The Spanish Antibiogram Committee (Comité Español del Antibiograma, COESANT) presents in this document a simple “roadmap” or decalogue of recommendations, with a view to facilitating the transition from the Clinical and Laboratory Standards Institute (CLSI) to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) antimicrobial susceptibility testing regulations to the Clinical Microbiology Spanish laboratories that still use the CLSI guidelines. The objectives are to adapt the closer European regulations to the Spanish clinical and epidemiological reality and to fully implement the EUCAST recommendations in all microbiology laboratories in Spain.
El Comité Español del Antibiograma (COESANT) presenta en este documento una sencilla «hoja de ruta» en forma de decálogo de recomendaciones cuya finalidad es facilitar la transición de la normativa del Clinical and Laboratory Standards Institute (CLSI) a la del European Committee on Antimirobial Susceptibility Testing (EUCAST) en los Servicios y Unidades de Microbiología Clínica que aún emplean los criterios del CLSI. Su objetivo es adaptar las directrices europeas, más próximas a la realidad clínico-epidemiológica española, y conseguir una implantación de los criterios del EUCAST en la totalidad de los laboratorios de Microbiología en España.
For almost fifty years, antimicrobial susceptibility studies in Spanish laboratories have been guided primarily by the standards recommended by the Clinical and Laboratory Standards Institute, or CLSI (formerly known as the National Committee for Clinical Laboratory Standards, or NCCLS). The Mesa Española de la Normalización de la Sensibilidad y Resistencia a los Antimicrobianos (MENSURA) [Spanish Bureau for the Standardisation of Antimicrobial Susceptibility and Resistance] was created in 1991, focusing on specific technical aspects related to the antibiogram, in the same vein as other European national committees such as the Comité de l’Antibiogramme of the Société Française de Microbiologie (CA-SFM) or the British Society for Antimicrobial Chemotherapy (BSAC) Susceptibility Testing Standing Committee. Both committees, together with those of other countries in northern Europe, were the spawn of the current European Committee on Antimicrobial Susceptibility Testing (EUCAST), which was organised in 1997 under the auspices of the European Centre for Diseases Prevention and Control (ECDC, www.ecdc.europa.eu) and the European Society for Clinical Microbiology and Infectious Diseases (ESCMID, https://www.escmid.org). EUCAST is currently accepted as the breakpoint committee for the European Medicines Agency (EMA) (http://www.ema.europa.eu) and the ECDC, with this guaranteeing the independence of criteria with respect to the pharmaceutical industry and the diagnosis, as it holds purely consultative powers.
With Spain now fully integrated into Europe, it makes no sense that, for the study of antimicrobial susceptibility, which is one of the central activities of Microbiology Departments and Units, some laboratories still use Reference documents from the United States (CLSI documents), especially taking into account the significant epidemiological differences. Currently, both committees establish clinical breakpoints based on microbiological aspects, including population analysis of minimum inhibitory concentrations, or MIC, on pharmacokinetic and pharmacodynamic parameters and on clinical outcomes (http://www.eucast.org/clinical_breakpoints/eucast_setting_breakpoints). EUCAST also provides epidemiological cut-off points, or ECOFF, which allow the microorganism to be classified according to whether or not it has an acquired resistance mechanism for a greater number of antibiotic/microorganism combinations than the CLSI. Regardless of the EMA and the ECDC recommendations, other differences supporting the definitive implementation of the EUCAST expert rules in Spain are the aforementioned EUCAST independence from the pharmaceutical industry and the fact that all the guidelines and the technical documentation from this committee are freely accessible on its website (http://www.eucast.org/). For the study of antifungal susceptibility with the EUCAST reference method, it should be noted that use of a spectrophotometric reading of the MIC eliminates the variability of the visual reading. Although EUCAST does not currently have breakpoints for all the antimicrobials marketed in Spain necessary to fully cover patient care (with some deficiencies particularly affecting rare microorganisms), recommendations are given on how proceed when this circumstance arises.1 However, this lack of data has a minimal impact on the day to day and will be resolved as sufficient information becomes available to establish these breakpoints with certainty.
