Bacteraemia caused by anaerobic bacteria is rare in the hospital setting. The Clostridium genus is the second most common cause of these infections, particularly Clostridium perfringens, which has a high mortality rate. However, reviews in the literature of these infections are scarce. The aim of this study was to retrospectively document the incidence, clinical characteristics and risk factors involved in the acquisition of bacteraemia caused by C. perfringens among patients treated at our hospital over a 10-year period. Twenty-eight patients with C. perfringens bacteraemia were included in the study. We evaluated pre-existing comorbidities, the source of bacteraemia, clinical features, the antimicrobial treatment administered and patient outcome. C. perfringens bacteraemia occurs rarely in our setting, but with a very high mortality rate. This rate is associated with old age and pre-existing, largely gastrointestinal malignancies. It presents with few specific symptoms but requires rapid and appropriate diagnosis and treatment to reduce the high mortality of this infection.
Las bacteriemias causadas por bacterias anaerobias no son frecuentes en el ámbito hospitalario, siendo el género Clostridium el segundo más frecuente en este tipo de infecciones, especialmente Clostridium perfringens, con un alta tasa de mortalidad, siendo escasas las revisiones en la literatura a este respecto. El objetivo de este estudio fue documentar retrospectivamente la incidencia, las características clínicas y los factores de riesgo implicados en la adquisición de la bacteriemia causada por C. perfringens entre los pacientes vistos en nuestro hospital a lo largo de un período de 10 años. Veintiocho pacientes con bacteriemia por C. perfringens fueron incluidos en el estudio, valorándose las comorbilidades preexistentes, el punto de partida de la bacteriemia, las características clínicas, el tratamiento antimicrobiano seguido y la evolución de los mismos. La bacteriemia por C. perfringens es un acontecimiento infrecuente en nuestro medio, pero con muy alta mortalidad, que se relaciona actualmente con pacientes de edad avanzada y neoplasias preexistentes, principalmente de origen digestivo, con poca expresividad específica en la clínica, pero que precisa de un diagnóstico y tratamiento rápido y adecuado para disminuir la alta letalidad de la infección.
Clostridium perfringens is a gram-positive, anaerobic and immobile bacillus that is part of the human gastrointestinal and genitourinary microbiota. Sometimes these micro-organisms can generate different types of exotoxins related to intestinal infectious processes, also exhibiting a significant enteroinvasive capacity.1 In addition, they can be involved in extraintestinal infections, preferably in skin and soft tissues, and be responsible for symptoms of crepitant cellulitis, fasciitis or myonecrosis.2 On the other hand, they may be causative agents of episodes of primary bacteraemia accompanied by acute haemolysis with a high mortality rate.3
Overall, bacteraemia caused by anaerobic bacteria are not frequent in the hospital setting, representing 0.5–12% of all episodes of bacteraemia, with the Clostridium genus being the second most frequent in this type of infections, with C. perfringens being the most frequent, representing between 20% and 50% of the total.4,5
Although there is a relatively high number of clinical reviews of bacteraemia caused by anaerobic bacteria as a whole, few are focused on those caused by C. perfringens in particular, despite the high and rapid mortality that accompanies them, of the order of 27–44%,5 since most of them do not show early specific clinical characteristics that allow immediate diagnosis and treatment.
The objective of this study was to retrospectively document the incidence, clinical characteristics and risk factors involved in the acquisition of C. perfringens bacteraemia among patients seen in our hospital over a period of 10 years, as well as assess the behaviour of antimicrobial treatment in relation to the clinical course of patients.
MethodsPatientsAll the patients treated in our hospital over 10 years (January 2005–January 2015) who presented with an episode of significant C. perfringens bacteraemia, identified in the Microbiology Department, were included in the study.
Each case was considered as such when the patient had at least one positive blood culture for C. perfringens and showed clinical characteristics compatible with systemic infection, excluding those cases in which the isolation was not accompanied by an indicative clinic picture and which were assessed as possible contaminants.
Microbiological identification and sensitivity testsThe identification was carried out by conventional methods (morphological, biochemical characteristics, etc.) for the first cases, later being substituted for mass spectrometry. At the start of the study, all strains were reidentified by mass spectrometry.
The preparation of the plates, the generation of the spectra and their subsequent diagnosis were carried out in accordance with the standards established and previously described for the Vitek® MS system (bioMérieux, France), using a software and an incorporated database. According to the manufacturer's instructions, a confidence value of between 60 and 99% for a given species was considered a good identification.
