Viruses are one of the most common causes of community-acquired pneumonia (CAP) in children. Early identification of respiratory viruses could result in a decrease in the use of antibiotics.
MethodsObservational, retrospective study from January 2014 to June 2018, that included paediatric patients admitted with a diagnosis of CAP in a tertiary hospital, in which antigenic tests and/or viral PCR on a respiratory sample was performed.
ResultsA total of 105 CAP episodes were included, with identification of a respiratory virus in 93 (88.6%) cases. Patients with respiratory syncytial virus (RSV) detection had a lower onset of empirical antibiotic therapy (35.1% vs. 55.9%, p-value=.042). In addition, cases with RSV or influenza identification required shorter duration of antibiotic therapy (receiving 45.6% ≥2 days vs. 68.8% of those not identified, p=.017).
ConclusionThe use of respiratory virus diagnostic techniques in our setting can optimise antibiotic use in children admitted with CAP.
Los virus son una de las causas más frecuentes de neumonía adquirida en la comunidad (NAC) en niños. La identificación precoz de virus respiratorios podría suponer una disminución en el consumo de antibióticos.
MétodosEstudio observacional, retrospectivo, desde enero del 2014 hasta junio del 2018, que incluyó a los pacientes pediátricos ingresados en un hospital terciario con diagnóstico de NAC, a los que se realizó test antigénico o PCR viral en muestra respiratoria.
ResultadosSe incluyeron 105 episodios de NAC, identificándose algún virus respiratorio en 93 (88,6%) casos. Los pacientes con detección de virus respiratorio sincitial (VRS) presentaron menor inicio de antibioterapia empírica (35,1% vs. 55,9%, p valour: 0,042). Además, los casos con identificación de VRS o influenza precisaron menor duración de antibioterapia (recibiendo el 45,6% ≥2 días frente al 68,8% de los que no se identificó, p=0,017).
ConclusiónEl uso de técnicas diagnósticas de virus respiratorios en nuestro medio puede optimizar el consumo de antibióticos en niños ingresados con NAC.
Pneumonia is a significant cause of morbidity in children under 5 years of age.1 Viruses represent one of the main aetiologies of community-acquired pneumonia (CAP), especially in children under 2 years of age, being identified in 83% of CAP in our setting.2 The increase in the incidence of infections by resistant microorganisms has increased interest in streamlining the prescription of antibiotics, advocating their use in cases with suspected or confirmed viral aetiology. The development of new microbiological diagnostic techniques that allow the identification of the causative microorganism in less time is allowing the optimisation of antimicrobial consumption. These tests include rapid virological diagnostic techniques in respiratory samples.3
There are conflicting data in the literature on the benefit of these techniques in the practice of paediatric patients. Several studies4–6 have demonstrated its usefulness in children by reducing the use of resources (performing complementary tests, duration of antibiotherapy, etc.), although others7,8 have not shown any benefit.
The main objective of this study was to analyse the impact of microbiological diagnostic tests of respiratory viruses on the use of antibiotics in paediatric patients admitted for CAP in a tertiary hospital.
Material and methodsAn observational, retrospective, single-centre study was conducted. Paediatric patients (<16 years old) who met the following criteria were included:
Admission between 01/01/2014 and 30/06/2018 with diagnosis of CAP, with radiographic evidence of pneumonia9: consolidation (pulmonary opacity with or without bronchogram), other infiltrates (interstitial densities) or pleural effusion. Community-acquired was considered to mean appearing at home (in the case of previous hospitalisation, more than 7 days after hospital discharge) or within the first 48h of hospital admission.
The performance during the first 48h of the admission of any of the following virological determinations in respiratory samples (exudate or nasopharyngeal aspirate):
- (a)
Multiplex polymerase chain reaction panel (mPCR) and subsequent visualisation using low-density arrays with CLART® technology (Clart® Pneumovir, Genómica S.A.U., Madrid, Spain), which allows the detection of the following respiratory viruses: respiratory syncytial virus (VRS) A and B, influenza A (subtypes H1N1/2009, H1N1 and H3N2), B and C, rhinovirus, adenovirus, metapneumovirus A and B, parainfluenza 1, 2, 3 and 4, bocavirus and coronavirus.
