Smoking remains the leading cause of morbidity and mortality worldwide. Because of its clear influence on cardiovascular and respiratory diseases, it is an important factor in internal medicine consultations. Although the rate of smoking cessation has been increasing in recent years, there is a percentage of patients who continue to smoke because they are unable or unwilling to quit, despite having tried existing pharmacological and non-pharmacological therapies. For this group of patients there are strategies based on interventions aimed at reducing the negative effects of smoking without the need for complete cessation. In this review it is shown that due to the absence of combustion of organic matter in conventional cigarettes, snus, e-cigarettes and heated tobacco products generate significantly lower levels of toxic substances.
El tabaquismo sigue siendo la principal causa de morbimortalidad a nivel mundial. Por su clara influencia en las enfermedades cardiovasculares y respiratorias es un factor importante en la consulta de medicina interna. Aunque la tasa de abandono del hábito tabáquico esta ascendiendo en los últimos años, existe un porcentaje de pacientes que continúan fumando porque no pueden o no quieren cesar el hábito, a pesar de haber probado las terapias farmacológicas y no farmacológicas existentes. Para este grupo de paciente existen unas estrategias que se basan en intervenciones destinadas a reducir los efectos negativos del tabaco sin la necesidad de extinguir por completo su consumo. En esta revisión se contempla como gracias a la ausencia de combustión de la materia orgánica que se da en el cigarrillo convencional, en snus, cigarrillo electrónico y productos de tabaco calentado se genera un nivel significativamente inferior de sustancias tóxicas.
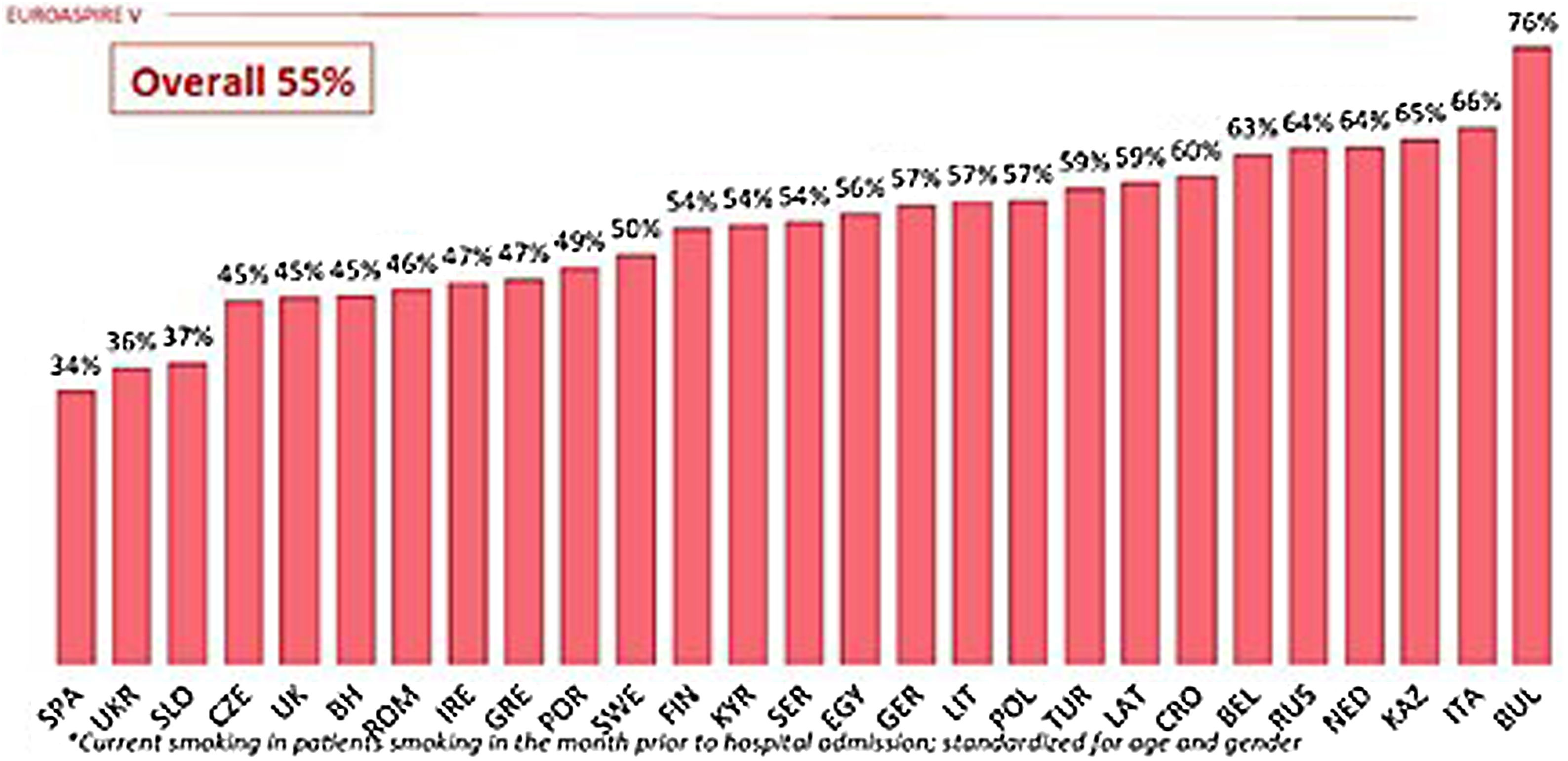
The latest WHO report indicates that between 2000 and 2015 there was a decline in tobacco use, with the global percentage of smokers falling from 33.3% to 24.9%. The same report also stated that estimates for the year 2025 stood at 20.9% of the world's population.1 Despite this reduction, smoking remains the leading cause of morbidity and mortality worldwide.2 Despite the implementation of legislative measures on smoking, which in many cases are coercive, a significant percentage of people, both sick and healthy, continue to smoke. However, although Spain is among the countries with the lowest rates of smokers at high or very high cardiovascular risk, data from the EUROASPIRE V survey indicate that 34% of these patients continue to smoke (Fig. 1).3
