Data of hepatitis C treatment with direct-acting antivirals (DAAs) in HIV infected patients are limited to a few number of antiretroviral therapies (ART). The aim of this study was to assess the effectiveness and safety of non-conventional ART as monotherapy or dual therapy (MDT) when combined with DAA.
MethodsRetrospective review of HIV/HCV-coinfected patients treated with DAAs during one year in 3 centers. Sustained virologic response 12 weeks after therapy (SVR) and maintenance of HIV viral suppression were compared between patients receiving triple ART (TT) or MDT.
ResultsOverall 485 patients were included (359 receiving TT and 126 MDT). HCV SVR was 93.4% (95%CI, 90.8% to 95.3%) in the intention-to-treat analysis without differences between groups: 92.8% on TT vs 95.2% on MDT (p=0.3). HCV virological failure was associated with lower CD4+cell count at baseline (for every 100-cell/μl increment: OR, 0.8; 95%CI, 0.7-0.9; p=0.01) and with liver stiffness (for every 10-unit increment: OR, 1.5; 95%CI 1.2-1.8; p<0.01). HIV-RNA during HCV treatment or 12 weeks after was detectable in 23 patients on TT (6.6%) and 9 (7.2%) patients on MDT (p=0.8). The median (IQR) change in CD4+cell count was not significantly different between the groups: 15 (–55 to 115) in TT vs –12 (–68 to 133) cells/μl in MDT (p=0.8).
ConclusionDAAs obtain high rates of SVR among HIV/HCV-coinfected patients independently of whether TT or non-conventional ART is used. Suppression of HIV was maintained in both groups.
Los datos sobre el tratamiento de la hepatitis C con antivirales de acción directa (AAD) en los pacientes infectados por VIH se limitan a un escaso número de terapias antirretrovirales (TARV). El objetivo de este estudio fue valorar la efectividad y seguridad de las TARV no convencionales, como monoterapia y terapia dual (MDT), al combinarse con AAD.
MétodosRevisión retrospectiva de pacientes co-infectados por VIH/VHC, tratados con AAD durante un año en 3 centros. Se comparó la respuesta virológica sostenida (RVS) a las 12 semanas de la terapia, y el mantenimiento de la supresión viral del VIH, entre los pacientes que recibieron triple TARV o MDT.
ResultadosSe incluyó a un total de 485 pacientes (359 que recibieron triple TARV y 126 que recibieron MDT). La RVS de VHC fue del 93,4% (IC 95%: 90,8-95,3%) en el análisis por intención de tratar, sin diferencias entre grupos: 92,8% en el grupo triple TARV vs. 95,2% en el grupo MDT (p=0,3). El fracaso virológico de VHC se asoció a un menor recuento basal de células CD4+ (para cada incremento de 100células/μl: OR: 0,8; IC 95%: 0,7-0,9; p=0,01) y a la rigidez hepática (para cada incremento de 10 unidades: OR: 1,5; IC 95%: 1,2-1,8; p<0,01). El ARN-VIH durante el tratamiento de VHC, o transcurridas 12 semanas, fue detectable en 23 pacientes en el grupo triple TARV (6,6%) y 9 (7,2%) pacientes en el grupo MDT (p=0,8). El cambio medio (RIC) en el recuento de células CD4+ no fue significativamente diferente entre ambos grupos: 15 (de –55-115) en el grupo triple TARV vs. –12 (de –68-133) células/μl en el grupo MDT (p=0,8).
ConclusiónLos AAD obtienen tasas altas de RVS entre los pacientes co-infectados de VIH/VHC, independientemente de si se utiliza triple TARV o TARV no convencional. La supresión de VIH se mantuvo en ambos grupos.
Oral combinations of direct-acting antivirals (DAA) have completely changed the treatment of chronic hepatitis C virus (HCV) infection. The combinations are well-tolerated, can be administered for shorter periods (between 8 and 12 weeks), and lead to a sustained virologic response (SVR) in over 90% of patients.1 The cure rate seems to be similar among HCV-monoinfected patients and patients coinfected by the HCV and human immunodeficiency virus (HIV).2,3 However, it is important to monitor interactions between antiretroviral therapy (ART) for HIV and DAA in this population.4
Most trials on treatment of HCV infection in HIV-infected patients have been performed with triple ART combinations. Regimens based on various antiretroviral drugs (eg, tenofovir/emtricitabine, efavirenz, ritonavir-boosted atazanavir, ritonavir-boosted darunavir, raltegravir, or rilpivirine) have been tested in combination with DAAs such as sofosbuvir (SOF), ledipasvir (LDV), daclatasvir (DCV), simeprevir (SIM), paritaprevir/ritonavir plus ombitasvir (PrO), and PrO plus dasabuvir.5–8 However, not all ART drugs have been tested with all DAAs. Moreover, only standard triple therapy with 2 nucleos(t)ide analogs and a third drug have been studied.