- (b)
Rapid diagnostic tests (RDT): antigenic detection by VRS TRU RSV® (Meridian Bioscience Inc., Palex Diagnóstica, Madrid, Spain) or isothermal amplification of influenza virus (Alere-i, Abbot, Spain) of influenza virus, with a positive result if not performed, and mPCR.
At the study site, during influenza and RSV epidemics, a TDR of RSV or influenza in respiratory samples is performed by protocol, according to the epidemic virus, and the result of which is usually obtained in <24h. If the tests are negative, viral mPCR is performed on respiratory samples. Outside of epidemic periods, mPCR is performed at the paediatrician's discretion.
Patients in whom pneumonia had been diagnosed in the preceding 30 days were excluded. Patients’ medical records were reviewed to obtain demographic, clinical, treatment and evolution information. The study was approved by the study site's Ethics Committee.
Descriptive statistics are presented as absolute frequencies or percentages in the case of categorical variables, and continuous variables as medians and interquartile ranges (IQR). Categorical variables were compared using the χ2 test or Fisher's test, as appropriate, and continuous variables using the Mann–Whitney U test. STATA® analysis software, version 15.1 (StataCorp, Texas, United States), was used for analysis. A value of p<0.05 was considered significant.
Results105 episodes of CAP were included (5 subjects presented 2 different episodes), 55 (52.4%) girls, median age of 21.5 months (RIC: 11.9–40.6). As for medical history, 27 (25.7%) presented recurrent bronchospasm, 11 (10.5%) prematurity and 12 (11.4%) other pathological histories.
An RSV test was performed in 73 (69.5%) cases, an influenza test was performed in 72 (68.6%), and mPCR was performed in 67 (63.8%). The 3 tests were performed in 38 (36.2%) cases, and RSV and influenza in 19 (18.1%). The RSV and influenza tests were performed in all cases within ≤48h of admission, and the mPCR was performed >48h of admission in only one case. In all cases, the result of the RSV and influenza tests was obtained within <24h, while only in 3/67 (4.5%) cases was the result of mPCR obtained within ≤48h of admission.
In 93 (88.6%) episodes some respiratory virus was identified. The most frequent was RSV in 37 (35.2%) children, followed by influenza in 21 (20%) children, rhinovirus in 15 (14.3%), metapneumovirus in 13 (12.4%) and others in 29 (27.6%). In 76 (72.4%) cases a single virus was identified, in 13 (12.4%) cases, 2 different viruses, in 3 (2.9%) cases, 3 viruses and in one case (1%), 4 viruses. The most frequent viral coinfections were rhinoviruses with bocavirus in 3 cases (3.2%) and RSV with adenovirus in 2 cases (2.2%).
Blood cultures were extracted in 79 (75.2%) children, isolating a microorganism considered pathogenic (Streptococcus pneumoniae) in just one case. Five (4.8%) cases associated pleural effusion, without requiring any drainage.
