Extended-spectrum beta-lactamase (ESBL) producing bacteria are infrequent pathogens of urinary tract infections in children. The objective of our study was to investigate the presence, clinically associated characteristics and risk factors for acquisition of urinary tract infection/acute pyelonephritis (UTI/APN) in hospitalised children <2years old caused by community-acquired ESBL.
MethodsA case-control study in a second level community hospital in Spain, in which 537 episodes of UTI/APN were investigated in a retrospective study between November 2005 and August 2014. Cases were patients with ESBL strains. For each case, four ESBL-negative controls were selected. A questionnaire with the variables of interest was completed for every patient, and the groups were compared.
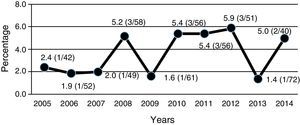
ResultsESBL-positive strains were found in 19 (3.5%) cultures. Of these 16 (84%) were Escherichia coli. Vesicoureteral reflux (VUR) of any grade was more frequent in the ESBL group (60 vs. 29%), although without statistical significance. Relapses were more frequent in the ESBL group (42% vs. 18%) (p=0.029; OR=3.2; 95%CI: 1.09–9.5). The prevalence of UTI/APN due to ESBL-positive strains increased slightly from 2.7% in the period 2005–2009 to 4.4% in the period 2010–2014.
ConclusionsESBL UTI/APN were associated with more frequent relapses. VUR of any grade was twice more frequent in the ESBL group. Piperacillin/tazobactam, fosfomycin and meropenem showed an excellent activity. Aminoglycosides may be a therapeutic option, and in our patients gentamicin was the antibiotic most used.
El objetivo de nuestro estudio fue investigar la presencia, las características clínicas y los factores de riesgo para la adquisición de infección urinaria febril/pielonefritis (ITU/PNA) de la comunidad por microorganismos productores de betalactamasas de espectro extendido (BLEE+) en niños <2años que fueron ingresados en el hospital.
MétodosEstudio de casos-controles en un hospital de segundo nivel en España. Se revisaron de forma retrospectiva 537 episodios de ITU/PNA entre noviembre de 2005 y agosto de 2014. Los casos fueron las ITU/PNA BLEE+. Por cada caso se escogieron 4 controles betalactamasas de espectro extendido negativos (BLEE−). Para cada paciente se rellenó un cuestionario con las variables de interés y se realizó la comparación entre los grupos.
ResultadosSe identificaron 19 casos (3,5%) BLEE+. De ellos, 16 (84%) fueron Escherichia coli. El reflujo vesicoureteral (RVU) de cualquier grado fue más frecuente en el grupo BLEE+ (60 vs. 29%), aunque la diferencia no alcanzó significación estadística. Las recurrencias fueron más frecuentes en el grupo BLEE+ (42% vs 18%) (p=0,029; OR=3,2; IC−95%: 1,09–9,5). La prevalencia de ITU/PNA BLEE+ se incrementó ligeramente desde el 2,7% en el periodo 2005–2009 al 4,4% en el periodo 2010–2014.
ConclusionesLas ITU/PNA BLEE+ se asociaron a recurrencias más frecuentes. El RVU fue el doble de frecuente en el grupo BLEE+. Piperacilina/tazobactam, meropenem y fosfomicina mostraron una excelente actividad. Los aminoglucósidos pueden ser una opción terapéutica, y en nuestra serie la gentamicina fue el antibiótico más utilizado.
Extended-spectrum beta-lactamases (ESBLs) are a group of plasmid-encoding enzymes produced mainly by enterobacteria. Their hydrolytic spectrum includes amino-, carboxy- and ureidopenicillins; monobactams; and first-, second- and third and fourth-generation cephalosporins1–3 with the exception of cephamycins. They do not hydrolyse carbapenems or combinations of beta-lactam antibiotics with beta-lactamase inhibitors such as clavulanic acid, tazobactam and sulbactam.4 These isolates often carry genes that encode resistance to other antimicrobials.1,3,5
Although ESBL-producing (ESBL+) microorganisms emerged as a cause of nosocomial infection in hospitals, community-acquired infections (especially urinary infections) have become a problem with an increasing incidence in clinical practice.1,5–12 The prevalence and distribution of ESBL+ microorganisms as a cause of community-acquired febrile urinary tract infection/acute pyelonephritis (UTI/APN) in children are not well known and are concerning due to the resistance of these isolates to many beta-lactam antibiotics and other antimicrobials.13 Knowledge of the risk factors for these infections may be useful to identify high-risk patients and thus administer empirical treatment that is more likely to be effective.8 We did not find any studies in Spain on the prevalence and risk factors for UTI/APN due to ESBL+ bacteria in children. The objective of our study was to determine the clinical and laboratory characteristics, antibiotic sensitivity and risk factors for UTI/APN due to ESBL+ bacteria in children versus UTI/APN caused by non-ESBL-producing (ESBL−) microorganisms.
