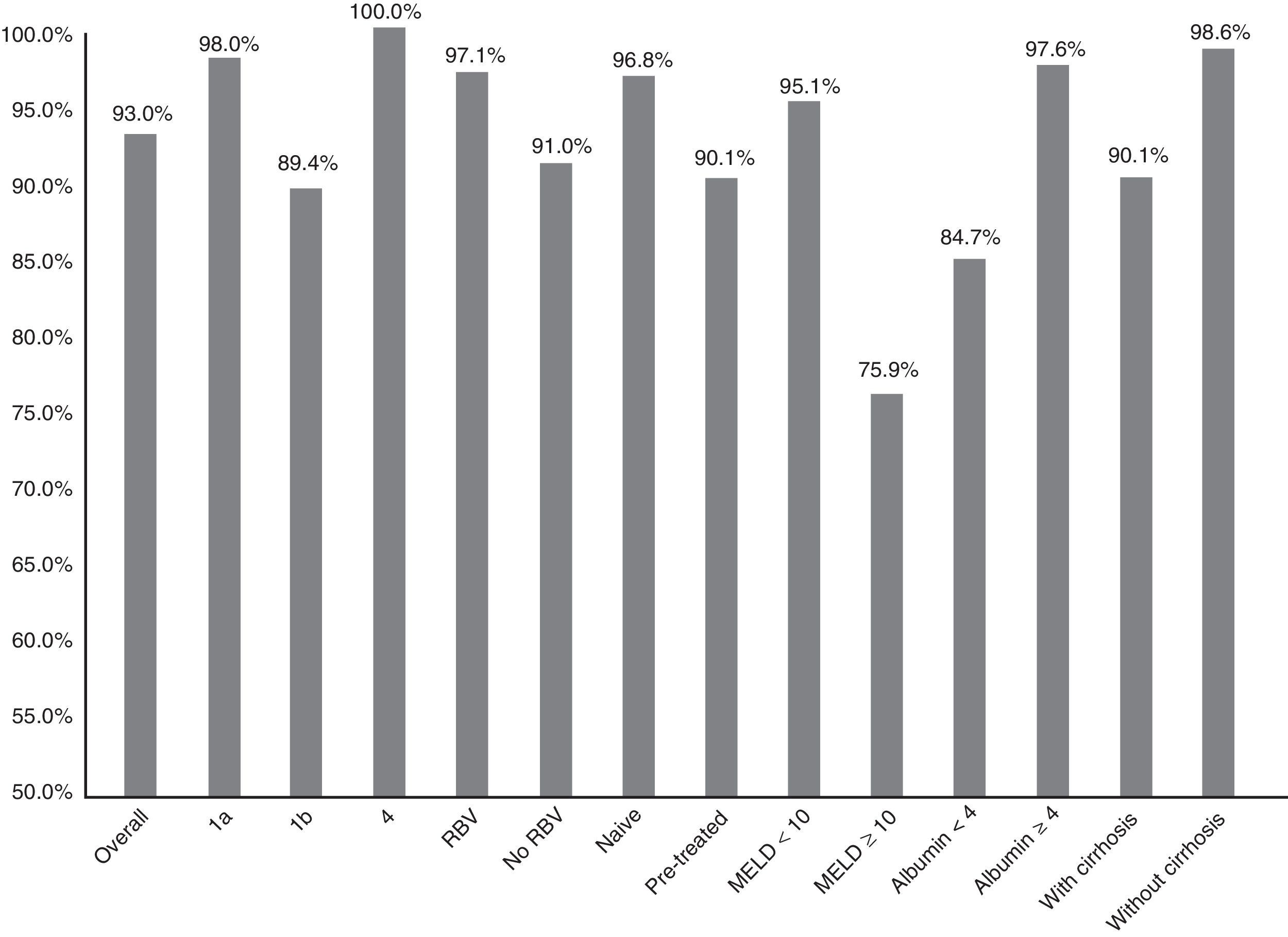
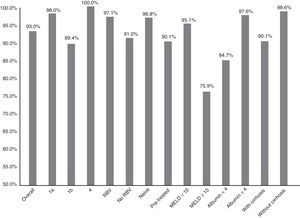
Fig. 1 summarises the SVR12 overall, by subtype, according to whether or not there was RBV association, by type of patient (naive o pre-treated), by MELD score (<10 or ≥10) and by baseline albumin level (<4 or ≥4).