Owing to toxicity, previous selection of drug resistance mutations, and simplification, ART with non-standard combinations such as boosted protease inhibitor (bPI) monotherapy or dual therapies are frequently used in clinical practice. It is important to assess whether these non-conventional ART schedules are as effective and safe as triple therapy (TT) for both eradication of HCV and suppression of HIV while treating HCV infection with DAA.
The objective of our study was to assess whether HCV SVR and maintenance of HIV suppression were similar in HIV-infected patients treated with non-conventional ART schedules and in patients with TT in clinical practice. We also described factors associated with poorer HCV SVR in HIV/HCV-coinfected patients treated with DAA.
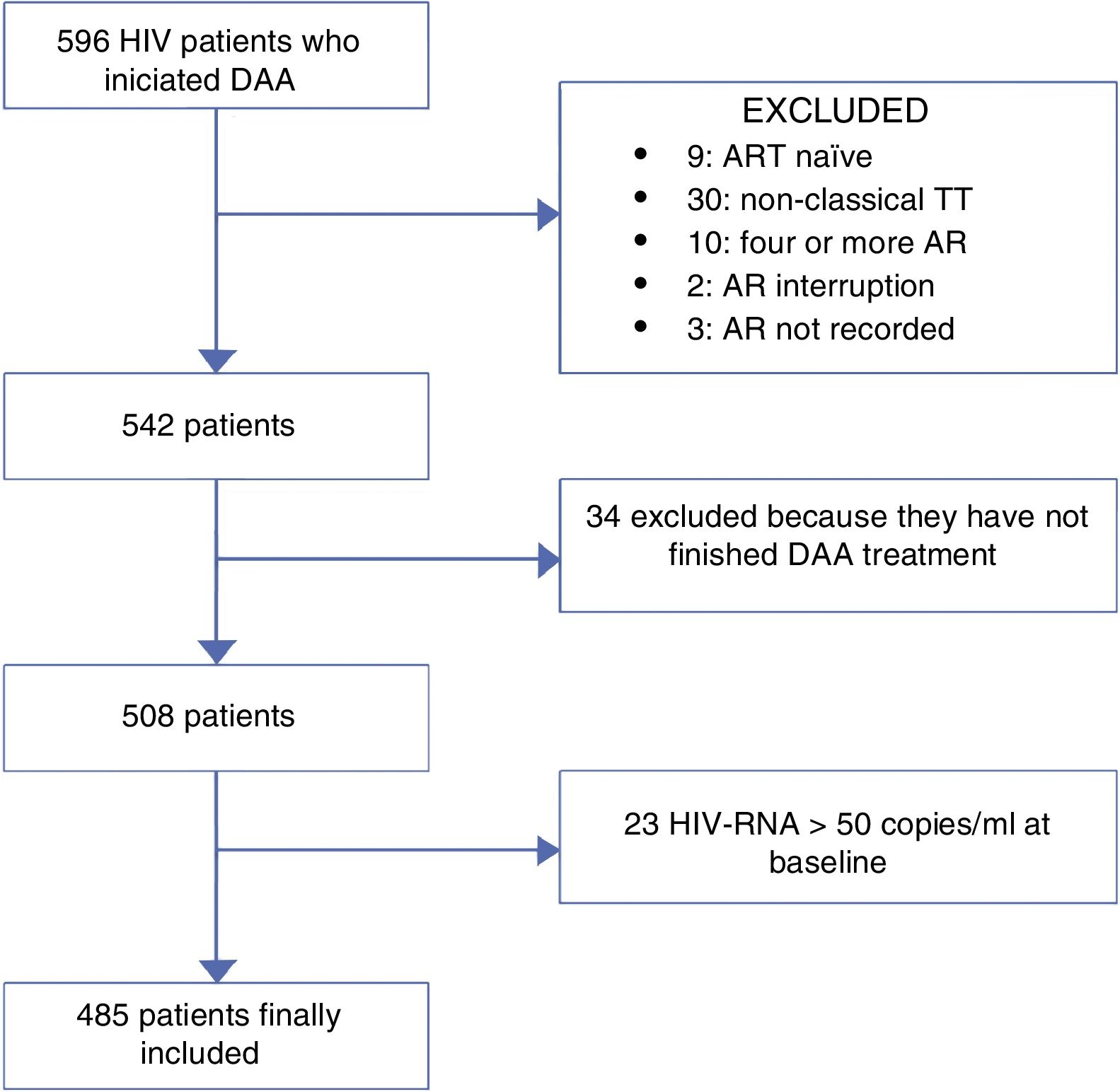
MethodsWe retrospectively reviewed all HIV/HCV-coinfected patients who started a DAA regimen for treatment of HCV from November 2014 to November 2015 at 3 centres in Madrid, Spain. Those patients who were still on HCV treatment or had competed it before 12 weeks of the day that the data were collected (no data of SVR) were excluded of the analysis. ART was recorded for each patient. The population was divided into patients receiving classic triple therapy and those receiving monotherapy or dual therapy (MDT). As the main objective of the study was to compare these two group of patients, patients who were naïve or were on other ART regimens were also excluded of the analysis. Fig. 1 represents the flowchart of the study.