MethodsThis was a descriptive, analytical, retrospective case–control study. The cases were the episodes of community-acquired UTI/APN caused by ESBL+ microorganisms in previously healthy children under 24 months of age in their first episode of hospitalisation from November 2005 to August 2014. This age group was chosen because it corresponds to the majority of the episodes of UTI/APN that were admitted during the study period and in which admission and follow-up on an outpatient basis provided a suitable record of the variables of interest.
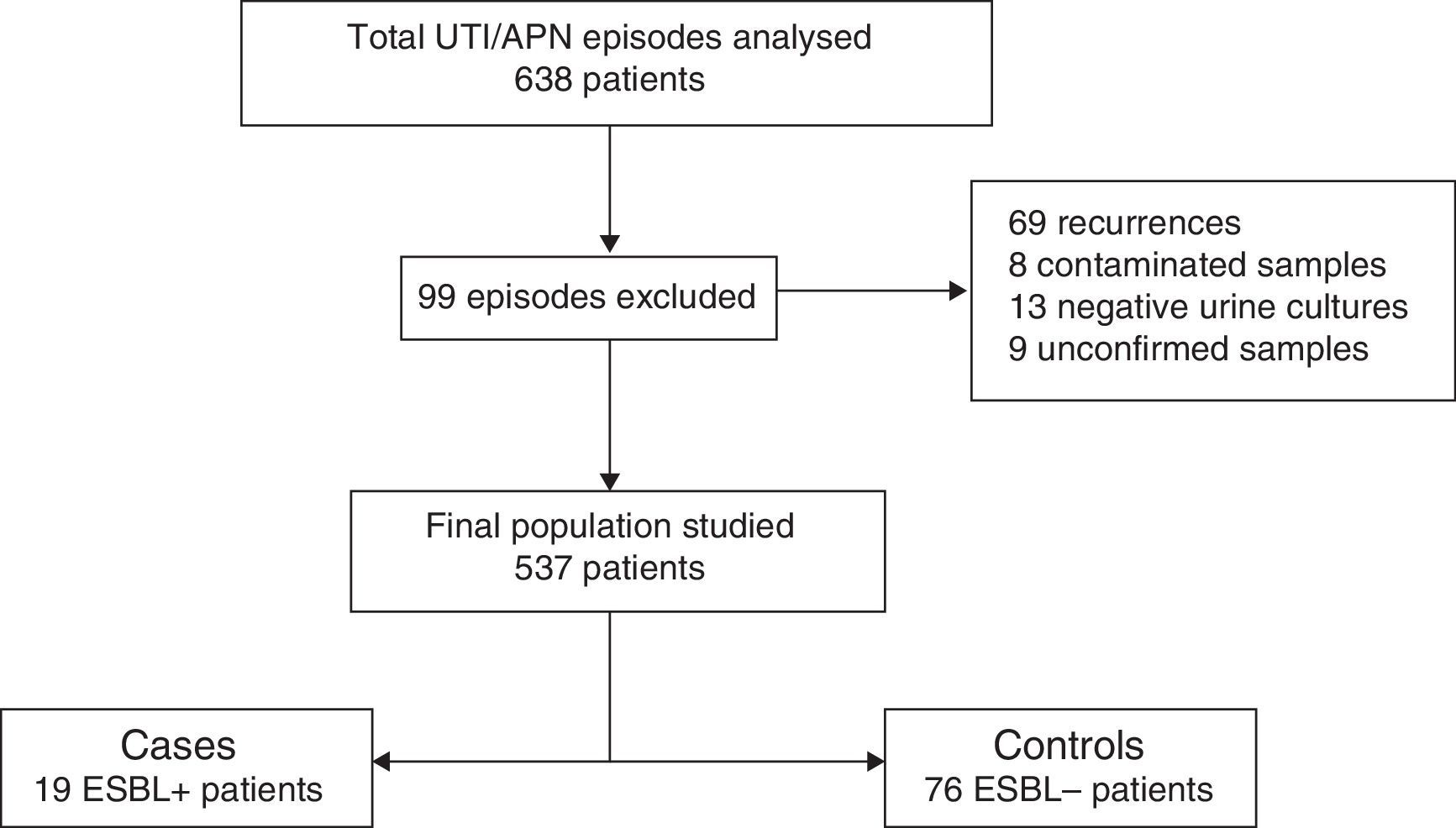
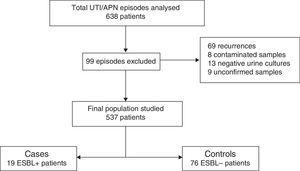
The study was conducted at Hospital Universitari Doctor Peset in Valencia, Spain, which is a secondary hospital that cares for a population of approximately 50,000 children. A total of 636 suspected episodes of UTI/APN obtained from hospital databases were reviewed. The following were excluded from the review: recurrences (only first episodes of UTI/APN were included), patients with contaminated urine samples, those in whom the urine culture turned out to be negative, those in whom the antibiogram was not available in the hospital database and the cases in which the results of the urine culture could not be known because it had been performed at another hospital and could not be accessed.
The diagnostic criteria for UTI depended on the method of urine collection according to the recommendations of the UTI Guidelines from the Spanish Association of Paediatrics.14,15 The collection of urine for culture in a collecting bag was not allowed.
Episodes of community-acquired UTI/APN were defined as those with a positive urine culture identified in the Emergency Unit at the hospital or within 48h of hospital admission. For each case of UTI/APN caused by ESBL+ microorganisms, 4 controls caused by ESBL− isolates were chosen due to closeness in time, the 2 before and the 2 after the case, which involved hospital admission.