Sensitivity tests were performed with a gradient diffusion system using E-test strips of the following antibiotics: penicillin, amoxicillin/clavulanic acid, piperacillin/tazobactam, clindamycin, meropenem, vancomycin and metronidazole.
Clinical dataThe data collected in the patients’ medical records were the basis of the demographic aspects, comorbidities and the starting point of the bacteraemia. The comorbidities identified in our patients included renal failure (defined as serum creatinine ≥1.4mg/dl or need for haemodialysis), cancer, diabetes mellitus, liver cirrhosis, stroke, heart failure, gallstone, immunosuppressive therapy or chemotherapy and recent surgery.
The presumed origin of the bacteraemia was determined from the clinical data collected in their records, surgical findings and microbiological identifications. The starting points of the bacteraemia were classified as of intraabdominal, respiratory tract, skin and soft tissue origin or primary bacteraemia without defined focus.
Antimicrobial treatment was considered appropriate when antibiotics that are effective against C. perfringens according to an antibiogram were used, and when they were administered within 48h after the onset of bacteraemia after receiving the blood culture result, and that they were continued for at least the following 3 days.
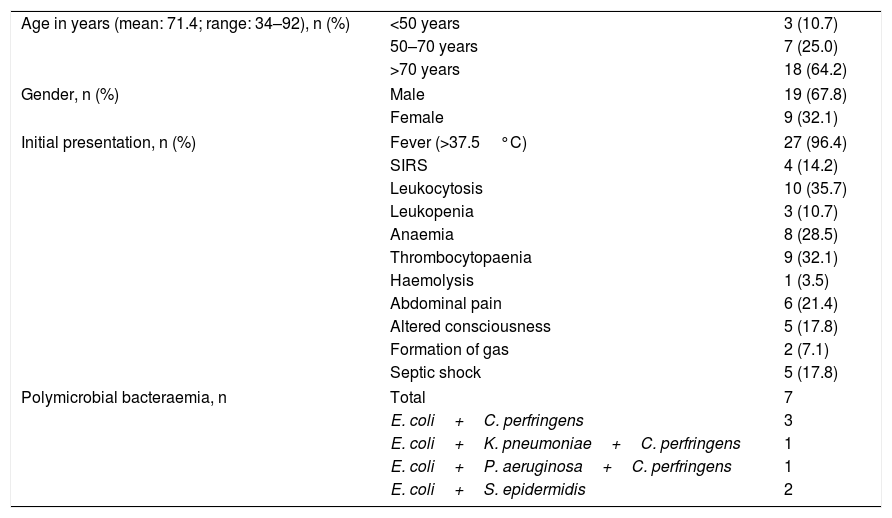
ResultsDemographic and clinical dataTwenty-eight patients with C. perfringens bacteraemia were included in the study. The corresponding demographic data and initial clinical findings are shown in Table 1. The mean age of the patients was 71 years, with a range of 34–92 years. Men predominated over women: 19/9.
Demographics and initial presentation of 28 patients with Clostridium perfringens bacteraemia.
| Age in years (mean: 71.4; range: 34–92), n (%) | <50 years | 3 (10.7) |
| 50–70 years | 7 (25.0) | |
| >70 years | 18 (64.2) | |
| Gender, n (%) | Male | 19 (67.8) |
| Female | 9 (32.1) | |
| Initial presentation, n (%) | Fever (>37.5°C) | 27 (96.4) |
| SIRS | 4 (14.2) | |
| Leukocytosis | 10 (35.7) | |
| Leukopenia | 3 (10.7) | |
| Anaemia | 8 (28.5) | |
| Thrombocytopaenia | 9 (32.1) | |
| Haemolysis | 1 (3.5) | |
| Abdominal pain | 6 (21.4) | |
| Altered consciousness | 5 (17.8) | |
| Formation of gas | 2 (7.1) | |
| Septic shock | 5 (17.8) | |
| Polymicrobial bacteraemia, n | Total | 7 |
| E. coli+C. perfringens | 3 | |
| E. coli+K. pneumoniae+C. perfringens | 1 | |
| E. coli+P. aeruginosa+C. perfringens | 1 | |
| E. coli+S. epidermidis | 2 | |
SIRS: systemic inflammatory response syndrome.
The distribution of cases over time during the study was one case in 2007, 2009 and 2013, and 4 cases in 2012.
Bacteraemia in one patient was of nosocomial origin, while in the remaining 27 it was considered a disease acquired in the community. Seven patients (14.3%) presented with polymicrobial bacteraemia, with the association with Escherichia coli being the most frequently found.