We recorded baseline demographic data (gender and age), HIV-related data (HIV-RNA, CD4+T-cell count, HIV treatment), HCV-related data (HCV-RNA, genotype, estimated grade of fibrosis, DAA regimen), and analytical and clinical data. Liver fibrosis was estimated using transient elastography (Fibroscan, Echo-Sens, Paris, France), considering cirrhosis as a liver stiffness value>14kPa.9
HCV SVR at week 12, suppression of HIV-RNA, and change in CD4+cell counts were compared between the groups. As the maintenance of undetectable HIV-RNA was one of the main objectives of our study, patients with baseline HIV-RNA>50 copies/ml were also excluded of the analysis.
EndpointsThe primary efficacy endpoints were SVR, defined as HCV RNA below 15 IU/ml, and maintenance of HIV-RNA below 50 copies/ml at 12 weeks after the end of HCV treatment. HCV-RNA was measured using the real-time quantitative assay (COBAS® TaqMan® HCV Test v.2, Roche Diagnostic, Switzerland) with a lower limit of quantification (LLOQ, across all genotypes) of 20 IU/mL. HIV-RNA was measured using the real-time quantitative assay (COBAS® TaqMan® HIV-1 Test v.2.0, Roche Diagnostic, Switzerland) with a LLOQ of 20 IU/ml.
Statistical analysisDescriptive statistics were expressed as median and interquartile range for continuous variables and as percentages for categorical variables. The baseline characteristics of both groups were compared using the chi-square test or the Mann-Whitney test, as needed. HCV SVR was assessed using intention-to-treat analysis (ITT), including all patients who started therapy with DAAs, and on-treatment analysis (OT), excluding patients who stopped treatment or were lost to follow-up.
Univariate logistic regression analysis was performed to calculate the odds ratio (OR) and 95% confidence intervals (95%CI) of the various parameters associated with HCV virological failure (not achieving SVR). Variables with a p value <0.1 or that have previously found associated with SVR, were included in a multivariate logistic regression analysis. To assess the main objective of our study, the TT group was compared with the MDT group in both univariate and multivariate logistic regression analysis. All analyses were performed using SPSS version 20.0 (IBM Corp, Armonk, New York, USA).
ResultsOverall, 485 patients were included in the study, 359 were receiving TT and 126 MDT. A total of 69 (14.2%) switched ART before starting DAAs owing to concerns over drug interactions.
Baseline characteristicsA total of 359 were receiving TT with 2 nucleos(t)ide analogs (NRTI) plus a third agent. The third agent was an integrase inhibitor (II) in 141 individuals, a non-nucleoside analog (NNRTI) in 116, and a bPI in 102. MT with a bPI was prescribed in 53 patients (36 darunavir and 17 lopinavir), bPI plus lamivudine in 45, and other dual therapies (15 PI+II, 8 PI+NNRTI, 5 NNRTI+II) in 28 patients