Empirical treatment guided by observation of Gram-negative bacilli in Gram staining of the urine sample was performed with gentamicin. The identification of ESBL+ isolates and the study of antibiotic sensitivity were performed by the Clinical Microbiology Laboratory according to the recommendations of the Clinical and Laboratory Standards Institute.16 The double-disk diffusion technique was used to confirm the ESBLs; in this technique, disks of cephalosporins (cefotaxime, ceftazidime, cefuroxime) and aztreonam were placed around another disk of amoxicillin–clavulanic acid. The presence of ESBL was demonstrated by the synergistic inhibition effect with the extension of a halo from one or several beta-lactam antibiotics.
A structured survey was used to collect the following variables from the children's medical records: sex, age, maximum intensity of fever, duration of signs and symptoms prior to admission expressed in hours, leukocyte count, neutrophil count, C-reactive protein (CRP) values expressed in mg/l, procalcitonin (PCT) values expressed in ng/ml, microbiological identification of the isolate, antibiogram, results of renal ultrasound, results of scintigraphy with dimercaptosuccinic acid (DMSA), results of the serial micturating cystourethrogram (MCUG) in the cases in which it was performed, number of recurrences, and indication of chemoprophylaxis on discharge.
For the statistical analysis, the chi-squared test was used to compare proportions in qualitative variables, Student's t test was used to compare 2 parametric variables and the Mann–Whitney U test was used to compare 2 non-parametric variables. Statistical analysis was performed using the SPSS (v. 22) program with a licence from the Universitat de València. Statistical significance was set at p≤0.05.
ResultsOf the 636 cases studied, 99 were excluded (Fig. 1), and so a final population of 537 children was analysed. A total of 19 ESBL+ isolates (3.5%; CI 95%: 2.1–5.5%) were identified. Among the 95 episodes studied (19 cases and 76 controls), the microorganisms isolated were Escherichia coli in 84 cases (88.4%), Klebsiella pneumoniae in 4 cases (4.2%), Citrobacter spp. in 3 cases (3.3%), Proteus mirabilis in one case (1.1%), Morganella morganii in one case (1.1%) and Enterobacter cloacae in one case (1.8%). Of the 19 ESBL+ isolates, 16 (84.2%) were E. coli, one (5.3%) was K. pneumoniae, one (5.3%) was Morganella morganii and one (5.3%) was Citrobacter spp. No difference in terms of frequency of isolation of Klebsiella spp. was observed between the ESBL+ group (5%) and the ESBL− group (4%).