Efficacy overall (SVR12), by subtype (1a, 1b and 4; p: 0.247), associated or not with ribavirin (RBV; p: 0.147), type of patient (naive or pre-treated; p: 0.06), MELD score (< or ≥10; p<0.001), baseline albumin levels (<4 or ≥4; p: 0.001) and presence or not of cirrhosis of the liver (p: 0.021).
The SVR12 was higher for genotype 1a (98%; 95% CI: 94–100%) than for 1b (89.4%, 95% CI: 84–95%). In patients with genotype 4, 100% SVR12 was achieved (Fig. 1). However, there were no significant differences (p: 0.247). Regarding the genotype of IL28B, the SVR12 was 96% (95% CI: 88–100%) (24/25) in C/C, 93.5% (95% CI 86–100%) (43/46) in C/T and 77.8% (95% CI: 44–100%), in T/T (p: 0.190). T/T patients showed a non-significant tendency towards a worse response (p: 0.075).
Overall effectiveness of patients who were treated for 12 weeks was 93.2% (95% CI: 90–97%) compared to 92.9% (95% CI: 77–100%) of the patients who were treated for 24 weeks (p=0.966).
Sixty-eight patients (33.8%) took RBV. SVR12 was achieved in 65 (97.1%, 95% CI: 93–100%) of those who received RBV and in 123 (91.1%, 95% CI: 86–96%) of those who did not receive it (Fig. 1). There was no statistical significance (p=0.147).
There was a non-significant tendency towards a worse response in pre-treated patients (SVR12: 90.1% (95% CI: 84–96%)—100/111—vs 96.8% (95% CI: 93–100%)—90/93—p: 0.06) in naive (Fig. 1). The 16 patients who had not responded previously to triple therapy with a protease inhibitor were cured of the infection (100 vs 92.6% of the rest; p: 0.608).
SVR12 was 94.1% (95% CI: 90–98%) (128/136) in individuals with a baseline viral load of HCV greater than 800,000IU/ml compared to 91.2% (95% CI: 84–98%) (62/68) of those with a lower load (p: 0.558). In terms of the negativity of the HCV viral load at 4 weeks from the start of treatment, SVR12 was 96% (95% CI: 93–99%) (120/125) in those who achieved it and 87.7% (95% CI: 80–95%) in those who did not (64/73) (p=0.042).