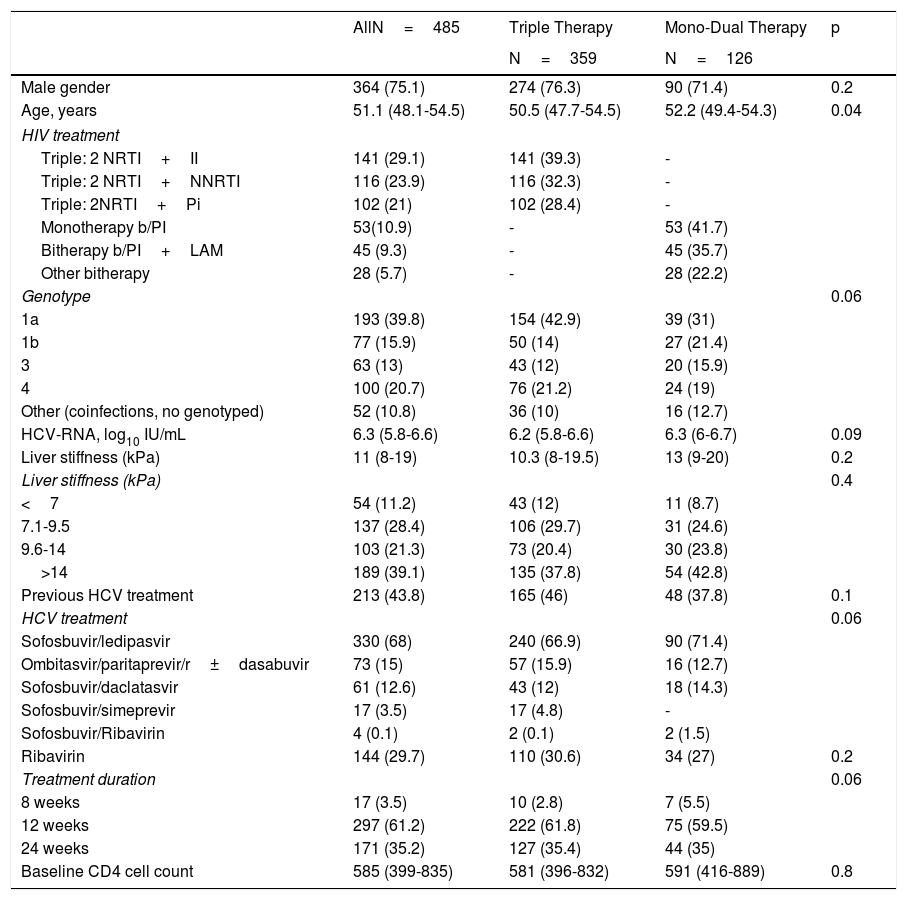
Baseline characteristics are shown in Table 1. Men accounted for 75.1% of patients, and the median age was 51 years. Genotype 1a was the most prevalent (39.8%), followed by genotype 4 (20.7%). Nearly 40% of patients were cirrhotic, and slightly more than 40% had received previous HCV treatment based on interferon. SOF/LDV was the most frequent combination used. Other schedules were PrO±D and SOF/DCV. As for HIV characteristics at baseline, the median CD4+cell count was 585. Baseline characteristics were similar between patients on TT and MDT, except for age, as patients on TT were younger than patients on MDT (50.5 vs 52.2 years; p=0.04).
Baseline characteristics of HIV/HCV-coinfected patients who received DAA.
| AllN=485 | Triple Therapy | Mono-Dual Therapy | p | |
|---|---|---|---|---|
| N=359 | N=126 | |||
| Male gender | 364 (75.1) | 274 (76.3) | 90 (71.4) | 0.2 |
| Age, years | 51.1 (48.1-54.5) | 50.5 (47.7-54.5) | 52.2 (49.4-54.3) | 0.04 |
| HIV treatment | ||||
| Triple: 2 NRTI+II | 141 (29.1) | 141 (39.3) | - | |
| Triple: 2 NRTI+NNRTI | 116 (23.9) | 116 (32.3) | - | |
| Triple: 2NRTI+Pi | 102 (21) | 102 (28.4) | - | |
| Monotherapy b/PI | 53(10.9) | - | 53 (41.7) | |
| Bitherapy b/PI+LAM | 45 (9.3) | - | 45 (35.7) | |
| Other bitherapy | 28 (5.7) | - | 28 (22.2) | |
| Genotype | 0.06 | |||
| 1a | 193 (39.8) | 154 (42.9) | 39 (31) | |
| 1b | 77 (15.9) | 50 (14) | 27 (21.4) | |
| 3 | 63 (13) | 43 (12) | 20 (15.9) | |
| 4 | 100 (20.7) | 76 (21.2) | 24 (19) | |
| Other (coinfections, no genotyped) | 52 (10.8) | 36 (10) | 16 (12.7) | |
| HCV-RNA, log10 IU/mL | 6.3 (5.8-6.6) | 6.2 (5.8-6.6) | 6.3 (6-6.7) | 0.09 |
| Liver stiffness (kPa) | 11 (8-19) | 10.3 (8-19.5) | 13 (9-20) | 0.2 |
| Liver stiffness (kPa) | 0.4 | |||
| <7 | 54 (11.2) | 43 (12) | 11 (8.7) | |
| 7.1-9.5 | 137 (28.4) | 106 (29.7) | 31 (24.6) | |
| 9.6-14 | 103 (21.3) | 73 (20.4) | 30 (23.8) | |
| >14 | 189 (39.1) | 135 (37.8) | 54 (42.8) | |
| Previous HCV treatment | 213 (43.8) | 165 (46) | 48 (37.8) | 0.1 |
| HCV treatment | 0.06 | |||
| Sofosbuvir/ledipasvir | 330 (68) | 240 (66.9) | 90 (71.4) | |
| Ombitasvir/paritaprevir/r±dasabuvir | 73 (15) | 57 (15.9) | 16 (12.7) | |
| Sofosbuvir/daclatasvir | 61 (12.6) | 43 (12) | 18 (14.3) | |
| Sofosbuvir/simeprevir | 17 (3.5) | 17 (4.8) | - | |
| Sofosbuvir/Ribavirin | 4 (0.1) | 2 (0.1) | 2 (1.5) | |
| Ribavirin | 144 (29.7) | 110 (30.6) | 34 (27) | 0.2 |
| Treatment duration | 0.06 | |||
| 8 weeks | 17 (3.5) | 10 (2.8) | 7 (5.5) | |
| 12 weeks | 297 (61.2) | 222 (61.8) | 75 (59.5) | |
| 24 weeks | 171 (35.2) | 127 (35.4) | 44 (35) | |
| Baseline CD4 cell count | 585 (399-835) | 581 (396-832) | 591 (416-889) | 0.8 |
Values are given as absolute number (%) or median (interquartile range).