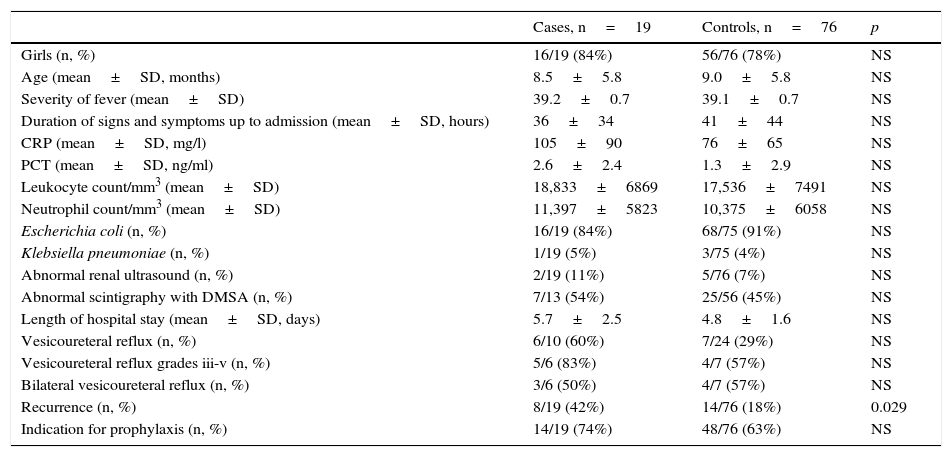
The case group and the control group were comparable with respect to distribution by sex, age and hours elapsed from onset of signs and symptoms to hospital admission (Table 1). There was no difference in terms of maximum intensity of fever. No differences were observed in terms of leukocyte count, neutrophil count or length of hospital stay. The difference between the mean CRP value for the cases (105mg/l) and for the controls (76mg/l), and between the mean PCT value for the cases (2.6ng/ml) and for the controls (1.3ng/ml), did not attain statistical significance.
Characteristics of cases and controls.
| Cases, n=19 | Controls, n=76 | p | |
|---|---|---|---|
| Girls (n, %) | 16/19 (84%) | 56/76 (78%) | NS |
| Age (mean±SD, months) | 8.5±5.8 | 9.0±5.8 | NS |
| Severity of fever (mean±SD) | 39.2±0.7 | 39.1±0.7 | NS |
| Duration of signs and symptoms up to admission (mean±SD, hours) | 36±34 | 41±44 | NS |
| CRP (mean±SD, mg/l) | 105±90 | 76±65 | NS |
| PCT (mean±SD, ng/ml) | 2.6±2.4 | 1.3±2.9 | NS |
| Leukocyte count/mm3 (mean±SD) | 18,833±6869 | 17,536±7491 | NS |
| Neutrophil count/mm3 (mean±SD) | 11,397±5823 | 10,375±6058 | NS |
| Escherichia coli (n, %) | 16/19 (84%) | 68/75 (91%) | NS |
| Klebsiella pneumoniae (n, %) | 1/19 (5%) | 3/75 (4%) | NS |
| Abnormal renal ultrasound (n, %) | 2/19 (11%) | 5/76 (7%) | NS |
| Abnormal scintigraphy with DMSA (n, %) | 7/13 (54%) | 25/56 (45%) | NS |
| Length of hospital stay (mean±SD, days) | 5.7±2.5 | 4.8±1.6 | NS |
| Vesicoureteral reflux (n, %) | 6/10 (60%) | 7/24 (29%) | NS |
| Vesicoureteral reflux grades iii-v (n, %) | 5/6 (83%) | 4/7 (57%) | NS |
| Bilateral vesicoureteral reflux (n, %) | 3/6 (50%) | 4/7 (57%) | NS |
| Recurrence (n, %) | 8/19 (42%) | 14/76 (18%) | 0.029 |
| Indication for prophylaxis (n, %) | 14/19 (74%) | 48/76 (63%) | NS |
SD: standard deviation; DMSA: dimercaptosuccinic acid; NS: non-significant; CRP: C-reactive protein; PCT: procalcitonin.