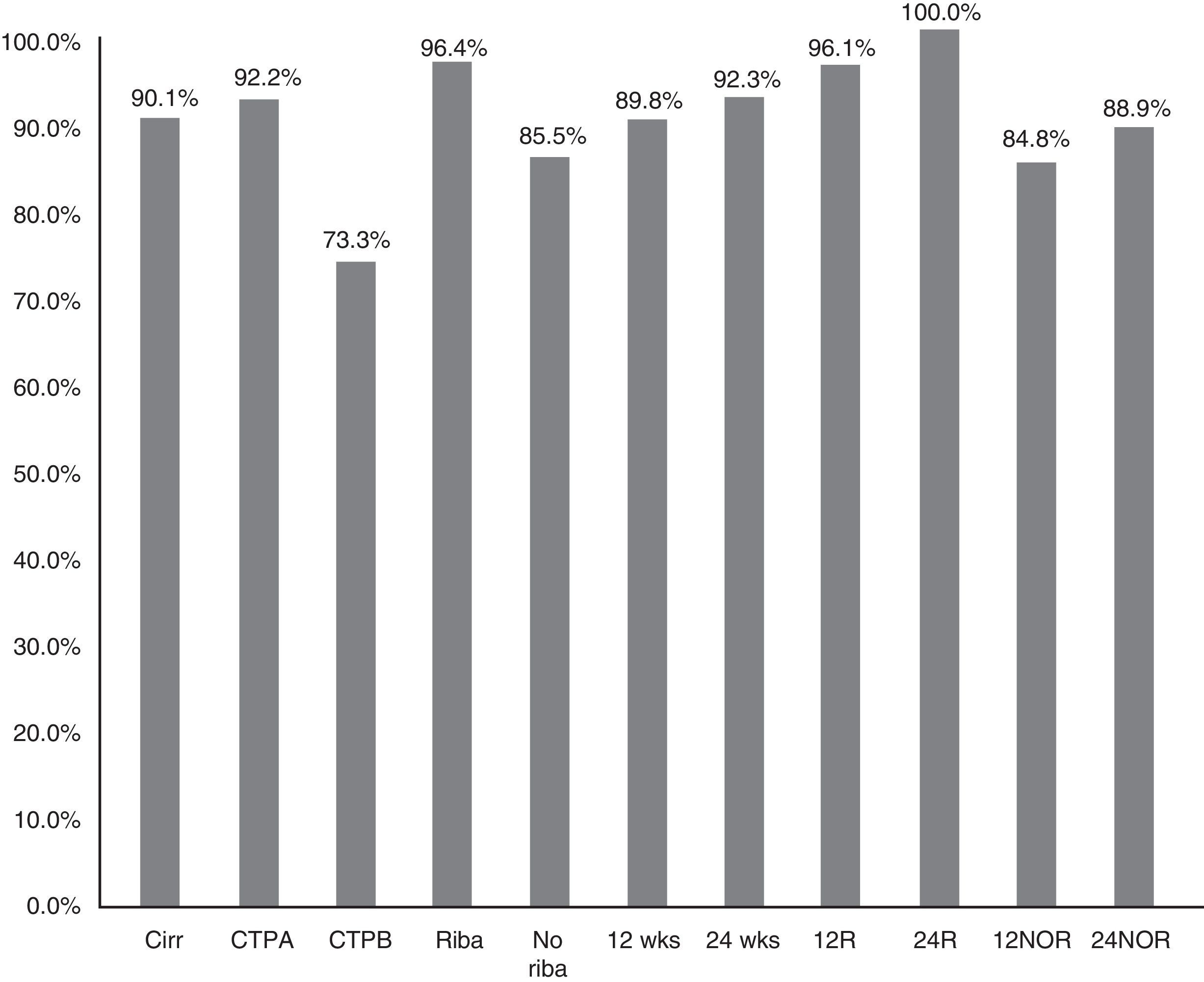
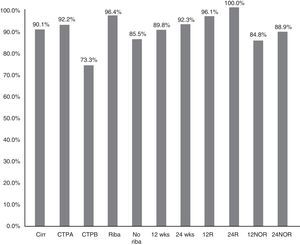
The SVR12 in patients without cirrhosis was 98.6% (95% CI: 96–100%), compared to 90.1% (95% CI: 85–95%) in those with cirrhosis (p=0.021) (Fig. 2). In Child-A patients it was 92.2% (95% CI: 85–96%) (106/115), 73.3% (95% CI: 48–99%) (11/15) in CTP-B and 100% in CTP-C (1/1) (p: 0.068). SVR in patients who had cirrhosis treated with RBV was 96.4% (54/56) (95% CI: 93–99%), and without RBV 85.5% (65/76) (95% CI: 80–95%); p: 0.038. SVR12 in patients who had cirrhosis treated for 12 and 24 weeks was 89.8% (95% CI: 77–94%) (106/118) vs 92.3% (95% CI: 91–100%) (12/13), respectively, without significant differences (p=0.777) (Fig. 2).
SVR12 was 84.7% (95% CI: 75–94%) (50/59) in patients with baseline albumin of ≤4g/dl vs 97.6% (95% CI: 95–100%) (120/123) if >4 (p: 0.001) (Fig. 1). SVR12 in those patients with more than 100,000platelets/mm3 was 93.6% (95% CI: 90–98%) (132/141) vs 91.5% (95% CI: 84–99%) (54/59) in those who had lower figures (p: 0.559).
SVR12 in patients with HIV co-infection was 96.2% (25/26), compared to 92.7% (165/178) (p: 0.515). The 9 transplant patients (liver or kidney) obtained SVR12 compared to 92.8% (181/195) of those without transplants (p: 0.405).
Finally, SVR12 in patients with cirrhosis with a MELD <10 was 95.1% (95% CI: 90–99%) (99/103) and in patients with MELD ≥10 it was 75.8% (95% CI: 58–92%) (22/29) (p: 0.002) (Fig. 1).
When conducting the Ward statistic with backward method in the subgroup of patients with cirrhosis, there was only a significant relationship between a baseline MELD score of lower than 10 and a greater SVR12. Patients with a baseline MELD score <10 have an OR of 6 (95% CI: 1.8–24) over a MELD ≥10 of reaching SVR12 (p=0.003). A naive patient status compared to pre-treated was closer to reaching statistical significance (OR: 3.3 [95% CI: 0.8–13.7]; p=0.097).
