The biofilm production (BP) of 200 clinical strains of Candida isolated during 2010–2013 were assessed using an in vitro model and a comparison of the results was made between species and between origins of the infections. The BP was assessed using the crystal violet assay, and the strains were classified as low, moderate, or high biofilm producers. Candida tropicalis had the highest values for BP, which varied depending on the origin of the infection. Assessment of BP is a key diagnostic tool that enables us to better understand Candida infections.
Desde 2010 a 2013 evaluamos la producción de biopelícula (PB) en 200 cepas clínicas de Candida y comparamos los resultados de las especies de Candida entre los orígenes de la infección mediante un modelo in vitro. La PB se determinó con el ensayo de cristal violeta y las cepas se clasificaron como baja, moderada o altamente productoras de biopelícula. C. tropicalis tuvo los valores más altos de PB, y la PB en Candida varió dependiendo del origen de la infección. La determinación de la PB es una herramienta diagnóstica importante para entender mejor las infecciones por Candida.
Candida albicans is the most common cause of oropharyngeal and cutaneous candidiasis, and non-albicans species are increasingly associated with invasive candidiasis.1Candida bloodstream infection is one of the main nosocomial infections, with high morbidity and mortality. The ease with which some Candida strains adhere to natural or artificial surfaces to create aggregates (biofilm) increases their virulence and the likelihood of chronic infection.2
Biofilm production (BP) by Candida species has been assessed mainly using the crystal violet binding assay. However, most studies report BP without a standard cut-off and are generally based on Candida species isolated from patients with candidemia.3–6 Data regarding BP in Candida species isolated from different sites are scarce.
Our objective was to test BP in clinical strains of Candida and compare the results between Candida species and between different sites of infection.
MethodsWe performed a prospective in vitro study of BP in 200 clinical strains of Candida species isolated from 155 patients admitted to our institution during 2010–2013. The Candida species were distributed as follows: C. albicans, 41 (20.5%); C. parapsilosis, 40 (20.0%); C. krusei, 40 (20.0%); C. glabrata, 39 (19.5%); C. tropicalis, 22 (11.0%); and C. guilliermondii, 18 (9.0%). The sources of the Candida isolates were as follows: urine, 33 (16.5%); biopsy specimens, 28 (14.0%); respiratory tract, 26 (13.0%); blood, 24 (12.0%); sterile liquids, 18 (9.0%); wound, 13 (6.5%); abscess, 10 (5.0%); catheter, 8 (4.0%); prosthetic material, 3 (1.5%); and other, 37 (18.5%).
The yeasts were identified using the ID 32C system (bioMérieux).
BP was assessed using the crystal violet binding assay.3,7
Laboratory procedureIsolates were grown on Sabouraud dextrose agar for 24h at 37°C. Two or three colonies from each plate were inoculated into 20mL of yeast peptone dextrose (YPD) medium and incubated for 18h at 30°C on an orbital shaker. They were then centrifuged at 3500rpm for 5min, washed twice with 10mL of phosphate-buffered saline, and re-suspended in RPMI 1640 medium. After being standardized to 1×106CFU/mL in RPMI, 100μL of the suspension was placed in the wells of a 96-well, flat-bottomed microtiter plate and incubated for 24h at 37°C. The suspensions were discarded, and the wells were washed 3 times with sterile phosphate-buffered saline and filled with crystal violet for 15min. The wells were then washed and the residue solubilized with acetic acid. The suspension was transferred to clean wells, and absorbance was detected in the spectrophotometer at 550nm. Each experiment was performed in triplicate, and the average value was used for the analysis.
Statistical analysisThe qualitative variables appear with their frequency distributions. Values for continuous variables are expressed as the mean (SD) with a 95% confidence interval (95% CI) when applicable. Categorical variables were evaluated using the chi-square or 2-tailed Fisher exact test. Normally distributed continuous variables were compared using the t test. We determined the cut-offs for BP using a ROC curve. The Games–Howell test was used to compare BP between the species and between the types of samples. The comparison between BP and origin of the infection was made irrespective of the Candida species.
Statistical significance was set at p <0.05 (2-tailed). All statistical tests were performed using SPSS version 21.0.
EthicsThe study was approved by the local ethics committee.
