The aim of our study was to describe and evaluate the clinical and metabolic characteristics of patients with MODY-3, MODY-2 or type 2 diabetes who presented I27L polymorphism in the HNF1α gene.
MethodsThe study included 31 previously diagnosed subjects under follow-up for MODY-3 (10 subjects from 5 families), MODY-2 (15 subjects from 9 families), or type 2 diabetes (6 subjects) with I27L polymorphism in the HNF1α gene. The demographic, clinical, metabolic, and genetic characteristics of all patients were analyzed.
ResultsNo differences were observed in distribution according to sex, age of onset, or form of diagnosis. All patients with MODY-2 or MODY-3 had a family history of diabetes. In contrast, 33.3% of patients with type 2 diabetes mellitus and I27L polymorphism in the HNF1α gene had no family history of diabetes (p < 0.05). No differences were observed in body mass index, prevalence of hypertension, or microvascular or macrovascular complications. Drug therapy was required by 100% of MODY-3 patients, but not required by 100% of MODY-2 patients or 16.7% of patients with type 2 diabetes mellitus and I27L polymorphism in the HNF1α gene (p < 0.05).
ConclusionsOccasional difficulties may be encountered when classifying patients with MODY-2, MODY-3 or type 2 diabetes of atypical characteristics, in this case patients who present I27L polymorphism in the HNF1α gene.
El objetivo de este estudio es describir y evaluar las características clínicas y metabólicas de pacientes diabéticos MODY 3, MODY 2 y con diabetes tipo 2 portadores del polimorfismo I27L en el gen HNF1α.
MétodosSe incluyó a 31 pacientes diagnosticados previamente y en seguimiento en consultas externas por diabetes tipo MODY 3, MODY 2 y diabetes tipo 2 portadores del polimorfismo I27L en el gen HNF1α: 10 pacientes diagnosticados de diabetes MODY 3 (pertenecientes a 5 familias); 15 pacientes con diabetes MODY 2 (pertenecientes a 9 familias) y 6 pacientes diagnosticados de diabetes tipo 2 portadores del polimorfismo I27L en el gen HNF1α. Se analizan las características clínicas, antropométricas y metabólicas de los pacientes.
ResultadosNo se objetivaron diferencias en la distribución por sexos y edad o forma de diagnóstico de la diabetes. Todos los pacientes con diabetes MODY 2 y MODY 3 tenían antecedentes familiares de diabetes. El 33,3% de los pacientes con diabetes tipo 2 portadores del polimorfismo I27L en el gen HNF1α no tenían antecedentes familiares de diabetes (p > 0,05). No se encontraron diferencias en el IMC, la prevalencia de hipertensión arterial o la incidencia de complicaciones microvasculares o macrovasculares. En cuanto al tratamiento, el 100% de los pacientes con diabetes MODY 3 necesitaban tratamiento farmacológico. El 100% de los pacientes con diabetes MODY 2 y el 16,7% de los pacientes con diabetes tipo 2 y el polimorfismo I27L en el gen HNF1α no necesitaban tratamiento farmacológico (p > 0,05).
ConclusionesEste artículo realza la dificultad en la correcta clasificación clínica de los pacientes con diabetes MODY 2, MODY 3 y diabéticos tipo 2 con características clínicas atípicas, en este caso portadores del polimorfismo I27L en el gen HNF1α.
Diabetes mellitus has been traditionally classified according to the age of symptom onset (juvenile diabetes and adultonset diabetes). Later, in 1979, the National Diabetes Data Group of the National Institutes of Health proposed a classification system based on the patient's therapeutic needs (insulin-dependent diabetes mellitus or non-insulindependent diabetes mellitus). In 1997, an international committee of experts analyzed the knowledge accumulated thus far and recommended a new classification based on the etiology and pathogenesis of diabetes which was subsequently endorsed by the American Diabetes Association and the World Health Organization1.
This diabetes mellitus classification, in force today, includes 2 main groups of diabetes (type 1 diabetes mellitus and type 2 diabetes mellitus), a third group known as other types of diabetes, and a fourth group that includes gestational diabetes. The third group of the current classification includes genetic defects that affect β-cell function, including maturity-onset diabetes of the young (MODY). Since 1991, the genetic basis of various subtypes of monogenic diabetes (which may explain the onset of diabetes in a young patient) has been described2-9.