Most of the patients initially presented with fever, leukocytosis, thrombocytopaenia and anaemia. Severe and diffuse abdominal pain and alterations in the level of consciousness were also relatively frequent. Systemic inflammatory response syndrome was seen in 4 patients. Five patients developed septic shock.
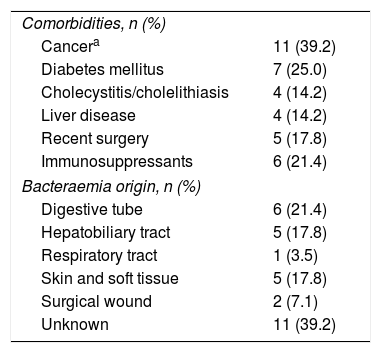
Comorbidities and starting point of bacteraemiaThe clinical profiles of the 28 patients with C. perfringens bacteraemia are shown in Table 2.
Clinical profiles of 28 patients with Clostridium perfringens bacteraemia.
| Comorbidities, n (%) | |
| Cancera | 11 (39.2) |
| Diabetes mellitus | 7 (25.0) |
| Cholecystitis/cholelithiasis | 4 (14.2) |
| Liver disease | 4 (14.2) |
| Recent surgery | 5 (17.8) |
| Immunosuppressants | 6 (21.4) |
| Bacteraemia origin, n (%) | |
| Digestive tube | 6 (21.4) |
| Hepatobiliary tract | 5 (17.8) |
| Respiratory tract | 1 (3.5) |
| Skin and soft tissue | 5 (17.8) |
| Surgical wound | 2 (7.1) |
| Unknown | 11 (39.2) |
The most frequent comorbidities were cancer (39.3%), predominantly in the digestive tract, diabetes mellitus (25%) and immunosuppressive treatment (21.4%).
Intra-abdominal infection was the origin of the bacteraemia in 11 patients (39.2%). The next most frequent starting point for bacteraemia was skin and soft tissue infection, including infection of the surgical wound in 7 cases; the infection of the lower respiratory tract could be the origin of one case, while on 11 occasions the starting point could not be credibly determined.
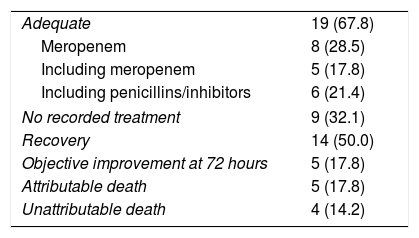
Sensitivity to antimicrobials, treatment and clinical courseResistance to penicillin was observed in one case, with an MIC of 2μg/ml. In another case, resistance to clindamycin was observed, with an MIC ≥16μg/ml.
The other antimicrobials tested—piperacillin/tazobactam, amoxicillin/clavulanic acid, meropenem, vancomycin and metronidazole—were active in vitro in all cases.
Table 3 summarises the aspects regarding antimicrobial treatment.
Antimicrobial treatment and clinical course.
| Adequate | 19 (67.8) |
| Meropenem | 8 (28.5) |
| Including meropenem | 5 (17.8) |
| Including penicillins/inhibitors | 6 (21.4) |
| No recorded treatment | 9 (32.1) |
| Recovery | 14 (50.0) |
| Objective improvement at 72 hours | 5 (17.8) |
| Attributable death | 5 (17.8) |
| Unattributable death | 4 (14.2) |
The data are expressed as n (%).
Of the 28 patients included, 19 received an antimicrobial treatment considered adequate. In these patients, meropenem was used in 8 cases, a combination of 2 or more antibiotics including meropenem in 5 cases and piperacillin/tazobactam alone or in combination with other agents in 6 other patients. In 9 cases, no antimicrobial treatment was established or it was considered marginal because of its short administration time.
The crude mortality of the series was 9 cases, although mortality attributable to bacteraemia was limited to 5 cases, which developed septic shock.
DiscussionC. perfringens bacteraemia is the result of multiple factors, such as intestinal or biliary tract surgery, liver abscesses, different underlying immunocompromised conditions or trauma and/or myonecrosis.6–8 On the other hand, the presence of the microorganism in the blood can also be due to transient leaks from the colonic flora, which lead to transient bacteraemia without clinical translation.1
As in other studies,1,6,7C. perfringens bacteraemia, as previously mentioned, is rarely detected. In our case it was detected in 0.040% of processed blood cultures, and occurs mostly in patients older than 70 years in almost two thirds of the series (64%), with an average of approximately 71 years and a marked predominance of male patients.