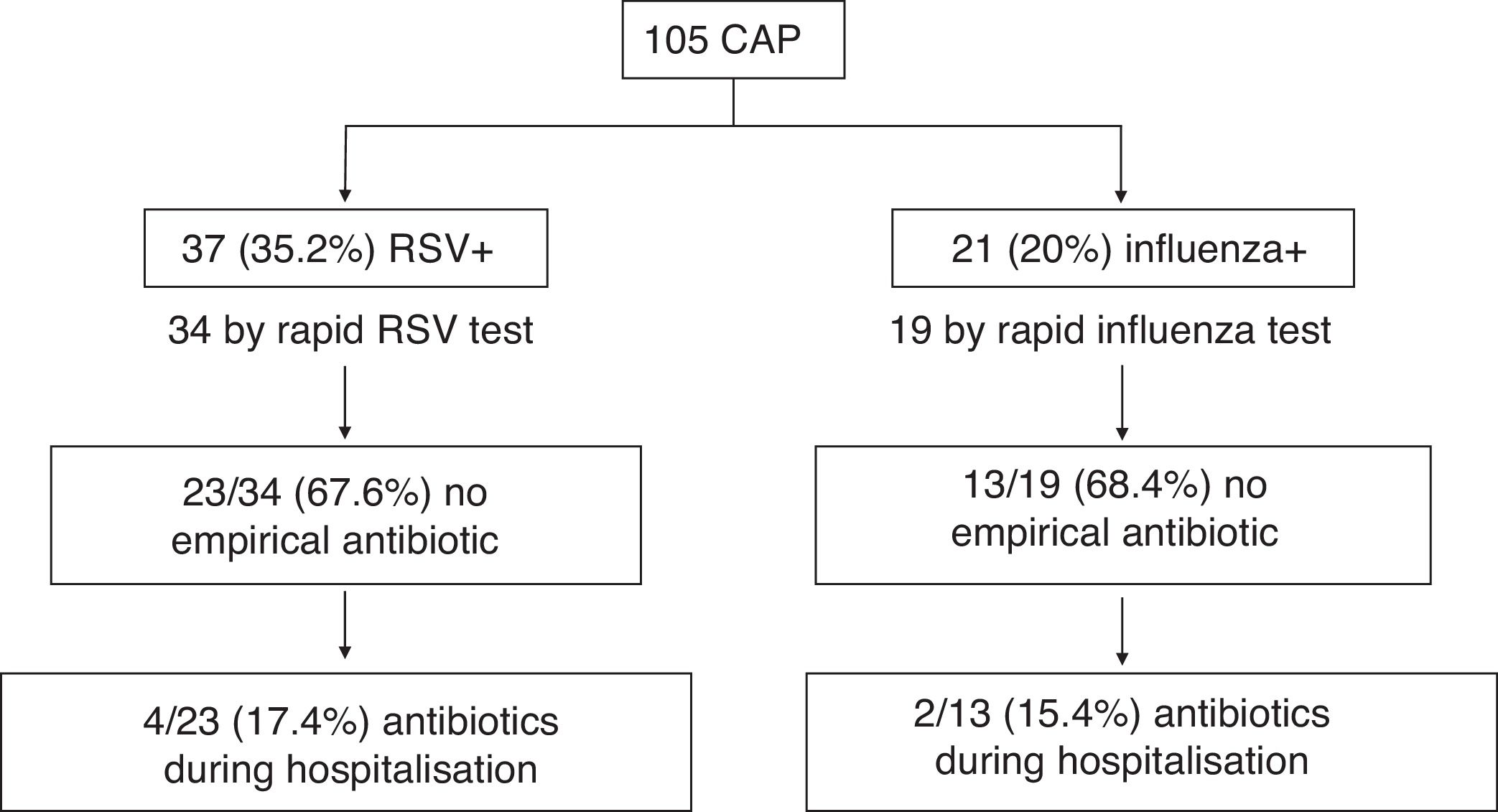
Fig. 1 shows the start of empirical antibiotic therapy according to the detection of RSV or influenza by TDR and the subsequent start during hospitalisation in cases where it was not empirically initiated. 55.9 and 57.9% of pneumonias with RSV or influenza detection by TDR, respectively, of the total CAPs with TDR, received no antibiotic therapy. Table 1 compares the characteristics, including initiation of antibiotic therapy and its duration, in the different groups according to the identification of different respiratory viruses. There is a notable decrease in the start of antibiotic therapy in cases with RSV detection as compared to those that were not detected (35.1% vs. 55.9%, OR: 0.43 [95% CI: 0.19–0.98]; p=0.042). In addition, cases with identification of RSV or influenza received a shorter duration of antibiotic therapy, receiving 45.6% ≥2 days compared to 68.8% of those which were not identified (OR: 0.38 [95% CI: 0.17–0.85]; p=0.017).