The percentage of abnormalities in renal ultrasound was 11% among the cases and 7% among the controls. The renal scintigraphy with DMSA was abnormal in 54% of the cases and 45% of the controls who underwent this test. When a MCUG was performed, vesicoureteral reflux (VUR) was found in 60% of the cases and 29% of the controls, and among them, the proportion of grade III–V VUR was 83% among the cases and 57% among the controls. However, none of these differences attained statistical significance. The proportion of recurrences among the cases (42%) was much higher than among the controls (18%) (p=0.029; OR=3.2; CI 95%: 1.1–9.5), with 2 or more recurrences in 26% in the ESBL+ group versus 3% in the ESBL− group (p<0.01; OR=13.1; CI 95%: 2.3–76.9).
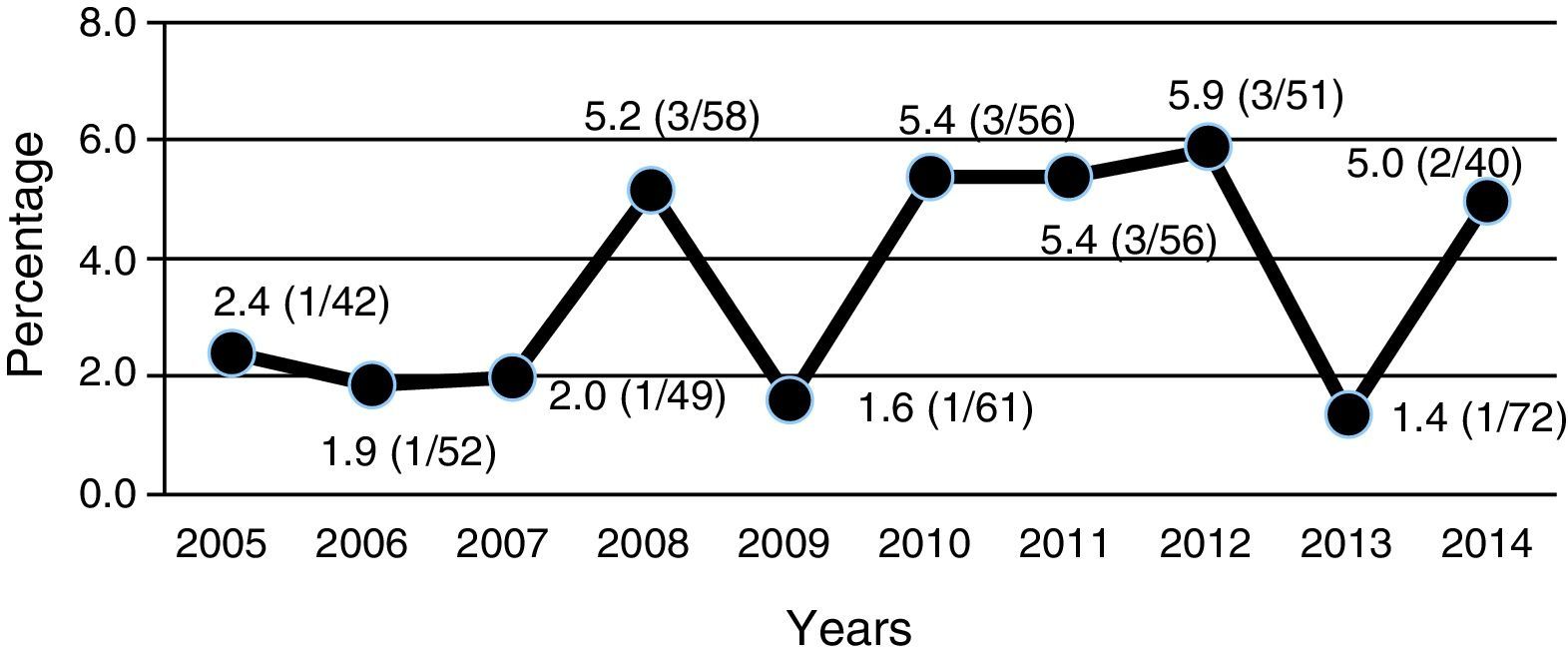
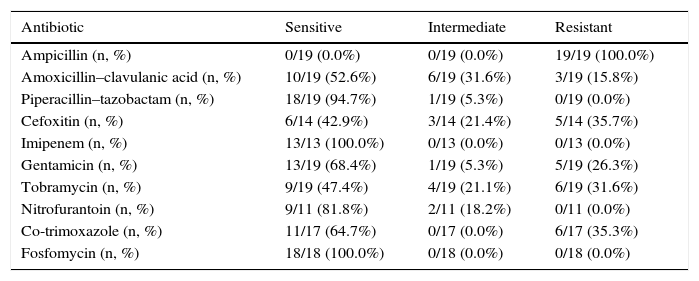
All ESBL+ isolates were resistant to ampicillin, 16% were resistant to amoxicillin–clavulanic acid, 36% were resistant to cefoxitin, 26% were resistant to gentamicin, 32% were resistant to tobramycin and 35% were resistant to co-trimoxazole (Table 2). Nearly 70% of ESBL+ isolates were sensitive to empirical treatment with gentamicin. There was no resistance to fosfomycin, piperacillin–tazobactam or meropenem. One episode of bacteraemia due to E. coli occurred in the ESBL+ group. No other complications (such as nephronia/renal abscess) were recorded; in addition, no mortality associated with the ESBL+ episodes was recorded. There was no difference in terms of indication of chemoprophylaxis on hospital discharge between cases and controls. The annual incidence of cases of UTI/APN due to ESBL+ bacteria is shown in Fig. 2. Grouping the years studied showed that from 2005 to 2009 the percentage of ESBL+ isolates was 2.7%, while from 2010 to 2014 it was 4.4%, although this increase did not attain statistical significance.
Antimicrobial sensitivity to ESBL+ isolates.
| Antibiotic | Sensitive | Intermediate | Resistant |
|---|---|---|---|
| Ampicillin (n, %) | 0/19 (0.0%) | 0/19 (0.0%) | 19/19 (100.0%) |