ResultsOf the 155 patients, 129 (83.2%) had only 1 Candida species isolated in 1 clinical specimen and 26 (16.8%) had 2 or more isolates at the same or different sites (mean [SD] no. of isolates per patient, 1.26 [0.75]). Only 6 patients (3.9%) had the same Candida species in different clinical specimens.
After the analysis using the crystal violet assay, we determined the following cut-offs for BP in Candida strains: low, <1; moderate, 1–2; and high, >2.
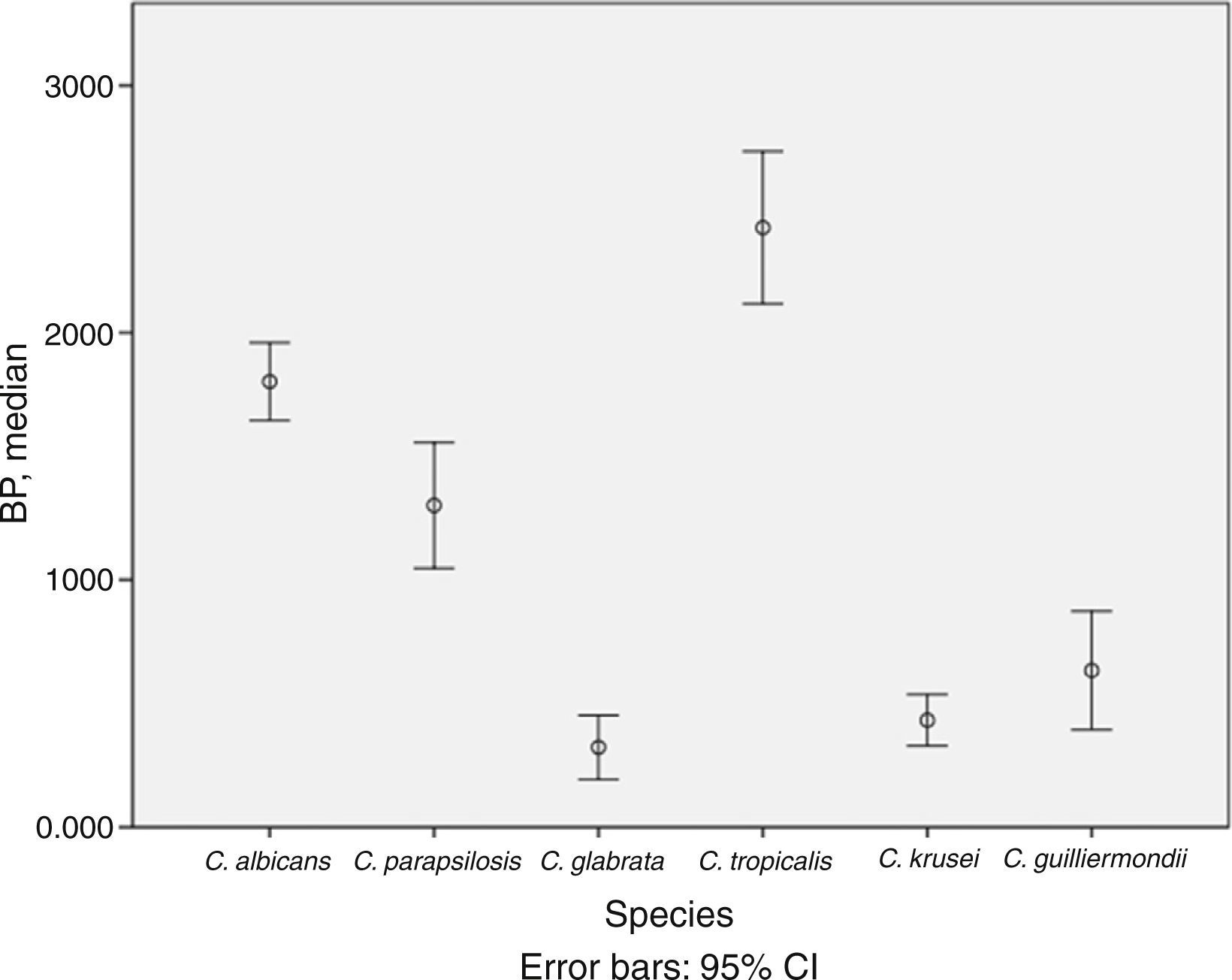
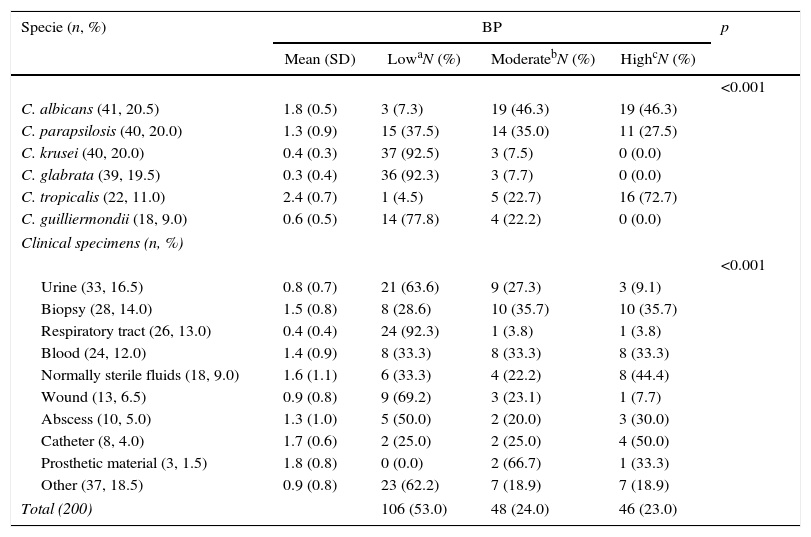
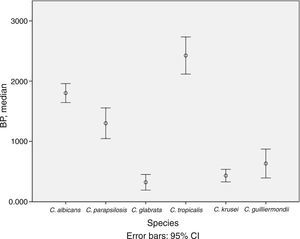
BP was low in 53.0% of isolates, moderate in 24.0%, and high in 23.0%. The mean (SD) BP for the different Candida species was as follows: C. albicans, 1.8 (0.5); C. parapsilosis, 1.3 (0.9); C. krusei, 0.4 (0.3); C. glabrata, 0.3 (0.4); C. tropicalis, 2.4 (0.7); and C. guilliermondii, 0.6 (0.5) (Table 1 and Fig. 1). Analysis of the degree of BP in the different Candida species showed that most C. albicans were high or moderate biofilm producers (92.6%) and that C. parapsilosis were mainly low or moderate biofilm producers (72.5%). In contrast, almost all C. krusei, C. glabrata, and C. guilliermondii were low biofilm producers (92.5%, 92.3%, and 77.8%, respectively). The highest BP values were found for C. tropicalis (72.7%).
Description of biofilm production according to Candida spp. and clinical specimens.
| Specie (n, %) | BP | p | |||