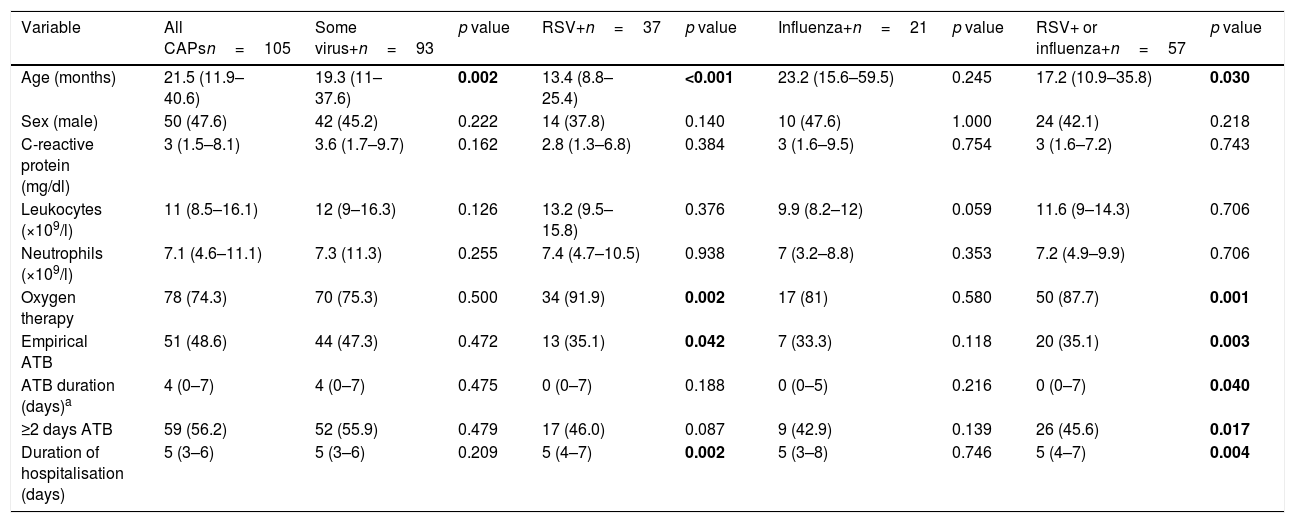
Comparison between community acquired pneumonias based on the identification of different respiratory viruses.
| Variable | All CAPsn=105 | Some virus+n=93 | p value | RSV+n=37 | p value | Influenza+n=21 | p value | RSV+ or influenza+n=57 | p value |
|---|---|---|---|---|---|---|---|---|---|
| Age (months) | 21.5 (11.9–40.6) | 19.3 (11–37.6) | 0.002 | 13.4 (8.8–25.4) | <0.001 | 23.2 (15.6–59.5) | 0.245 | 17.2 (10.9–35.8) | 0.030 |
| Sex (male) | 50 (47.6) | 42 (45.2) | 0.222 | 14 (37.8) | 0.140 | 10 (47.6) | 1.000 | 24 (42.1) | 0.218 |
| C-reactive protein (mg/dl) | 3 (1.5–8.1) | 3.6 (1.7–9.7) | 0.162 | 2.8 (1.3–6.8) | 0.384 | 3 (1.6–9.5) | 0.754 | 3 (1.6–7.2) | 0.743 |
| Leukocytes (×109/l) | 11 (8.5–16.1) | 12 (9–16.3) | 0.126 | 13.2 (9.5–15.8) | 0.376 | 9.9 (8.2–12) | 0.059 | 11.6 (9–14.3) | 0.706 |
| Neutrophils (×109/l) | 7.1 (4.6–11.1) | 7.3 (11.3) | 0.255 | 7.4 (4.7–10.5) | 0.938 | 7 (3.2–8.8) | 0.353 | 7.2 (4.9–9.9) | 0.706 |
| Oxygen therapy | 78 (74.3) | 70 (75.3) | 0.500 | 34 (91.9) | 0.002 | 17 (81) | 0.580 | 50 (87.7) | 0.001 |
| Empirical ATB | 51 (48.6) | 44 (47.3) | 0.472 | 13 (35.1) | 0.042 | 7 (33.3) | 0.118 | 20 (35.1) | 0.003 |
| ATB duration (days)a | 4 (0–7) | 4 (0–7) | 0.475 | 0 (0–7) | 0.188 | 0 (0–5) | 0.216 | 0 (0–7) | 0.040 |
| ≥2 days ATB | 59 (56.2) | 52 (55.9) | 0.479 | 17 (46.0) | 0.087 | 9 (42.9) | 0.139 | 26 (45.6) | 0.017 |
| Duration of hospitalisation (days) | 5 (3–6) | 5 (3–6) | 0.209 | 5 (4–7) | 0.002 | 5 (3–8) | 0.746 | 5 (4–7) | 0.004 |
Continuous variables are expressed as median (interquartile range) and categorical variables as absolute frequencies (percentage). The p value is calculated using the CAP group as a reference, with a negative result in each of the viruses evaluated.