At the present time, MODY-2 (Mendelian Inheritance in Man 125851) and MODY-3 (Mendelian Inheritance in Man 600496) are the most common monogenic diabetes, although the relative frequency varies according to the study population. In Spain, Costa et al10 observed a higher relative frequency of MODY-3. A later study by Barrio et al11 carried out in children reported a higher relative frequency of MODY-2, and Estalella et al12 found a higher relative frequency of MODY-2. However, no prevalence studies have been conducted to ascertain the relative frequencies of each subtype of MODY and, therefore, estimates must be used13.
Despite the importance of proper classification, it is not always easy to classify the juvenile-onset diabetes patients for several reasons. Onset of type 1 diabetes still accounts for a high proportion of the total cases in the intermedíate ages14, the increase in the prevalence of obesity is leading to an increase in the incidence of type 2 diabetes at increasingly younger ages15, and further developments are being made in the diagnosis of specific forms of diabetes associated with genetic defects13.
Another aspect to consider is the emergence in recent years of common genetic variants from normality that cannot themselves explain the disease, but do represent a mild-to-moderate risk of developing type 2 diabetes in some cases16-19. Specifically, the I27L amino acid polymorphism in the hepatocyte nuclear factor 1α (HNF1α) gene has been associated with increased insulin resistance in the hyperglycemic clamp20 and has been confirmed as a polymorphic variant of risk for type 2 diabetes in population studies21. In our experience, this polymorphism is a common finding in patients studied because of unusual features for type 1 and type 2 diabetes in which the presence of mutations consistent with the diagnosis of MODY-2 or MODY-3 is not confirmed.
The aim of our study was to describe and compare clinical and metabolic characteristics of patients with MODY-2, MODY-3 or type 2 diabetes with I27L polymorphism in the HNFIα gene.
Patients and methodsThe study was based on a retrospective design and included 31 diabetic patients who had been diagnosed with monogenic diabetes MODY-3, MODY-2, or type 2 diabetes and in whom the I27L polymorphism in the HNFIα gene had been observed in a genetic study of the 10 coding exons and the promoter region of the GCK gene and the 10 coding exons and promoter region of the HNFIα gene. The genetic study had been carried out in all patients due to atypical features for type 1 and type 2 diabetes. The patients were recruited from the outpatient clinic of the endocrinology and nutrition section at the General Hospital of Albacete (patients followed by pediatrics were not included), the General Hospital of Villarrobledo, the General Hospital of Almansa, and the Hospital of Hellín (Albacete, Spain), which represent a catchment area of nearly 400 000 people.
All genetic studies were performed using 5-10 ml of uncoagulated whole blood with ethylenediaminetetraacetic acid (EDTA), drawn from each patient at the Human Genetic Laboratory, School of Medicine of Albacete (University of Castilla-La Mancha) by automated sequencing of the 10 coding exons (1a, 2-10) and the promoter region (−1 to −870) of the GCK gene (MODY-2) and the 10 coding exons and promoter region (−1 to −291) of the HNFIα gene (MODY-3). All patients were informed of the aims and possible uses of the genetic study and gave written informed consent.
The following variables were collected at the last followup visit for all subjects: sex, familial aggregation of diabetes, age at diagnosis, reason for suspicion of diagnosis –including: a) genetic study due to monogenic diabetes diagnosis in a familial index-case; b) incidental finding in a laboratory test; c) diagnosis based on the features of diabetes, and d) gestational diabetes–, body mass index (BMI), calculated as weight (in kg) / height2 (in m), diagnosis of hypertension (blood pressure ≥ 130/80 mmHg in at least two measurements or need for antihypertensive medication), glycated hemoglobin (HbA1c) (determined by highperformance liquid chromatography), high-density lipoprotein cholesterol (HDLc) and triglycerides (determined by enzymatic colorimetric assay), current patient treatment –including: a) diet and exercise; b) oral agents; c) oral agents and insulin, or d) insulin–, presence of microvascular –retinopathy, nephropathy and diabetic neuropathy (yes/no)– and macrovascular complications (yes/no), and genetic diagnosis.
A descriptive data analysis was then performed, and quantitative variables were expressed as mean ± SD and qualitative variables as percentages. The Kruskal-Wallis nonparametric test was used to compare quantitative variables between the 3 groups and the χ2 test to compare proportions. All data were analyzed using SPSS 7.5 for Windows.