NRTI: nucleos(t)ide reverse transcriptase inhibitor; II: integrase inhibitor; NNRTI: non-analog nucleoside reverse transcriptase inhibitor; PI: protease inhibitor, b/PI: boosted PI; LAM: lamivudine: HCV: hepatitis C virus; kPa: kilopascals.
During follow-up, 8 patients died (all with causes associated with advanced liver disease), and 4 patients stopped treatment voluntarily or were lost to follow-up. Meanwhile, 485 and 481 patients were included in the ITT and OT analysis, respectively.
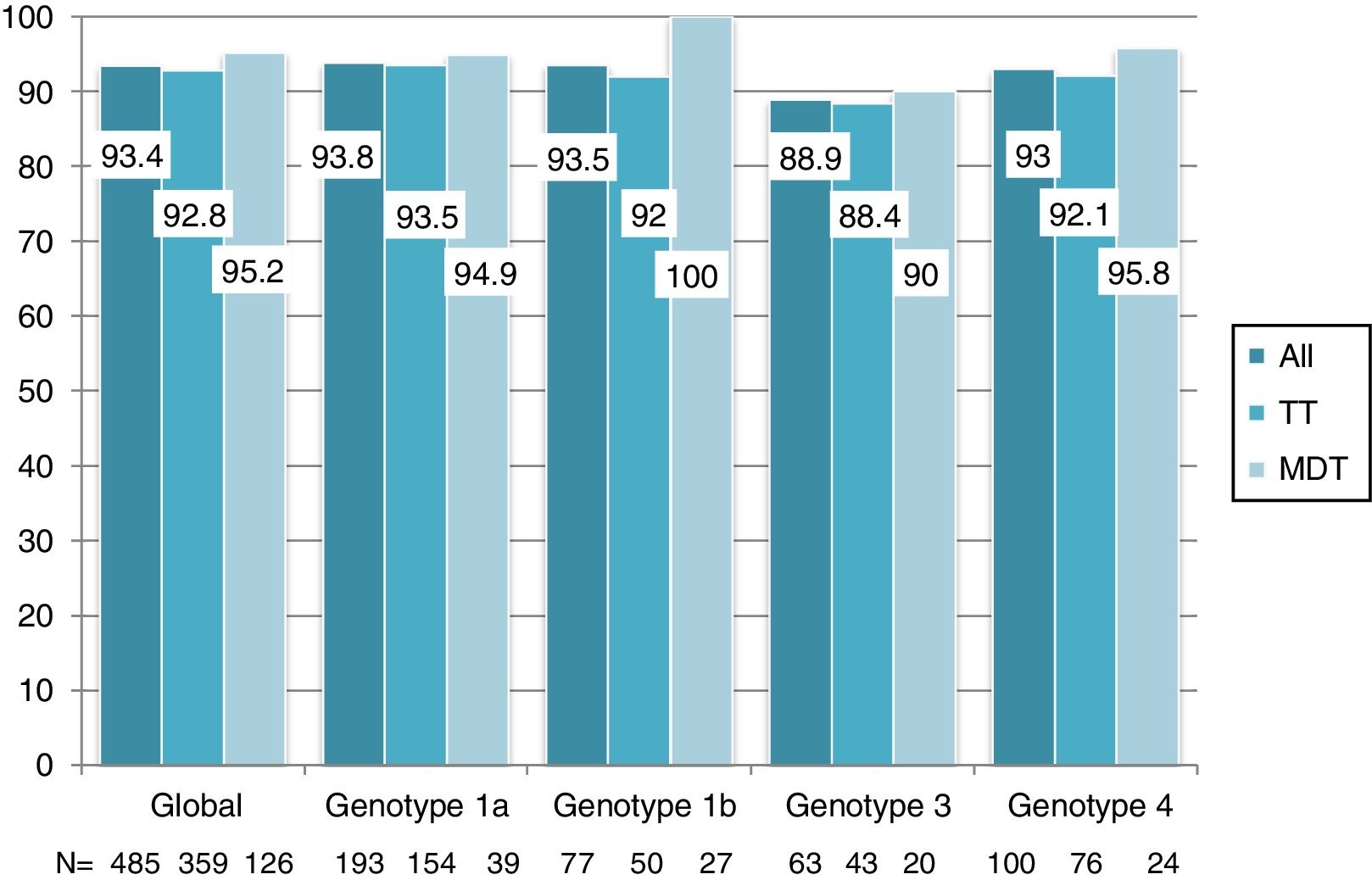