| Amoxicillin–clavulanic acid (n, %) | 10/19 (52.6%) | 6/19 (31.6%) | 3/19 (15.8%) |
| Piperacillin–tazobactam (n, %) | 18/19 (94.7%) | 1/19 (5.3%) | 0/19 (0.0%) |
| Cefoxitin (n, %) | 6/14 (42.9%) | 3/14 (21.4%) | 5/14 (35.7%) |
| Imipenem (n, %) | 13/13 (100.0%) | 0/13 (0.0%) | 0/13 (0.0%) |
| Gentamicin (n, %) | 13/19 (68.4%) | 1/19 (5.3%) | 5/19 (26.3%) |
| Tobramycin (n, %) | 9/19 (47.4%) | 4/19 (21.1%) | 6/19 (31.6%) |
| Nitrofurantoin (n, %) | 9/11 (81.8%) | 2/11 (18.2%) | 0/11 (0.0%) |
| Co-trimoxazole (n, %) | 11/17 (64.7%) | 0/17 (0.0%) | 6/17 (35.3%) |
| Fosfomycin (n, %) | 18/18 (100.0%) | 0/18 (0.0%) | 0/18 (0.0%) |
Interest in ESBL+ bacteria has been growing in Spain in the last 20years. The prevalence of these bacteria in children is not as well known as in adults. Just one recent publication in the Basque Country, Spain,13 showed a prevalence of ESBL+ E. coli carriers in the faeces of 24% of 125 children from 8 to 16 months of age. The origin of these isolates could have been the abuse of antibiotics in humans and animals, cross-infections between humans or transmission from contaminated pets or other animals to humans through the food chain.17,18
In our study, ESBL+ isolates were an uncommon cause (3.5%) of UTI/APN in hospitalised children under 2years of age. In Mediterranean countries, a study conducted in Greece found an incidence of UTIs of 10.4% in children under 14years of age19; another study in Israel found an incidence of UTIs of 5% in children under 18years of age9; and yet another study in Turkey, also in children, found an incidence of 43%.20
Maximum severity of fever, duration of signs and symptoms expressed in hours, and haematological variables, such as leukocyte count and neutrophil count, did not differ between the 2 groups, as in other studies.8,9,11,20 Acute phase reactants (CRP and PCT) were not significantly higher in the ESBL+ cases versus the controls; in addition, the proportion of acute injury was not statistically higher in renal scintigraphy with DMSA.