Safety of treatmentOf the 204 patients included in the study, at the end of follow-up, four (2%) had died due to causes considered to be unrelated to the medication: one suicide during treatment, one advanced hepatocellular carcinoma relapse (8 weeks after discontinuation), one case of lymphoma in a patient who had been cured of the infection (24 weeks after conclusion) and one case of sepsis in a patient with cirrhosis (6 weeks after conclusion).
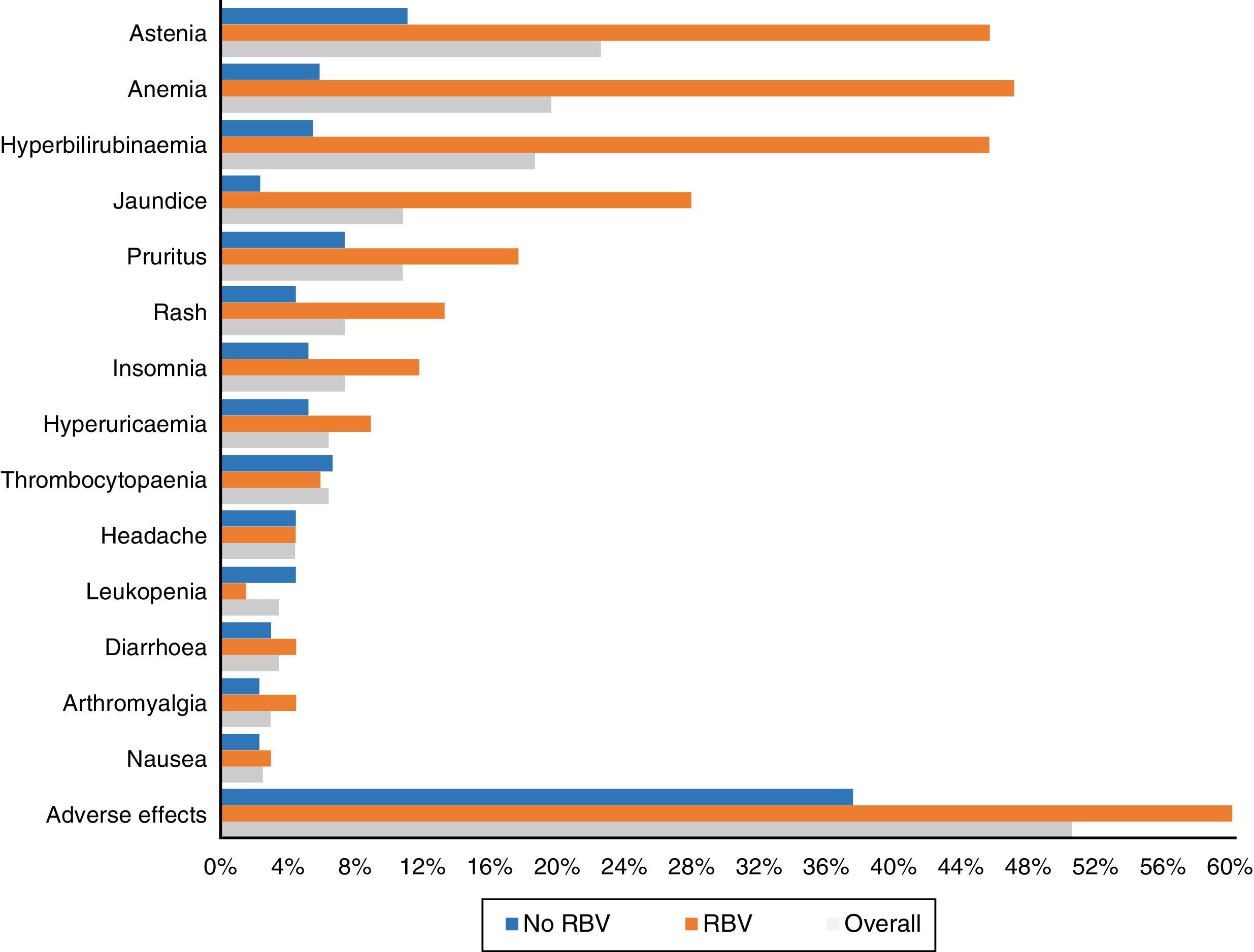
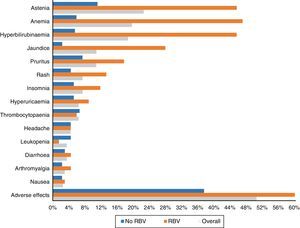
One hundred and three patients (50.5%) experienced adverse effects during treatment. These occurred more frequently in those patients who received RBV (76.5%—52/68) than in those who did not take it (37.5%—51/136) (p<0.0001). They were also more common in those who had cirrhosis (56.1%—74/132—vs 40.3%—29/72) (p: 0.023). The most frequent side effects overall and according to use of RBV are shown in Fig. 3.