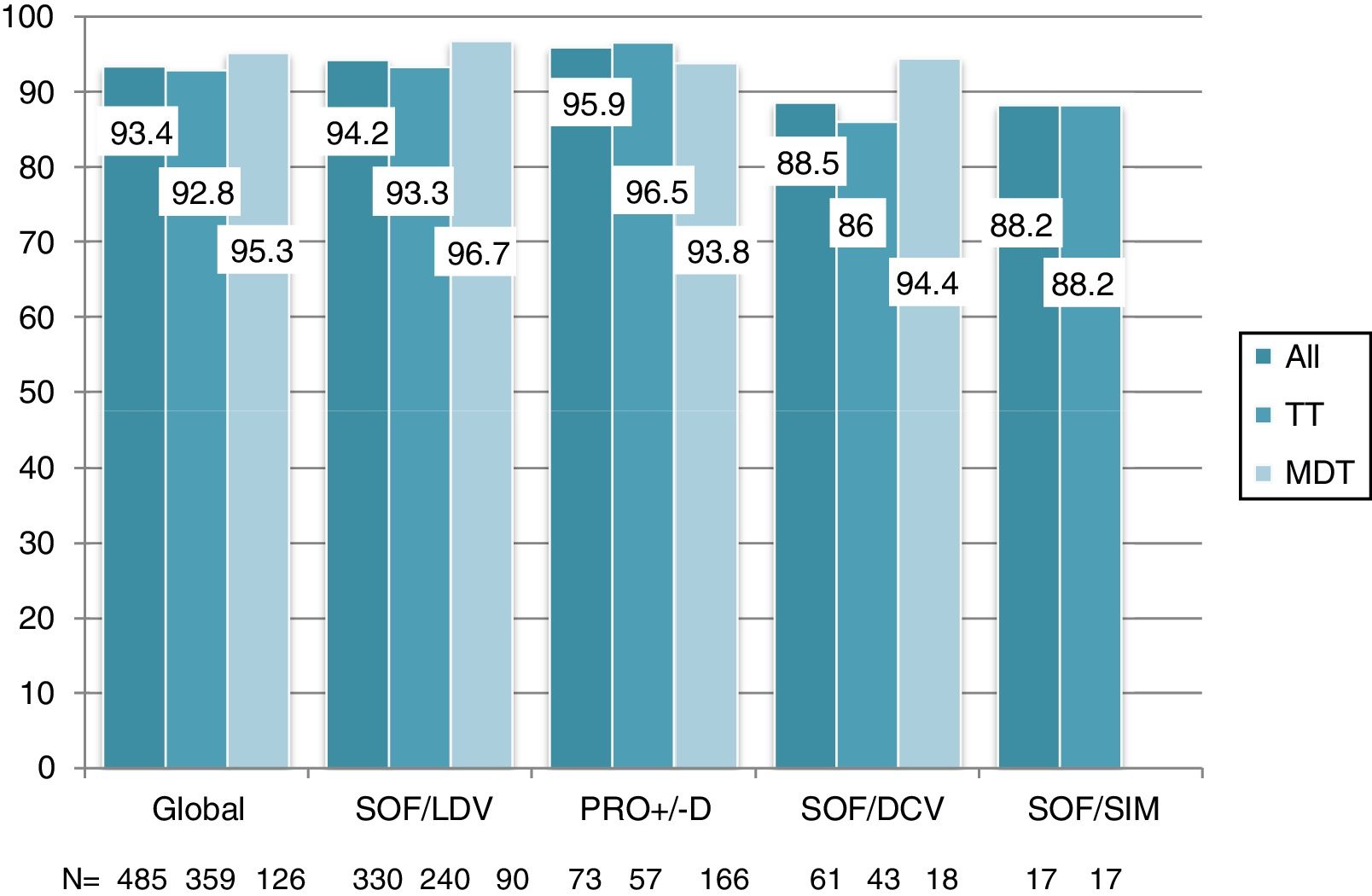
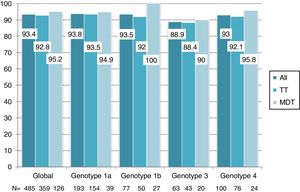
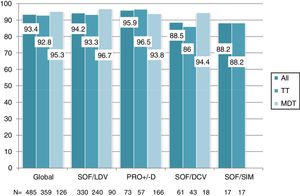
Overall, 453 patients obtained SVR: SVR 12 was 93.4% (95%CI, 90.8%-95.3%) and 94.2% (95%CI, 91.7%-96%) in the ITT and OT analysis, respectively. No differences in SVR were seen in patients on TT or MDT: ITT (92.8% vs 95.2%; p=0.3), OT (93.5% vs 96%; p=0.3). Response rate by HCV genotype (figure 2) and by DAA combination (figure 3) was also similar between the TT and MDT groups.