Regarding risk factors for UTI/APN, renal ultrasound was modestly more abnormal in the cases (11% vs 7%) than in the controls. The presence of VUR of any grade was twice as high among the cases versus the controls (60% vs 29%). High-grade VUR (83% vs 57%) and the indication for prophylaxis (74% vs 63%) were higher in the ESBL+ group, although not statistically significantly. Only the higher proportion of recurrences attained statistical significance (42% vs 18%), as in other studies in children.8,10,11,20 Four studies in children found that anatomical and functional urinary tract abnormalities such as VUR were risk factors for ESBL+ infections.8,9,11,21 One study found a higher proportion of anatomical and functional abnormalities, but not a higher proportion of reflux.19 Based on all this, isolation of ESBL+ bacteria could be considered a potential indicator of nephro-urological abnormalities, especially high-grade VUR.
Several studies have identified use of cephalosporins and other antibiotics in the previous 1–3months as a risk factor,6,8–11,19,20,22 and we believe it is important to note that the use of cephalosporins as prophylaxis should be avoided to prevent the selection of ESBL+ microorganisms.20 Given the retrospective nature of our study, prior use of antibiotics could not be analysed. Recent hospitalisation or underlying diseases (metabolic, onco-haematological, etc.), identified as risk factors in other studies,9–11,20 were not analysed as first episodes of hospitalisation due to UTI/APN in previously healthy children were included as cases.
Some studies have found a higher relative frequency of isolation of Klebsiella spp. in the ESBL+ group, and it has been proposed that identification of Klebsiella could be a predictor of potential ESBL+ microorganism isolation8,9,19,22; however, this did not occur in our study.
ESBL+ microorganisms encode resistance to third-generation cephalosporins, which are the treatment of choice in UTI/APN in non-hospitalised children and an option for empirical treatment in admitted children.14,15 This may represent inappropriate antibiotic handling before the results of the urine culture are available.20 After empirical treatment with third-generation cephalosporins has been started, it is advisable to monitor the clinical response, as an unfavourable clinical response may be an early indicator of an aetiology of ESBL+ bacteria.
These isolates show cross-resistance with other non-beta-lactam antibiotics.23 Four of the 19 isolates (21%) in our study showed resistance to 2 or more non-beta-lactam antibiotics. All this tends to represent the need for hospitalisation for treatment20 and, given the profile of multi-drug resistance, treatment options may be limited. In our study, fosfomycin, piperacillin–tazobactam and meropenem showed excellent activity, as in other studies.6,24 However, notably, there was a high percentage of strains with resistance and intermediate-resistance to cefoxitin; this may have been a sign of the presence of AmpC plasmidic cephamycinases.
There are few data in the literature to make recommendations on the treatment of these infections in children. For the treatment of ESBL+ infections, Curello and MacDougall5 propose a carbapenem as a first option in patients with septic shock or immunocompromised patients and in clinically stable patients with pyelonephritis. To prevent the use of carbapenems and the emergence of resistance, Park et al.25 suggest the use of aminoglycosides, fluoroquinolones and combinations of a beta-lactam/beta-lactamase inhibitor (especially piperacillin/tazobactam and to quite a lesser extent amoxicillin/clavulanic acid due to its clearly higher proportion of resistances) for the treatment of community-acquired acute pyelonephritis in adults.2 In their review, they report that aminoglycosides were the most commonly used non-carbapenem antibiotics in patients with cases of uncomplicated pyelonephritis who had little or no comorbidity, and they observed no clinical or microbiological failures.25
The disadvantage of piperacillin/tazobactam is that, according to its summary of product characteristics, it is approved for use in children over 2years of age26 and its usefulness may be affected by the inoculum effect (substantial increase in MIC in presence of high bacterial inocula).7 Cephamycins easily develop resistance and are not recommended.23 The use of quinolones is not authorised in those under 18years of age, although they have been used without substantial side effects in multi-drug resistant infections.27
Therefore, aminoglycosides may be a definitive empirical treatment option28 in cases of UTI/APN in children, preferred due to their high concentration in renal tissue and conditional on the local resistance profile in ESBL+ isolates and the sensitivity of the isolate in each case. In our series, gentamicin was the most commonly used antibiotic as it was the empirical treatment of choice to which nearly 70% of ESBL+ isolates were sensitive. This could help explain the lack of differences in terms of injury severity in the scintigraphy with DMSA, since in many cases there was no delay in starting effective treatment. However, the percentage of resistance to gentamicin was 26%. This means that the sensitivity of these isolates must be monitored on the antibiogram. Amikacin is the aminoglycoside least affected by resistances in ESBL+ microorganisms,7,23,24 although our study lacked data on sensitivity to amikacin.