The main focus of the Comité Español del Antibiograma (COESANT) [Spanish Antibiogram Committee] (http://coesant-seimc.org), sponsored by the Sociedad Española de Enfermedades Infecciosas y Microbiology Clínica (SEIMC) [Spanish Society of Infectious Diseases and Clinical Microbiology] and the Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) [Spanish Agency of Medicines and Medical Devices], is to promote the implementation of the EUCAST expert rules in the Clinical Microbiology Departments and Units of Spain, providing any necessary help and support that may facilitate the change of reference system for antibiotic susceptibility tests.2 It should also be noted that one of the aims of the Plan Nacional frente a la Resistencia a los Antibióticos (PRAN) [Spanish Plan against Antibiotic Resistance] is to promote uniform implementation of the use of susceptibility tests with EUCAST criteria. Among other functions, COESANT is responsible for reviewing EUCAST documents and proposals, and for channelling and managing specific consultations between microbiologists who are experts in the study of antimicrobial susceptibility and the European committee. This allows needs to be identified and priorities established, such as the document set out below. From our experience of having overcome the process of migrating from CLSI to EUCAST, the aim of this simple “road map” is to resolve the initial doubts that arise and facilitate the transition as far as possible by simplifying the actions to be taken.
Today, according to data from a survey on the status of Microbiology Departments and Units in Spain (unpublished data), as well as SEIMC Quality Control and multicentre studies,3 50–65% of Microbiology Departments/Units in Spain have already implemented the EUCAST expert rules as a single reference or complemented by some CLSI criteria.
The guidelines presented below have been simplified into ten points (Table 1), the aim being to create a document which is more practical than theoretical, but citing the sources of origin so that those who want to delve further into the subject are able to do so.
Ten fundamental steps to adopt the EUCAST expert rules as reference in the reading of antibiograms.
| 1. Review the EUCAST manual of breakpoints and the rules of interpretation |
| 2. Notify the suppliers of commercial support systems in order to plan the necessary software changes |
| 3. Pay close attention to the specific recommendations for the disc-diffusion method |
| 4. Anticipate the change in concentration of some disks and of culture medium for some microorganisms |
| 5. Decide the breakpoints to be adopted in cases where EUCAST has not set specific criteria |
| 6. Verify that the appropriate control strains are available |
| 7. Adapt the laboratory's documentary information and computer support system |
| 8. Inform the physicians responsible for the patient and the pertinent Committees of possible variations in the interpretation of susceptibility |
| 9. Take into account the differences in the analysis of cumulative susceptibility reports |
| 10. Consult COESANT about any doubts or queries that may arise |
- 1.
Review of the EUCAST manual of breakpoints and the rules of interpretation.4–6
In this first step, certain aspects need to be taken into account such as those listed below:
- •
For EUCAST, the resistant category (R) is defined as a value “greater than” (>), while the CLSI defines it as “greater than or equal to” (≥).
- •
EUCAST does not individually refer to the intermediate susceptibility category (I), purely to simplify the tables. This category obviously exists and refers to the zone between the minimum inhibitory concentrations or the diameters of inhibition halos between the susceptible (S) and resistant (R) cut-off points. EUCAST recently proposed a redefinition of the “intermediate” category (Susceptible, increased exposure) that reflects only the aspects related to the exposure of the microorganism to the antibiotic (by adjusting the dosage regimen or by concentration at the site of infection), and not the techniques derived from the laboratory susceptibility study itself.7 The interpretation of category I is proposed as “Susceptible, increased exposure”.
- •
A breakpoint of S≥50mm is an arbitrary scale for situations in which wild strains are categorised as intermediate; for example, trimethoprim-sulfamethoxazole and Enterococcus spp.
- •
For the MIC evaluation of the combinations of amoxicillin-clavulanic acid and ampicillin-sulbactam, the inhibitor concentration is set for clavulanic acid at 2mg/l and for sulbactam at 4mg/l and is not a ratio (2:1) as in the case of CLSI. For piperacillin-tazobactam, ceftolozane-tazobactam and ceftazidime-avibactam, the concentration is the same for both committees (2mg/l in the first case and 4mg/l in the other combinations).8 That implies a higher percentage of resistance, but better clinical correlation. It should also be noted that the EUCAST breakpoints for amoxicillin-clavulanic acid are different in urinary tract infection an in systemic infection.
- •
For quinolones, the EUCAST breakpoints are more restrictive than those defined by the CLSI. In Salmonella enterica, the MIC of ciprofloxacin, levofloxacin or ofloxacin should be determined to detect the presence of strains with a low level of resistance to quinolones, including strains that produce Qnr proteins or with the variant of the bifunctional enzyme AAC(6′)-Ib-cr.8,9 COESANT considers that this recommendation should be extended to the other Enterobacteriaceae, at least when they produce invasive infections. In the Departments/Units that mainly use the disc-diffusion method, the study of susceptibility to nalidixic acid facilitates the phenotypic recognition of part of these resistance mechanisms against quinolones.