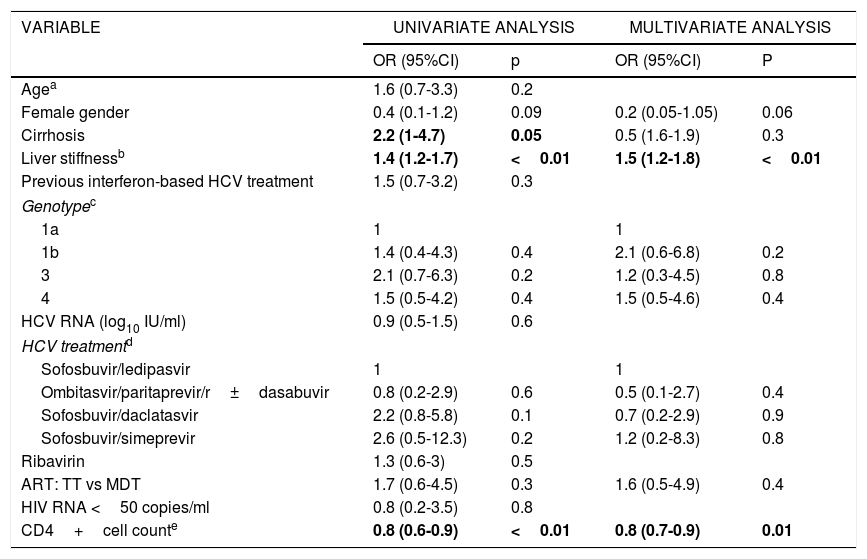
ART (TT vs MDT) was not associated with failure of HCV treatment in the univariate or multivariate logistic regression analysis (Table 2). The only factors associated with failure of HCV treatment in the multivariate analysis were having a lower CD4 cell count at baseline (for every 100 cells increment, OR, 0.8; 95%CI, 0.7-0.9; p=0.01) and liver stiffness (for every 10 units increment, OR, 1.5; 95%CI, 1.2-1.8; p<0.01).
Factors associated with failure of HCV treatment (not achieving SVR at week 12).
| VARIABLE | UNIVARIATE ANALYSIS | MULTIVARIATE ANALYSIS | ||
|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | P | |
| Agea | 1.6 (0.7-3.3) | 0.2 | ||
| Female gender | 0.4 (0.1-1.2) | 0.09 | 0.2 (0.05-1.05) | 0.06 |
| Cirrhosis | 2.2 (1-4.7) | 0.05 | 0.5 (1.6-1.9) | 0.3 |
| Liver stiffnessb | 1.4 (1.2-1.7) | <0.01 | 1.5 (1.2-1.8) | <0.01 |
| Previous interferon-based HCV treatment | 1.5 (0.7-3.2) | 0.3 | ||
| Genotypec | ||||
| 1a | 1 | 1 | ||
| 1b | 1.4 (0.4-4.3) | 0.4 | 2.1 (0.6-6.8) | 0.2 |
| 3 | 2.1 (0.7-6.3) | 0.2 | 1.2 (0.3-4.5) | 0.8 |
| 4 | 1.5 (0.5-4.2) | 0.4 | 1.5 (0.5-4.6) | 0.4 |
| HCV RNA (log10 IU/ml) | 0.9 (0.5-1.5) | 0.6 | ||
| HCV treatmentd | ||||
| Sofosbuvir/ledipasvir | 1 | 1 | ||
| Ombitasvir/paritaprevir/r±dasabuvir | 0.8 (0.2-2.9) | 0.6 | 0.5 (0.1-2.7) | 0.4 |
| Sofosbuvir/daclatasvir | 2.2 (0.8-5.8) | 0.1 | 0.7 (0.2-2.9) | 0.9 |
| Sofosbuvir/simeprevir | 2.6 (0.5-12.3) | 0.2 | 1.2 (0.2-8.3) | 0.8 |
| Ribavirin | 1.3 (0.6-3) | 0.5 | ||
| ART: TT vs MDT | 1.7 (0.6-4.5) | 0.3 | 1.6 (0.5-4.9) | 0.4 |
| HIV RNA <50 copies/ml | 0.8 (0.2-3.5) | 0.8 | ||
| CD4+cell counte | 0.8 (0.6-0.9) | <0.01 | 0.8 (0.7-0.9) | 0.01 |
HCV RNA: hepatitis C viral load; HIV RNA: HIV viral load; OR: odds ratio; CI: confidence interval; ART: antiretroviral therapy; TT: triple therapy; MDT: monotherapy or dual therapy.