Fosfomycin does not have cross-resistance with other antibiotics.29 In a review by Falagas et al.,30 96.8% of 1657 ESBL+ E. coli isolates and 81.3% of 748 ESBL+ K. pneumoniae isolates were sensitive to fosfomycin. Its use seems to be increasingly common in these cases. However, experience in the use of fosfomycin in serious urinary tract infections in children is limited. When there is suspected renal or systemic impairment, it should be used intravenously; the oral route should be reserved for uncomplicated lower urinary infections.29
Everything mentioned greatly limits oral treatment options on hospital discharge, which could prolong the hospital stay.9–11,19 In our series, the mean length of hospital stay of the cases was just one day longer than that of the controls. We attributed this to the fact that nearly 70% of ESBL+ isolates were sensitive to initial treatment with gentamicin and to the fact that an oral alternative was available for hospital discharge in 8 cases. In 7 cases, the initial treatment was modified with the data from the antibiogram. It was replaced with carbapenems in 3 cases, with amoxicillin–clavulanic acid in 2 cases, with co-trimoxazole in one case and with gentamicin in the case that had started cefixime. On discharge, treatment involved co-trimoxazole in 5 cases, amoxicillin–clavulanic acid in 3 cases, cefixime (erroneously) in 2 cases and fosfomycin (which could be considered suboptimal) in one case. The ESBL+ isolates were sensitive to these oral treatments, except in the cases treated with cefixime, although all followed a favourable clinical course. There is a striking article in which, although only 4 of 28 patients with febrile UTI due to ESBL+ isolates received appropriate empirical treatment, 95% followed a favourable clinical and microbiological course.21 The researchers attributed this to the fact that, although the treatment may have been inappropriate, with intravenous administration, favourable pharmacokinetic and pharmacodynamic objectives were achieved in the urinary tract as an extremely high concentration of the antibiotic.7,21
Our study, although it gathered data from a 9-year period, had the disadvantage of its small number of cases, given the rarity of these isolates. This probably led to some of the differences observed, especially in terms of frequency of reflux of any grade and perhaps of high-grade reflux, not attaining statistical significance. Another disadvantage was the fact that the study was limited to children under 2years of age for whom the diagnosis of febrile UTI or pyelonephritis had for many years been an indication for admission to our hospital. The study was retrospective and some variables of interest, such as a history of recent use of antimicrobials, were not collected in the medical records and could not be analysed.
From the literature review and our study data it could be deduced that, in the treatment of episodes of UTI/APN with hospitalisation wherein patients have received antibiotics or have been admitted in the previous 1–3months, have underlying diseases or anatomical or functional urinary tract abnormalities, have a higher frequency of recurrences or have received chemoprophylaxis, the option of covering ESBL+ microorganisms should be considered to prevent suboptimal treatment and the possibility of renal damage. Despite the limitations of the study, given the limited data in the literature on children and the ever-increasing prevalence of these isolates, we believe that the information provided by this study may be useful. It would be desirable to design a large-scale prospective study to better define in children the frequency and characteristics in terms of clinical course of these infections in our setting and to overcome the disadvantages of retrospective studies with limitations in terms of data collection and number of ESBL+ isolates.9
FundingThis study did not receive any funding.
Conflicts of interestThe authors of this study do not have conflicts of interest.
Please cite this article as: Hernández Marco R, Guillén Olmos E, Bretón-Martínez JR, Giner Pérez L, Casado Sánchez B, Fujkova J, et al. Infección urinaria febril adquirida en la comunidad por bacterias productoras de betalactamasas de espectro extendido en niños hospitalizados. Enferm Infecc Microbiol Clin. 2017;35:287–292.