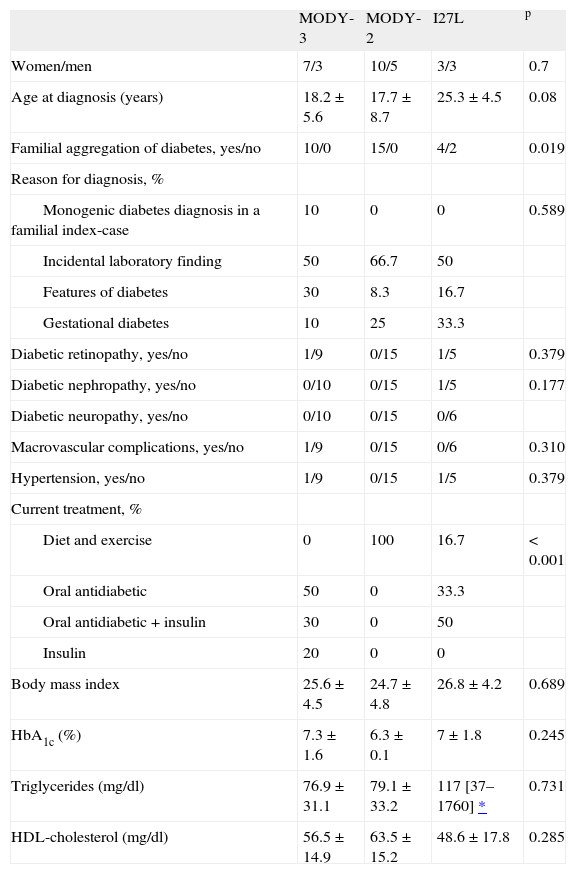
ResultsI27L polymorphism in the HNFIα gene was found in 10 subjects with MODY-3, 15 with MODY-2, and 6 with type 2 diabetes mellitus. The clinical characteristics of all subjects are shown in table 1.
Clinical features of the patients
| MODY-3 | MODY-2 | I27L | p | |
| Women/men | 7/3 | 10/5 | 3/3 | 0.7 |
| Age at diagnosis (years) | 18.2 ± 5.6 | 17.7 ± 8.7 | 25.3 ± 4.5 | 0.08 |
| Familial aggregation of diabetes, yes/no | 10/0 | 15/0 | 4/2 | 0.019 |
| Reason for diagnosis, % | ||||
| Monogenic diabetes diagnosis in a familial index-case | 10 | 0 | 0 | 0.589 |
| Incidental laboratory finding | 50 | 66.7 | 50 | |
| Features of diabetes | 30 | 8.3 | 16.7 | |
| Gestational diabetes | 10 | 25 | 33.3 | |
| Diabetic retinopathy, yes/no | 1/9 | 0/15 | 1/5 | 0.379 |
| Diabetic nephropathy, yes/no | 0/10 | 0/15 | 1/5 | 0.177 |
| Diabetic neuropathy, yes/no | 0/10 | 0/15 | 0/6 | |
| Macrovascular complications, yes/no | 1/9 | 0/15 | 0/6 | 0.310 |
| Hypertension, yes/no | 1/9 | 0/15 | 1/5 | 0.379 |
| Current treatment, % | ||||
| Diet and exercise | 0 | 100 | 16.7 | < 0.001 |
| Oral antidiabetic | 50 | 0 | 33.3 | |
| Oral antidiabetic + insulin | 30 | 0 | 50 | |
| Insulin | 20 | 0 | 0 | |
| Body mass index | 25.6 ± 4.5 | 24.7 ± 4.8 | 26.8 ± 4.2 | 0.689 |
| HbA1c (%) | 7.3 ± 1.6 | 6.3 ± 0.1 | 7 ± 1.8 | 0.245 |
| Triglycerides (mg/dl) | 76.9 ± 31.1 | 79.1 ± 33.2 | 117 [37–1760] * | 0.731 |
| HDL-cholesterol (mg/dl) | 56.5 ± 14.9 | 63.5 ± 15.2 | 48.6 ± 17.8 | 0.285 |
Because the subjects were presumed to be potentially from the same family, a relationship was found once pedigree was assessed for three generations. The MODY-2 group included 15 patients from 9 different families, whereas the MODY-3 group included 10 subjects from 5 different families.
In the case of type 2 diabetes with I27L polymorphism, the group included 6 patients. All genetic studies were performed because the patient presented atypical features for type 1 and type 2 diabetes (young, nonobese adults with no insulin resistance data or young patients with no insulin requirement and with C-peptide levels within normal limits).