|---|---|---|---|---|---|
| Mean (SD) | LowaN (%) | ModeratebN (%) | HighcN (%) | ||
| <0.001 | |||||
| C. albicans (41, 20.5) | 1.8 (0.5) | 3 (7.3) | 19 (46.3) | 19 (46.3) | |
| C. parapsilosis (40, 20.0) | 1.3 (0.9) | 15 (37.5) | 14 (35.0) | 11 (27.5) | |
| C. krusei (40, 20.0) | 0.4 (0.3) | 37 (92.5) | 3 (7.5) | 0 (0.0) | |
| C. glabrata (39, 19.5) | 0.3 (0.4) | 36 (92.3) | 3 (7.7) | 0 (0.0) | |
| C. tropicalis (22, 11.0) | 2.4 (0.7) | 1 (4.5) | 5 (22.7) | 16 (72.7) | |
| C. guilliermondii (18, 9.0) | 0.6 (0.5) | 14 (77.8) | 4 (22.2) | 0 (0.0) | |
| Clinical specimens (n, %) | |||||
| <0.001 | |||||
| Urine (33, 16.5) | 0.8 (0.7) | 21 (63.6) | 9 (27.3) | 3 (9.1) | |
| Biopsy (28, 14.0) | 1.5 (0.8) | 8 (28.6) | 10 (35.7) | 10 (35.7) | |
| Respiratory tract (26, 13.0) | 0.4 (0.4) | 24 (92.3) | 1 (3.8) | 1 (3.8) | |
| Blood (24, 12.0) | 1.4 (0.9) | 8 (33.3) | 8 (33.3) | 8 (33.3) | |
| Normally sterile fluids (18, 9.0) | 1.6 (1.1) | 6 (33.3) | 4 (22.2) | 8 (44.4) | |
| Wound (13, 6.5) | 0.9 (0.8) | 9 (69.2) | 3 (23.1) | 1 (7.7) | |
| Abscess (10, 5.0) | 1.3 (1.0) | 5 (50.0) | 2 (20.0) | 3 (30.0) | |
| Catheter (8, 4.0) | 1.7 (0.6) | 2 (25.0) | 2 (25.0) | 4 (50.0) | |
| Prosthetic material (3, 1.5) | 1.8 (0.8) | 0 (0.0) | 2 (66.7) | 1 (33.3) | |
| Other (37, 18.5) | 0.9 (0.8) | 23 (62.2) | 7 (18.9) | 7 (18.9) | |
| Total (200) | 106 (53.0) | 48 (24.0) | 46 (23.0) | ||
BP, biofilm production; SD, standard deviation.
