The objective was to compare incidence rates of whooping cough in children under the age of 1 in Castelló, before and after the introduction of vaccination of pregnant women in January 2015.
MethodsThe incidence of the POST-vaccine period (2015-2018) was compared with the PRE-vaccine period (2011-2014) in all ages, in children from 3 to 11 months and under 3 months. The relative risks (RR) and their 95% confidence intervals were calculated.
ResultsThe overall rate of pertussis in all ages was higher in the POST-vaccine period (0.23 per 1000 person-years) than in the PRE-vaccine period (0.15), but decreased in those under 3 months. The RR were: 1.56 (1.34 – 1.82) in all ages; 1.73 (0.87-3.57) for children aged 3 to 11 months, and 0.35 (0.16-0.69) for children under 3 months. A similar pattern was observed for hospitalised children.
ConclusionsThe incidence rate in children under 3 months was reduced by 65% in the period after the intervention, the hospitalisation risk rate by 71%, suggesting that the measure has been effective and specific for this age group.
Se analiza el cambio en las tasas de incidencia de tosferina en menores de 1 año en Castelló, antes y después de la introducción de la vacunación a embarazadas en enero de 2015.
MétodosSe han comparado las tasas de incidencia del periodo POST-vacunal (2015-2018) con el PRE-vacunal (2011-2014) en todas las edades, niños de 3 a 11 meses y menores de 3 meses. Se han calculado los riesgos relativos (RR) y sus intervalos de confianza al 95%.
ResultadosLa tasa global fue superior en el periodo POSTvac que en el PREvac (0,23 vs 0,15 por 1000 personas-año) pero disminuyó en los menores de 3 meses. Los RR fueron: 1,56 (1,34 - 1,82) para todas las edades; 1,73 (0,87-3,57) para 3 a 11 meses, y 0,35 (0,16-0,69) para menores de 3 meses. Un patrón similar se observó para niños hospitalizados.
ConclusionesLa tasa de incidencia en menores de 3 meses se redujo en un 65%, y el riesgo de hospitalización en un 71%, lo que sugiere que la medida ha sido efectiva. Esta reducción de la incidencia ocurrió de forma específica en este grupo de edad y no en otros.
Pertussis is currently considered to be a re-emerging disease, even in populations with high vaccine coverage,1,2 such as Spain.3 Children under one year of age are the most vulnerable population, particularly those under 2 months old, as they have yet to be given their first dose of vaccine.4,5
To protect neonates from the transplacental transfer of antibodies, immunisation with the tetanus-diphtheria-acellular pertussis (Tdap) vaccine was introduced to pregnant mothers in the third trimester.6–8 Observational studies have shown the effectiveness of the vaccine to be greater than 90%.9,10 In Valencia Region, this practice was implemented systematically in January 2015.11 However, there was no reduction in the overall incidence of whooping cough in Castelló.
The aim of this study was to determine whether there were indeed any changes in the incidence rates of whooping cough among children under one year of age in Castelló in the periods before and after introduction of the pertussis vaccination programme in pregnant women.
MethodsPopulation and periodArea of the Castelló Public Health Centre (Valencia Region), from 2011 to 2018. The populations were taken from Instituto Valenciano de Estadística [Valencian Institute of Statistics].12
Study subjectsPertussis cases reported in the Epidemiological Surveillance System according to clinical, epidemiological and microbiological criteria of the Valencia Region Pertussis Protocol, coinciding with the Red Nacional de Vigilancia Epidemiológica [Spanish Epidemiological Surveillance Network].13
DesignPre-post intervention study, comparison of two different periods, in all ages and in children under one year of age. Children under one year of age were divided into two groups: those aged 3 to 11 months and under 3 months. This cut-off point (< 3 months of age) is consistent with the start of primary vaccination with Tdap in infants and has been used in other Spanish studies.4
Statistical methodsFirst, the characteristics of the cases in the pre-vaccine (PREvac) and post-vaccine (POSTvac) periods were compared using the Chi test2, Fisher's test, Student's t test or the Mann-Whitney U test, according to whether or not they met conditions of application. Next, the incidence rates were calculated, in addition to relative risks and their 95% confidence intervals (95% CI) for the intervention period (POSTvac) versus the previous period (PREvac). The total number of person-years in these periods was used as denominator. The calculations were made with the SPSS® version 14 and EPIDAT® version 4.2 programs.