Unfortunately, in a high number of patients the initial presentation of the clinical symptoms does not show differential characteristics with other septic processes, so the clinical suspicion and diagnosis are not established as early enough as would be desirable when establishing effective therapeutic actions, hyperbaric therapy, early antimicrobial treatment or removal of the focus of the infection,2,8 unless more specific symptoms are seen, such as gas formation close to the lesions or the initial stages of massive haemolysis syndromes,3,9 which, as already mentioned, is infrequent, hindering the prognosis of the infection.
In our series, which covers clinically significant cases over a period of 10 years, different neoplasms, especially of the digestive tract, were present in almost 40% of cases. Hepatobiliary tract diseases, cholecystitis/cholelithiasis, cholangiocarcinoma and other hepatopathies, regardless of their clinical definition, were among the most frequently detected comorbidities in these patients, as expected, given the ecology of the micro-organism in the human microbiota.10 The existence, added or not, of diabetes was also an important factor in the characterisation of these patients, since it was present in a quarter of them.11 A similar percentage occurred in those patients undergoing immunosuppressive therapy. Another factor related to bacteraemia was the existence of recent surgery in the abdominal area, present in 17% of cases.
An interesting piece of information is, in our series, the relatively low incidence of skin and soft tissue infections as a starting point for bacteraemia, taking into account the ubiquity of the bacterial agent in the environment and its known relationship with traumas, wounds and the abundant catalogue of toxins and other lipoproteins which are very effective in the capacity for cellular destruction that it possesses.1 The massive haemolysis that accompanies these clostridial symptoms, therefore, was rare and only in one case could its existence be established in our series, although it is true that when it occurs, the accompanying mortality rate is extremely high, being, according to the authors, between 80% and 100% of cases.12,13
We could not objectify in any of the cases of hepatobiliary infections the existence of emphysematous infections, although, given the virulence characteristics of the micro-organism, it must have been present in some cases, but as it was a retrospective series we did not obtain conclusive data in many of them.
For the same reason it was not possible to analyse another section like the mortality section between neoplastic and non-neoplastic patients, or those that were only treated medicinally against those in whom surgery was also used.
In a quarter of our cases (7 patients), the bacteraemia detected was polymicrobial, with the isolation of C. perfringens being accompanied by different gram-negative bacilli, such as E. coli, Klebsiella pneumoniae or Pseudomonas aeruginosa. These polymicrobial bacteraemia did not alter the course or prognosis of the infection when compared with the individualised isolates of C. perfringens, so its clinical significance does not appear to be relevant.
The prognosis of the infection, on the other hand, is clearly influenced by the implementation of an early and appropriate antimicrobial treatment.14 Among our 28 patients, 19 received an appropriate treatment, considered as such when the chosen antimicrobial showed activity against C. perfringens and started it immediately after obtaining the result in the blood culture. The treatment included a carbapenemic preparation, in our case meropenem, either as monotherapy or associated with other antimicrobials. The other option consisted of a penicillin associated with an inhibitor, in our cases, mainly piperacillin/tazobactam.
Sensitivity to tetracyclines was not carried out, since there seems to be no correlation between the MICs of these antibiotics and the clinical course of the patients, so no cut-off points have been established for these antimicrobials.
Similarly, for the same reasons, the MIC of linezolid could not be assessed, although the values of vancomycin were known, with all strains being sensitive to MIC ≤2μg/ml to this antimicrobial.
In the remaining 9 patients, an effective regimen was not established or there was not enough time to implement the antimicrobial treatment that could have been useful. In 14 patients, half of those included in the series, all of which treated correctly, the clinical picture was cured, and in another 5, a measurable improvement was observed on the third day. Five other patients died as a result of the septic shock produced and in 4 other cases death was due to circumstances not directly related to bacteraemia.
In conclusion, C. perfringens bacteraemia is an uncommon event in our environment, but one with very high mortality, which is currently related to elderly patients and pre-existing neoplasms, mainly of digestive origin, with little specific expression in the clinical picture, but which requires rapid and adequate diagnosis and treatment to reduce the high lethality of the infection.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Lopez-Fabal MF, Sanz N, Ruiz-Bastian M, Barros C, Gomez-Garces J-L. Bacteriemia por Clostridium perfringens. Un análisis de 28 casos durante 10 años en un hospital universitario de Madrid. Enferm Infecc Microbiol Clin. 2018;36:225–228.