The adverse effects were mild in 79 patients (38.7%), moderate in 17 (8.3%) and severe in seven (3.4%), requiring complete discontinuation of treatment in only four patients (2%). The reasons for suspension of treatment were suicide, psychotic episode, severe hyperbilirubinaemia (10mg/dl) and advanced hepatocellular carcinoma relapse with refractory ascites. There was a total of 5 cases of decompensated liver disease (3.8% of those with cirrhosis). Aside from the ascites mentioned, three patients presented with hepatic encephalopathy and another with gastrointestinal bleeding due to oesophageal varices.
Asthenia was the most common adverse effect, experienced by 46 patients (22.5%). In 38 patients it was mild, 7 moderate and one severe. Twenty-two (10.8%) patients presented with jaundice, and another 22 (10.8%) pruritus, which was severe in only four cases. Fifteen (7.4%) presented with exanthema, 13 localised and two extensive, and another 15 (7.4%) with insomnia.
Forty patients (19.6%) had anaemia, although only six (2.9%) required transfusions. The anaemia was classified as mild in 30 patients (14.7%), moderate, with a change in dose of RBV in 16 (7.8%) – 4 discontinuations and 12 decreases in dose to a maximum of 50% of the starting dose in 3 patients.
Thirty-eight patients (18.6%) presented with an increase in bilirubin levels. Thirty-one of the 68 patients (45.6%) had been treated with RBV, compared with only 7 of the 136 who did not take it (5.1%; p<0.0001). In one patient, it was decided to discontinue treatment for this reason, with subsequent improvement. Thirty-four of the patients with an increase in bilirubin had cirrhosis (34/132: 25.8%) and only 4 (4/72: 5.6%) did not (p: 0.001).
DiscussionIn this real-life study, the effectiveness and safety of a regimen that combined the protease inhibitor SMV with the polymerase inhibitor SOF was assessed in patients infected with hepatitis C genotypes 1 and 4. An SVR12 of 93% was achieved, and it was well tolerated by the majority of patients.
In our series, as happens in Spain in patients without HIV co-infection, for the most part patients are infected with genotype 1 (91.2%), above all with subtype 1b (61.8%). As in other studies, the majority are male, with a mean age of around 50.19 Given that our geographical area is one with a low immigrant population, the majority of those treated were Spanish. Under our health system, patients are prioritised for treatment according to severity of liver disease, for which reason two third of our patients had cirrhosis.
This therapeutic combination achieved high rates of cured infection in patients with genotype 1 (1a: 98% vs 1b: 89.4%). The infection was cured in all patients infected with genotype 4. Although there was no statistical significance, in our series, in contrast to other studies,15,18,20 HCV genotype 1a patients obtained higher rates of response than those with genotype 1b. In the OPTIMIST-217 study, patients with both subtypes responded the same, but those with genotype 1a without Q80K polymorphism responded better than those with 1b. It is likely that the reason our patients infected with genotype 1a responded better is due to the low incidence of said polymorphism in the Spanish population (7%). In other studies, just as in ours16,21 the response in both subtypes was similar. Although there were few patients, the response obtained in HCV genotype 4 patients was excellent.
In our study, T/T patients showed a non-significant trend of a worse response (p: 0.075). Although the number of patients is low, these results are consistent with the OPTIMIST-217 study.
Our study includes patients treated at almost all the hospitals in our autonomous community. The RBV indication was established by the responsible physician. Only a third of our patients received said drug, and even though this association could achieve higher cure rates (97.1 vs 91.1%), there was no statistical significance. However, in our series, the rate of response did increase in patients with cirrhosis of the liver (96.4 vs 85.5%). There is continued controversy regarding the use of this drug, which substantially worsens tolerance to therapy.8,15 In other studies,15,18,21 its addition increased the SVR rate.