In bold: statistically significant differences.
CAP: community acquired pneumonia; RSV: respiratory syncytial virus. RSV: respiratory syncytial virus.
In this study, conducted in children admitted for CAP, we observed a lower use of antibiotics in those with an early detection of RSV in a respiratory sample. Similarly, we observed a shorter duration of antibiotic therapy in those in whom RSV or influenza was detected. However, no differences were found with the detection of other respiratory viruses. The lack of impact of the identification of other respiratory viruses in antibiotic therapy may be due to excessive time until the result is obtained.
Several previous studies have evaluated the consumption of antibiotics through the use of respiratory virus tests. Similar to our results, the identification of RSV in hospitalised children has been associated with lower antibiotic use.10 The study by Subramony et al.6 compared the period prior to the incorporation of an mPCR respiratory panel with the consecutive period, demonstrating a decrease in the duration of antibiotic therapy, the performance of chest radiographs and the greater use of isolation precautions. Similarly, the study by Schulert et al.4 demonstrated a decrease in the duration of intravenous antibiotic therapy in children with pneumonia and identification of a respiratory virus through an mPCR panel.
However, other similar studies have demonstrated no significant impact on the decrease in antibiotic use in children admitted for CAP8 or other respiratory infections.7
One of the difficulties in interpreting the detection of a respiratory virus in respiratory samples is establishing the causal relationship with the clinical syndrome. Several studies11,12 in which the detection of respiratory viruses in children with CAP was compared with asymptomatic controls have shown a greater association with the identification of influenza, parainfluenza, RSV and metapneumovirus viruses, and the diagnosis of pneumonia.
On the other hand, the identification of a respiratory virus does not exclude bacterial coinfection,13 with bacterial coinfection with some viruses having been described as a factor associated with greater severity.14 A recent study15 evaluated the use of C-reactive protein to differentiate bacterial pneumonias from pneumonia produced by RSV in children <5 years of age, demonstrating an adequate discriminative capacity.
The limitations of the study are its retrospective nature and the difficult assessment of the detection of respiratory viruses to differentiate infection colonisation in this population. In addition, the insufficient statistical power due to the small sample size precludes the demonstration of significant differences in several comparisons made. As advantages, we would highlight the use of strict inclusion criteria that allow increasing sensitivity in the detection of respiratory viruses, together with specific radiological criteria. Additionally, the non-interventionist methodology followed allows the evaluation of the use of these techniques in routine clinical practice.
In conclusion, the use of respiratory virus diagnostic techniques in our setting can optimise the use of antibiotics in children admitted with CAP. The decrease in the execution times of the mPCR panels could offer an additional benefit, by allowing the duration of antibiotic therapy to be shortened. Moreover, the use of predictive models, which take into account the detection of respiratory viruses along with clinical and analytical parameters, could improve the identification of children with CAP in whom the use of antibiotics can be safely dispensed with.
Conflicts of interestWe declare that we have no conflicts of interest.
Please cite this article as: Aguilera-Alonso D, Illán-Ramos M, Daoud Z, Guinea V, Culebras E, Ramos JT. Análisis del impacto de los test de diagnóstico virológico en el consumo de antibióticos en pacientes pediátricos ingresados por neumonía adquirida en la comunidad. Enferm Infecc Microbiol Clin. 2020;38:230–233.