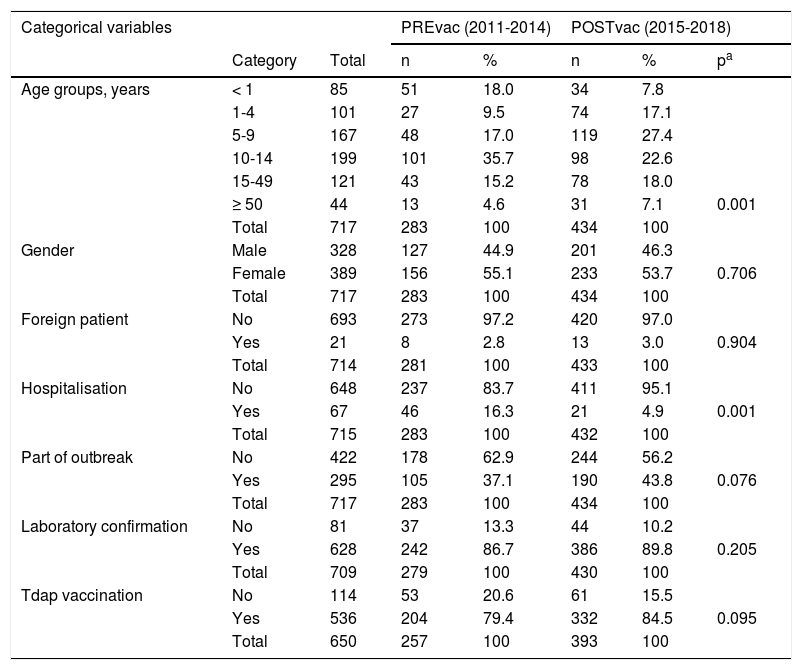
ResultsA total of 717 cases of pertussis were reported, 283 from the PREvac period and 434 the POSTvac period. Except for the distribution by age groups and hospital admission rate, there were no substantial differences in the characteristics of the cases in the two periods (Table 1). The vast majority of the cases had microbiological confirmation.
Comparison of the characteristics of the cases in the pre-vaccination and post-vaccination periods.
| Categorical variables | PREvac (2011-2014) | POSTvac (2015-2018) | |||||
|---|---|---|---|---|---|---|---|
| Category | Total | n | % | n | % | pa | |
| Age groups, years | < 1 | 85 | 51 | 18.0 | 34 | 7.8 | |
| 1-4 | 101 | 27 | 9.5 | 74 | 17.1 | ||
| 5-9 | 167 | 48 | 17.0 | 119 | 27.4 | ||
| 10-14 | 199 | 101 | 35.7 | 98 | 22.6 | ||
| 15-49 | 121 | 43 | 15.2 | 78 | 18.0 | ||
| ≥ 50 | 44 | 13 | 4.6 | 31 | 7.1 | 0.001 | |
| Total | 717 | 283 | 100 | 434 | 100 | ||
| Gender | Male | 328 | 127 | 44.9 | 201 | 46.3 | |
| Female | 389 | 156 | 55.1 | 233 | 53.7 | 0.706 | |
| Total | 717 | 283 | 100 | 434 | 100 | ||
| Foreign patient | No | 693 | 273 | 97.2 | 420 | 97.0 | |
| Yes | 21 | 8 | 2.8 | 13 | 3.0 | 0.904 | |
| Total | 714 | 281 | 100 | 433 | 100 | ||
| Hospitalisation | No | 648 | 237 | 83.7 | 411 | 95.1 | |
| Yes | 67 | 46 | 16.3 | 21 | 4.9 | 0.001 | |
| Total | 715 | 283 | 100 | 432 | 100 | ||
| Part of outbreak | No | 422 | 178 | 62.9 | 244 | 56.2 | |
| Yes | 295 | 105 | 37.1 | 190 | 43.8 | 0.076 | |
| Total | 717 | 283 | 100 | 434 | 100 | ||
| Laboratory confirmation | No | 81 | 37 | 13.3 | 44 | 10.2 | |
| Yes | 628 | 242 | 86.7 | 386 | 89.8 | 0.205 | |
| Total | 709 | 279 | 100 | 430 | 100 | ||
| Tdap vaccination | No | 114 | 53 | 20.6 | 61 | 15.5 | |
| Yes | 536 | 204 | 79.4 | 332 | 84.5 | 0.095 | |
| Total | 650 | 257 | 100 | 393 | 100 | ||
| Quantitative variables | ||||||
|---|---|---|---|---|---|---|
| n | Mean | n | Mean | p | ||
| Age (years) | 717 | 283 | 13.6 | 434 | 16.0 | 0.065b |
| Delay in diagnosis (days)c | 715 | 282 | 27.1 | 432 | 26.1 | 0.723 |
| Delay in declaration (days)c | 715 | 282 | 30.5 | 432 | 27.6 | 0.330 |
| Time since last Tdap vaccination (years)c | 530 | 205 | 4.4 | 325 | 4.4 | 0.834 |
| Hospital stay (days)c | 67 | 46 | 8.6 | 21 | 7.0 | 0.325 |
POSTvac: post-vaccine period; PREvac: pre-vaccine period.