Se destacan en negrita los valores que han resultado estadísticamente significativos.
During HCV treatment or 12 weeks after, 32 (6.6%) patients had at least 1 detectable HIV-RNA value, of them, 21 had low HIV-RNA (50-200 copies/ml). Of these patients, 11 reported poor adherence. HIV-RNA was detectable in 23 TT patients (6.6%) and 9 MDT patients (7.2%) (p=0.8). The CD4+cell count did not change significantly during HCV treatment in either group: the median (IQR) difference between CD4 counts 12 weeks after the end of HCV therapy and baseline was 15 (–55 to 115) in the TT group and –12 (–68 to 133) in the MDT group (p=0.8).
DiscussionTreatment of HCV with pegylated-interferon plus ribavirin was previously more effective in HIV-negative than in HIV-positive patients.4 However, this difference has disappeared with the introduction of oral DAAs.2,3 Consistent with these findings, we recorded SVR rates greater than 90% in HIV-positive patients in a real-world setting.
Many different combinations of ART are used in daily clinical practice. Non-conventional ART such as MDT has proven rates of efficacy similar to those of triple therapy in some scenarios and is widely used because of toxicity, resistance, simplification, and cost.10,11 Evenmore, ART with only two drugs have been included as a reasonable option in recent guidelines.12 HIV/HCV-coinfected patients in the south of Europe constitute a special population infected mainly through intravenous drug use during the first decades of the HIV epidemic. Therefore, most are now elderly people with comorbidities who are likely to be polymedicated and prone to drug toxicity and resistance 13 and in whom the use of non-conventional ART is not infrequent. Our study shows that these combinations are effective and safe when combined with the new DAAs and achieve similar rates of HCV SVR and HIV control when compared with classic triple therapies.
Drug interactions between DDAs and antiretroviral drugs are one of the main concerns in HIV/HCV-coinfected patients and must be carefully evaluated. In our series, this is reflected by the number of patients who switched ART before HCV treatment (15%) and by clinicians’ preference for IIs (25% of patients), as raltegravir and dolutegravir do not induce, inhibit, or are significantly metabolized by cytochrome p450 (the main enzyme responsible for drug interactions).
The multivariate logistic regression analysis showed lower baseline CD4 cell count and liver stiffness to be the only predictors of failure of HCV treatment in HIV/HCV-coinfected patients. Our study found an inverse association between CD4+cell count and SVR in patients treated with DAA. This association was previously reported in patients treated with interferon 14 but it is no so clear in patients treated with DAAs. Another recent study of the German GECCO cohort has also found worst response with lower CD4 cell counts, although this difference was lost when adjusting by cirrhosis.15 Surprisingly, we found this association in patients without severe immunosuppression (median CD4+cell count, 593 cells/μl). We were not able to elucidate whether a lower CD4 cell count was the reason for the poorer response or whether lower CD4+cell counts were a consequence of poorer liver function, which also led to lower DAAs response rates.
It is known that cirrhosis is one of the main factors associated with failure of DAAs, especially in patients with decompensated cirrhosis.16 Our study went further and revealed liver stiffness to be a continuous variable that predicts failure of HCV treatment, probably because it is a more accurate variable for identification of the most severely ill patients. Liver stiffness as a continuous variable has also been associated with mortality, esophageal varices, and portal hypertension in both HCV-monoinfected and HIV/HCV-coinfected patients.17–21
In contrast with previous studies, we did not find genotype 3 to be a poor predictor of response, probably because of the choice of DAA (DCV/SOF) and because most of our patients, following local and European guidelines, were treated for 24 weeks. Consistent with our findings, Welzel et al 22 describe a high SVR (above 90%) among a cohort of patients with severe liver disease treated with DCV/SOF with or without ribavirin. More than 85% were treated for more than 20 weeks. High and similar SVRs were seen in patients infected with genotype 3. We would like to highlight the high prevalence of genotype 4 in our study (20%), reflecting the overrepresentation of this genotype among the HIV population.13
The main limitation of our study is that it is not randomized. Consequently, there are differences between the MDT and TT groups. However, given the paucity of data on interactions between DAAs and ART, we believe our results are helpful. Another limitation of the study is that both groups, specially the MDT group, were very heterogeneous; patients, as it happens in real live, were taken many different combinations, and we can not confirm that all of them are equally save and effective when combined with DAA.
In conclusion, very high HCV cure rates are obtained with DAA in a real-world setting in HIV-positive patients. Non-conventional ART such as monotherapy with a bPI or dual therapy is as effective and safe as triple therapy when combined with DAA. It is not necessary to switch ART before the use of DAA in coinfected patients. Liver fibrosis and lower CD4 cell counts are associated with failure of HCV treatment.
Financial supportNone to be declared.
Conflict of interestNone to be declared.