- •
The EUCAST breakpoints for imipenem and meropenem in Enterobacteriaceae are less restrictive in terms of their translation in the clinical category; not so the carbapenemase screening breakpoint, which aims to increase the sensitivity for detection.10–12 If the presence of this resistance mechanism is confirmed, it is mandatory to give the result of the MIC for the carbapenems, given the possibility of using them in combination (lower failure rate than other alternatives), provided that the MIC is ≤8mg/l according to Pk/Pd models and clinical outcomes.13,14
- •
For trimethoprim-sulfamethoxazole at a 1:19 ratio, the MIC values are expressed as trimethoprim concentration.
- •
There are significant variations in the breakpoints established by EUCAST and CLSI for antibiotics such as aztreonam15 and fosfomycin.
- •
The breakpoints for azoles and echinocandins are species-specific and depend on the different combinations of antifungal and Candida/Aspergillus species.16–18 EUCAST does not currently recommend antifungal susceptibility testing of caspofungin against isolates of Candida spp. due to the high variability in the results obtained between different laboratories. The susceptibility of Candida spp. to caspofungin can be extrapolated from the results of the study of anidulafungin and micafungin, and it is recommended that both echinocandins be tested in order to detect uncommon phenotypes of strains resistant only to one or the other.
- 2.
Notification of the suppliers of commercial antibiogram systems of the intention to implement the EUCAST expert rules, in order to plan the necessary software changes.
If a laboratory boasts a commercial antimicrobial susceptibility testing system, it is recommended to inform the supplier in advance of the intention to adopt the EUCAST criteria, in order to plan the adaptation of the software and the configuration of the panels (currently under review).19 If a semi-automatic disc-diffusion test reader is used, the supplier must also be informed of the need to adapt the expert systems and breakpoints to the EUCAST criteria.
- 3.
Review the specific recommendations for the disc-diffusion method.
The specific EUCAST recommendations for the disc-diffusion method20,21 must be taken into account (for example, to incubate the plates for no less than 16h but no more than 20h, with specific exceptions, such as Corynebacterium spp.).
- 4.
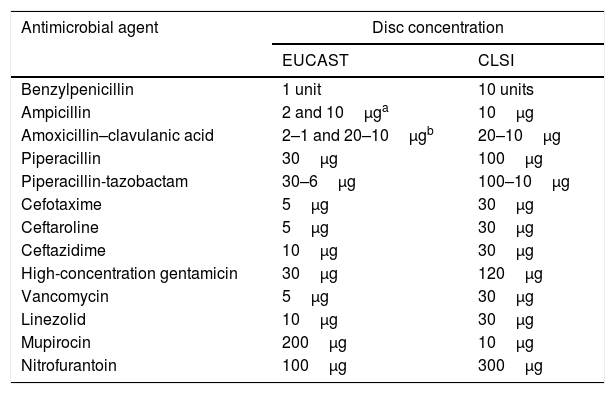
Anticipate the change in concentration of some disks and of culture medium for some microorganisms.
In the disc-diffusion method, keep in mind that the antibiotic concentration on some disks changes (Table 2). In addition, in the case of streptococci and other bacterial species, such as Moraxella catarrhalis, Listeria monocytogenes, Pasteurella multocida, Campylobacter jejuni, Campylobacter coli, Corynebacterium spp., Aerococcus sanguinicola and Aerococcus urinae, as well as Kingella kingae, Mueller-Hinton agar is recommended supplemented with 5% defibrinated horse blood and 20mg/l of β-nicotinamide adenine dinucleotide (NAD).22
- 5.
Decide the breakpoints to be adopted in cases where EUCAST has not ruled.
Differences in disc concentration between EUCAST and CLSI.
| Antimicrobial agent | Disc concentration | |
|---|---|---|
| EUCAST | CLSI | |
| Benzylpenicillin | 1 unit | 10 units |
| Ampicillin | 2 and 10μga | 10μg |
| Amoxicillin–clavulanic acid | 2–1 and 20–10μgb | 20–10μg |
| Piperacillin | 30μg | 100μg |
| Piperacillin-tazobactam | 30–6μg | 100–10μg |
| Cefotaxime | 5μg | 30μg |
| Ceftaroline | 5μg | 30μg |
| Ceftazidime | 10μg | 30μg |
| High-concentration gentamicin | 30μg | 120μg |
| Vancomycin | 5μg | 30μg |
| Linezolid | 10μg | 30μg |
| Mupirocin | 200μg | 10μg |
| Nitrofurantoin | 100μg | 300μg |
Each Microbiology Department/Unit must decide which breakpoints apply in cases where EUCAST has not yet established criteria. The EUCAST document referred to above should be taken into account for that purpose.1
- •
Pk/Pd breakpoints not related to the microbial species.