In children under one year of age, the mean age was 2.9 months and 4.3 months in the PREvac and POSTvac periods respectively, with a difference of 1.5 months (95% CI 0.2 to 2.7). There were 85 cases under one year of age, 51 were before introduction of the vaccination programme and 34 were after. Thirty-eight were over 3 months old (15 before 2015 and 23 after) and 47 were younger than 3 months (36 before and 11 after).
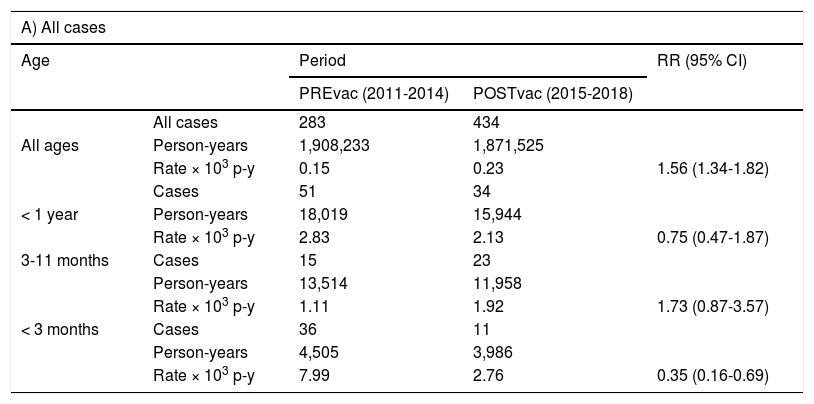
The incidence rates in the PREvac and POSTvac periods (Table 2) in all ages were 0.15 and 0.23 cases per 1,000 person-years respectively; in children aged 3 to 11 months they were 1.11 and 1.92, and in children under 3 months old, 7.99 and 2.76 respectively. The relative risks in the POSTvac versus PREvac period were 1.56 (95% CI 1.34-1.82) for all ages; 1.73 (95% CI 0.87-3.57) for children aged 3 to 11 months and 0.35 (95% CI 0.16-0.69) for children under 3 months old.
Incidence rates per 1,000 person-years in children under one year of age, 3-11 months of age and under 3 months of age, in the pre-vaccine and post-vaccine periods.
| A) All cases | ||||
|---|---|---|---|---|
| Age | Period | RR (95% CI) | ||
| PREvac (2011-2014) | POSTvac (2015-2018) | |||
| All cases | 283 | 434 | ||
| All ages | Person-years | 1,908,233 | 1,871,525 | |
| Rate × 103 p-y | 0.15 | 0.23 | 1.56 (1.34-1.82) | |
| Cases | 51 | 34 | ||
| < 1 year | Person-years | 18,019 | 15,944 | |
| Rate × 103 p-y | 2.83 | 2.13 | 0.75 (0.47-1.87) | |
| 3-11 months | Cases | 15 | 23 | |
| Person-years | 13,514 | 11,958 | ||
| Rate × 103 p-y | 1.11 | 1.92 | 1.73 (0.87-3.57) | |
| < 3 months | Cases | 36 | 11 | |
| Person-years | 4,505 | 3,986 | ||
| Rate × 103 p-y | 7.99 | 2.76 | 0.35 (0.16-0.69) | |
| B) Hospitalised only | ||||
|---|---|---|---|---|
| Age | Period | RR (95% CI) | ||
| PREvac (2011-2014) | POSTvac (2015-2018) | |||
| All ages | Hospitalised cases | 46 | 21 | |
| Person-years | 1,908,233 | 1,871,525 | ||
| Rate × 103 p-y | 0.024 | 0.011 | 0.47 (0.26-0.80) | |
| < 1 year | Cases | 38 | 17 | |
| Person-years | 18,019 | 15,944 | ||
| Rate × 103 p-y | 2.11 | 1.07 | 0.51 (0.27-0.92) | |
| 3-11 months | Cases | 7 | 9 | |
| Person-years | 13,514 | 11,958 | ||
| Rate × 103 p-y | 0.52 | 0.75 | 1.45 (0.48-4.59) | |
| < 3 months | Cases | 31 | 8 | |
| Person-years | 4,505 | 3,986 | ||
| Rate × 103 p-y | 6.88 | 2.01 | 0.29 (0.12-0.65) | |
95% CI: 95% confidence interval; p-y: person-years; POSTvac: post-vaccine period; PREvac: pre-vaccine period; RR: relative risk.