Despite the fact that the majority of our patients were treated for 12 weeks, in our study, as in the COSMOS15 study, lengthening the therapy period to 24 weeks did not improve SVR. However, as it was a non-randomised real-life study, it is likely that some physicians treated those patients with a higher risk of therapeutic failure for 24 weeks instead of 12.
Although in our study there was no statistical significance, as in other studies15,17,18 our naive subjects obtained higher cure rates than those who had been pre-treated (96.8 vs 90.1%; NS). However, there are other studies21 in which the patients treated for the first time did not achieve a higher SVR12 rate.
In our study, as in that of Aqel et al.,21 the baseline viral load was not associated with a different response to treatment. Although it was not confirmed in the multivariate analysis, in the univariate analysis, the obtainment of a rapid virologic response (VL negative after 4 weeks) was associated with a greater SVR12. These data contrast with the COSMOS15 study.
Almost two thirds of our patients had cirrhosis, although the majority were at an early stage in the Child–Pugh scoring system. The effectiveness in patients who had cirrhosis decreased from 98.6 to 90.1% (p=0.021). These results are similar to those from Sulkowski et al.18 SVR12 in patients with CTP-A cirrhosis was 92.2%, and 73.3% in CTP-B. In the study by Aqel et al.,21 there was also a lower SVR12 in patients with advanced disease (CTP-B), compared to those with compensated cirrhosis (CTP-A).
Response to treatment was lower in patients with a baseline albumin of below 4 (84.7 vs 97.6%—p: 0.001). These results were not confirmed in the multivariate study. SVR12 was also analysed according to the baseline platelet count, with no differences found. In the OPTIMIST-217 study, there was a lower SVR12 in those patients with albumin under 4 and with a platelet count of less than 90,000/mm3.
Finally, the severity of liver disease, assessed using the MELD score had an important impact on SVR12. Patients with a baseline MELD of less than 9 have an OR of 15 over MELD ≥10 of achieving SVR12 (p<0.001). In the study by Aqel,21 an MELD score of ≤10 was a very important predictor of SVR12.
As is logical, tolerance to oral antiviral regimens is better than tolerance to older regimens with interferon and the majority of side effects in our series were of mild intensity, requiring only symptomatic treatment. Half of the patients experienced some kind of adverse effect during treatment, although most of them were mild. These occurred twice as frequently in those patients who received RBV (76.5%) than in those who did not take it (38.1%). They were also more common in those who had cirrhosis (56.9 vs 40.3%). These data are consistent with what has previously been published.15,18,21
As in other studies,15,18,21 asthenia and anaemia, in general mild and related to the use of RBV, were the most commonly recorded adverse effects. Less common side effects were hyperbilirubinaemia and jaundice, pruritus, rash, headache and insomnia.
Ultimately, the combination of SMV and SOF is very effective in patients infected with hepatitis C genotypes 1 and 4. The best candidates to receive this combination are those patients with a MELD score of less than 10. Furthermore, its a safe combination; the addition of RBV predetermines poor tolerance to treatment, although it could increase the rate of response in patients with cirrhosis of the liver.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We would like to express our sincere thanks to the collaborators who participated in this study:
Ponciano Martínez-Rodenas (Gastroenterology Unit, Hospital de Almansa, Albacete).
María Montealegre Barrejón (Gastroenterology Unit, Hospital de Villarrobledo, Albacete).
Eduardo Sánz de Villalobos (Gastroenterology Unit, Hospital Universitario de Guadalajara).
Tomás Artaza Varasa (Gastroenterology Unit, Complejo Hospitalario de Toledo).
Ricardo Pérez-Flores (Gastroenterology Unit, Complejo Hospitalario Universitario de Albacete; Universidad de Castilla La Mancha).
Sami Aoufi (Gastroenterology Unit, Hospital de Alcázar de San Juan, Ciudad Real).
Ignacio Marañés Antonanzas (Internal Medicine Unit, Hospital de Hellín, Albacete).
Please cite this article as: Moreno-Planas JM, Larrubia-Marfil JR, Sánchez-Ruano JJ, Morillas-Ariño J, Patón-Arenas R, Sáiz-Chumillas RM, et al. Influencia de la puntuación MELD basal en la eficacia del tratamiento de la hepatitis C con sofosbuvir y simeprevir. Enferm Infecc Microbiol Clin. 2018;36:277–283.