- •
In the absence of Pk/Pd breakpoints, interpret the result based on whether they belong to the wild-type (susceptible) population or to the population that may have a resistance mechanism (resistant).
- •
For specific queries, consult COESANT.
- 6.
Verify that the appropriate control strains are available.
Verify that the main appropriate control strains are available in the laboratory both for routine controls (Escherichia coli ATCC 25922, Escherichia coli ATCC 35218, Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 29213, Enterococcus faecalis ATCC 29212, Streptococcus pneumoniae ATCC 49619, Haemophilus influenzae ATCC 49766, Campylobacter jejuni ATCC 33560, Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019) and for the detection of the main resistance mechanisms.6
- a.
Extended-spectrum beta-lactamases (ESBL) and resistance of some combinations of beta-lactam-beta-lactamase inhibitor such as ceftazidime-avibactam, ceftolozane-tazobactam and piperacillin-tazobactam: Klebsiella pneumoniae ATCC 700603, which produces SHV-18.
- b.
Methicillin-resistant Staphylococcus aureus (mecA gene): Staphylococcus aureus NCTC 12493.
- c.
Vancomycin-resistant enterococci (VanB phenotype): Enterococcus faecalis ATCC 51299.
- d.
Enterococci with high-level gentamicin resistance: Enterococcus faecalis ATCC 51299.
- e.
Haemophilus influenzae with decreased susceptibility to ampicillin due to PBP mutations: Haemophilus influenzae ATCC 49247.
- f.
Colistin resistance: Escherichia coli NCTC 13846 (mcr-1).
- 7.
Adapt the laboratory's documentary information and computer support system.
Make all necessary changes in the documents or working procedures in the laboratory (technical documentation and quality manuals), and in the laboratory's computer support system and the results which are sent to the patients’ medical records.
- 8.
Information on the possible wide-ranging variations in the interpretation of susceptibility.
Inform the physicians responsible for the patient and the Infections and/or Antibiotic and Pharmacy Policy Committees of possible variations in the interpretation of susceptibility.23 Also communicate if it is decided not to report the interpreted antibiogram in the case of certain resistance mechanisms such as ESBL-producing strains; in such cases, add a comment in the laboratory report as recommended by EUCAST.
- 9.
Consider differences in the analysis of cumulative susceptibility reports.
This information should be borne in mind when analysing the results of cumulative susceptibility reports, which may vary in certain aspects from those of other years (decreased susceptibility in species/antibiotic combinations where there is variation in breakpoints) and the reports should be prepared according to EUCAST guidelines23–27 and the recommendations of the SEIMC Clinical Microbiology Procedures.28
- 10.
For difficulties or queries, consult COESANT.
For any questions deriving from the process of transitioning from CLSI to EUCAST, consult COESANT (http://coesant-seimc.org/).
FundingNo specific funding was received to draft this document. COESANT's activities are partly funded by the Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC) [Spanish Society of Infectious Diseases and Clinical Microbiology] and the Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) [Spanish Agency of Medicines and Medical Devices]. The research by MNL, RC, FFC, AO and LMM is funded by the Plan Estatal de Investigación Científica y Técnica y de Innovación [State Plan for Scientific and Technical Research and Innovation] 2013–2016 and the Instituto de Salud Carlos III [Carlos III Health Institute], Subdirección General de Redes y Centros de Investigación Cooperativa [General Sub-directorate of Networks and Centres for Cooperative Research], Ministerio de Ciencia, Innovación y Universidades [Spanish Ministry of Science, Innovation and Universities], Red Española de Investigación en Patología Infecciosa [Spanish Network for Research in Infectious Diseases] (REIPI RD16/0016/0003; RD16/0016/0011; RD16/0016/0001; RD16/0016/0004; RD16/0016/0008), co-funded by the European Regional Development Fund “A way to make Europe”, Smart Growth Operational Programme 2014–2020.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Larrosa MN, Benito N, Cantón R, Canut A, Cercenado E, Fernández-Cuenca F, et al. Del CLSI al EUCAST, una transición necesaria en los laboratorios españoles. Enferm Infecc Microbiol Clin. 2020;38:79–83.