Because the study populations were small, the study failed to show statistically significant differences between groups regarding sex, diagnosis, presence of microvascular and macrovascular complications, and presence of arterial hypertension. Although the BMI and average age were slightly higher in patients with type 2 diabetes, the difference was not statistically significant. In addition, no statistically significant differences were found between groups in HbA1c, triglyceride, or HDLc levels. Nonetheless, significant differences were observed in the percentage of patients with no familial aggregation of diabetes (2 of 6 patients with type 2 diabetes had no familial aggregation). There were also significant differences in treatment: 100% of MODY-2 cases and 16.7% of type 2 diabetes cases were managed with diet and exercise while the MODY-3 group had no patients treated by diet and exercise alone.
DiscussionIn 1997, an international committee of experts analyzed the knowledge accumulated to date and recommended a new classification based on the etiology and pathogenesis of diabetes, which was endorsed by the American Diabetes Association and the World Health Organization1.
This diabetes mellitus classification is still used and includes genetic defects that affect (β-cell function including MODY within the third group2-9. In Spain, MODY-2 and MODY-3 are the most common monogenic diabetes, although the percentage varies according to the population studied10-12. It is believed that the increased frequency of MODY-2 found in pediatric studies may be explained because MODY-3 patients may manifest the disease at somewhat older ages. Therefore, studies in adults should provide a more realistic approximation of the different relative frequencies among the MODY subtypes.
In contrast with previous data obtained from the Spanish adult population10-12, our study found a higher relative frequency of patients with MODY-2 (9 families) than MODY-3 (5 families). We believe this discrepancy is due to differences in recruitment or clinical suspicion rather than to genetic load between different populations.
A notable limitation of our study is that the genetic study of patients with the I27L polymorphism in the HNF1α gene was not extended to other MODY subtypes. However, the clinical characteristics of these patients (no renal defect that might suggest MODY-522) and the low prevalence in Spain of other MODY subtypes (eg, MODY-110-12) led us to believe that patients were more likely to present type 2 diabetes with atypical features than other types of MODY.
Earlier studies have already assessed and clearly defined the clinical differences between patients with MODY-2 and MODY-310-13. Hence, our interest focused on assessing the difficulty of clinically documenting a specific case with diabetes onset at intermediate ages (between 20 and 30 years), a situation presenting a wide range of diagnostic possibilities (type 1, type 2, or MODY diabetes). In our study, we analyzed the clinical and metabolic characteristics of patients with MODY-2, MODY-3, and type 2 diabetes mellitus with atypical features and I27L polymorphism in the HNF1α gene. The present study was unable to infer the relationship between the presence of this polymorphism and the presence of atypical features; hence, a specific design to assess this possibility is currently being prepared. A pilot study by Conget et al23 assessed the clinical differences between patients with MODY-3 and young patients with type 2 diabetes and found significant differences in HDLc levels, triglycerides, and BMI. However, the clinical characteristics of patients with type 2 diabetes were considerably different in the patients included in our study (mean age, 52 vs 25 years; BMI, 31 vs 26; triglycerides, 184 vs 117 mg/dl; HDLc, 43 vs 48 mg/dl), which explains the absence of significant differences.
The monogenic diabetes working group of the Spanish Society of Diabetes establishes several conditions for studying MODY in addition to the presence of diabetes: a) presence of an autosomal dominant inheritance (at least 2 generations); b) at least 1 subject diagnosed before age 25, and c) no need for insulin or normal levels of C-peptide for at least 5 years after diagnosis. In 2008, Murphy et al13 reviewed the literature and recommended that HNF1α mutation be ruled out in young adults with features of type 1 diabetes when a parent had similar characteristics, antibodies were negative at diagnosis, and particularly if C-peptide response was preserved. These authors also recommended ruling out monogenic diabetes mellitus in young adults who apparently have type 2 diabetes but are not obese and have no other insulin-resistance features.
Our study demonstrates the importance of familial aggregation in excluding MODY-2 and MODY-3 (100% of patients with MODY-3 or MODY-2 had familial aggregation and neither patient without familial aggregation presented MODY) and the limitation of other clinical factors in differentiating MODY-2, MODY-3, and type 2 diabetes with atypical features (age at diagnosis, reason for diagnosis, BMI, and laboratory workup results similar in all groups).
To Maria Luisa Casas Oñate and Dolores Montoya Martínez, nurses of the endocrinology and nutrition outpatient clinic, for their assistance.
To the Castilla-La Mancha Foundation for Diabetes (FUCAMDI), for financial aid provided to the Laboratory of Human Genetics, School of Medicine of Albacete.