Comparison of Candida BP between clinical specimens revealed that high-biofilm-producing species were those colonizing normally sterile body fluids, biopsy specimens, and catheter samples, whereas low-biofilm-producing species of Candida strains were those colonizing urine and respiratory tract samples (Table 1). Comparison of BP according to the origin of the isolates revealed statistically significant differences only between BP in blood and respiratory tract samples (mean BP, 1.4 vs. 0.4; p=0.002) and between respiratory tract and sterile fluids (mean BP, 0.4 vs. 1.6; p=0.010), biopsy specimens (mean BP, 0.4 vs. 1.5; p<0.001), and catheter (mean BP, 0.4 vs. 1.7; p=0.009).
DiscussionBP in Candida varies with the species and is highest with C. tropicalis. In addition, higher BP correlates with deep-seated infection.
BP is an important virulence factor of Candida species, and the methodology used to test it has not been well standardized. A recent study by Marcos-Zambrano et al.3 was the first to assess the BP of Candida species isolated from blood by comparing biomass production and metabolic activity to establish tentative cut-offs so that isolates could be classed as low-, moderate-, or high-biofilm-producing. In our study, we used the crystal violet assay to detect BP and obtained similar cut-offs to classify Candida strains according to BP.
Analysis of differences in the degree of BP by Candida species revealed that C. tropicalis was the highest biofilm producer and that C. albicans and C. parapsilosis were moderate or high producers, as previously described.8,9
BP is highly dependent on the conditions under which the biofilm is formed (e.g., type of implanted device and its location); therefore, its values can differ between sites of infection.10,11 Shin et al.12 compared BP in Candida isolated from blood and from other anatomical sites and demonstrated that Candida species isolated from blood produced higher amounts of biofilm than Candida species isolated from other sites (57% vs. 32%, p<0.001). However, they did not classify BP as low, moderate, or high. In our study, we only found statistically significant differences in BP between blood and respiratory tract samples (mean BP, 1.4 vs. 0.4; p=0.002). Moreover, we showed that Candida species isolated from samples from patients with deep-seated infection (normally sterile fluids, biopsy specimens, and catheter samples) produced more biofilm than those isolated from urine and respiratory tract samples (mean BP, 1.5 vs. 0.8; p<0.001).
Therefore, our findings demonstrate that, as BP was higher among C. tropicalis and among clinical specimens from deep tissues, it may play a key role in clinical outcome by acting as a reservoir for re-infection. Early detection of Candida BP can be useful in predicting the severity of the infection and in clinical decision making.
One of the main limitations of the study was that the methodology for testing BP could have influenced the results, as we did not test BP in parallel with the metabolic activity of Candida biofilms. Moreover, as the methodologies differ between studies, the results may not be comparable. Another limitation was that we could not compare the differences between species according to the clinical specimens because of the small numbers of isolates in the subgroups. However, our study is the first in which Candida species isolated from several clinical samples are classified according to the degree of BP and in which BP was evaluated according to species of Candida and type of clinical specimens. Research is currently under way in studies based on the XTT assay and larger samples.
Our data demonstrate that BP varies according to the Candida species and the site of the infection. Consequently, the capacity of Candida species to produce biofilm may be a reflection of the pathogenic potential of the isolates.
Financial supportM. Guembe is supported by the Miguel Servet Program (ISCIII-MICINN) from the Health Research Fund (FIS), of the Carlos III Health Institute (ISCIII), Madrid Spain, partially financed by the by the European Regional Development Fund (FEDER) “A way of making Europe”. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interestThe authors declare no conflicts of interest.
We thank Thomas O’Boyle for his help in the preparation of the manuscript.