The hospitalisation rates overall and in children under one year of age decreased by 53%, from 0.024 in the PREvac period to 0.011 in the POSTvac period per 1,000 person-years, with the decrease mainly affecting those under 3 months of age (Table 2). It is in this age group where the reduction was most notable, from 6.88 to 2.01, a 71% reduction (relative risk 0.29; 95% CI 0.12-0.65). In the PREvac period, 74.5% of children under one year of age were hospitalised, while in the POSTvac period 50% were hospitalised.
DiscussionOur results reveal a decrease in the incidence rate of pertussis specifically in children under 3 months of age after the implementation of the vaccination programme in pregnant women, in an area where the overall incidence of this disease increased significantly. The reduction translated into 65% for incidence rates and 71% for hospitalisation rates. We found no similar reduction in the 3-11 month-old group.
Figures for vaccination during pregnancy show that national coverage was 80.7% in 2017, and that coverage in our area for the study period was 87.4% (estimate based on data provided by the Health Promotion Department of the Conselleria de Sanitat Universal i Salut Pública [Ministry of Universal Health and Public Health]).
At the same time, an increase in the pertussis incidence rate in all ages was observed in Castelló (from 0.15 to 0.23 cases per 1,000 person-years), reflecting greater circulation of Bordetella pertussis in our area in the POSTvac period. We see that the incidence rate increased in the 3-to-11-month-old age group in line with the increase in other ages, while, in children under 3 months of age the incidence decreased substantially. This is an interesting aspect of our study that differentiates it from the one recently published by the Spanish government's Centro Nacional de Epidemiología [National Centre for Epidemiology]4 where there was a decrease in the incidence of pertussis from 2015 to 2016 at virtually all ages. This shows that in Castelló the decrease in our study in children under 3 months of age is specific to that age group, and not as a result of the lower circulation of Bordetella pertussis.
The epidemiological pattern reflected in the overall comparison of the case characteristics in the two periods shows no significant differences in percentages of cases in the variables of gender, outbreaks, foreigners or vaccination or in the delay in diagnosis or declaration times. We can also rule out a better diagnosis bias in one period or another, as the percentage of confirmation was very high and without significant differences between the two periods. This supports the comparability of the PREvac and POSTvac periods. Differences were found in the percentage hospitalised and in age, both consistent with the decrease in cases in the youngest age group during the POSTvac period. The lower hospitalisation rate in the POSTvac period may be the result of there being fewer cases in children under 3 months of age, who are more often hospitalised.4 It may also be an effect of the maternal vaccine, which would lead to a lower degree of severity in cases involving children of vaccinated mothers.
In addition to the study by Spain's National Centre for Epidemiology,4,14 other authors have reported similar findings. A pre-post study conducted between 2003 and 2016 in Argentina on hospital admissions for pertussis15 found that the average age of cases in children aged under one year went from 3 months in the period before the intervention to 9 months in the period afterwards.
One of the opportunities for research with this type of case-centred study is to estimate vaccine effectiveness by the screening method,16 based on the prevalence of maternal vaccination in cases and vaccine-coverage data for the population. That, however, was not the goal of this study, but rather a proposal for the future which our group hopes to pursue.
In conclusion, we can state that the risk of whooping cough in children under 3 months of age decreased substantially in the period after vaccination of pregnant women. The risk of hospitalisation also decreased. Moreover, this occurred in an epidemic environment with a higher incidence of whooping cough in the general population. Despite the limitations of ecological studies like this, the results show that there has been a specific impact in children under 3 months of age following pertussis vaccination during pregnancy in Castelló.
Conflicts of interestThe authors declare that they have no conflicts of interest.
A M. Ángeles Romeu García, Lourdes Safont Adsuara and Juan Carlos Gascó Laborda, from the Epidemiology Section of the Centro de Salud Pública de Castelló, and M. Dolores Tirado Balaguer, from the Microbiology Service at Hospital General Universitari de Castelló.
Please cite this article as: Chong-Valbuena A, Garay-Moya Á, Vizcaíno A, Meseguer-Ferrer N, Sabater-Vidal S, Bellido-Blasco J. Impacto en niños menores de un año del programa de vacunación con dTpa en embarazadas en Castelló. Enferm Infecc Microbiol Clin. 2020;38:485–488.