Prevalence of smoking among patients who smoked in the month prior to hospitalisation due to a cardiovascular event.3
Variations in smoking prevalence in the population are marked by initiation, cessation, and relapse rates. These rates should therefore be considered when assessing and planning smoking control policies and future strategies.4 Apart from concerns regarding the high prevalence of smoking and low cessation rates, the impact of smoking on individual and collective health (active and passive smokers) should be highlighted.5
Smoking plays a fundamental role in increasing the incidence of cardiovascular diseases, and its treatment should therefore be made a priority in plans for the prevention of these diseases.6 In fact, smoking is a major preventable cause of morbidity and mortality, and a factor that increases the risk of developing these diseases.7 However, the impact of the habit on cardiovascular disease is often underestimated. This results in treatments that focus on the disease, overlooking the implementation of measures to promote smoking cessation in patients. Smoking cessation has been shown to have a rapid and significant cardiovascular benefit for smokers, making it the most cost-effective intervention in the prevention of cardiovascular disease.8 For patients at high cardiovascular risk, this measure has greater efficacy compared to the prescription of statins, aspirin, angiotensin-converting enzyme inhibitors, or beta-blockers, drugs whose efficacy is supported by extensive scientific evidence. Lastly, there is the harm that smoking causes passive smokers by increasing their cardiovascular risk.
Cigarette combustion is the main basis for the damage caused by tobacco. This process results in the emission of more than 7000 substances, of which approximately 100 have been classified as harmful or potentially harmful to health. Tobacco ignites at temperatures of about 800 °C. Data show that the production of harmful constituents increases with temperature. However, at temperatures below 400 °C the formation of these compounds decreases significantly.
Clinical trials evaluating the efficacy of different interventions aimed at smoking cessation show that the combination of pharmacological and non-pharmacological measures is the most effective. Treatments in the first group include nicotine replacement therapies, varenicline, bupropion, nortriptyline, or cytisine, and those in the second group comprise approaches ranging from brief counselling to behavioural support.9
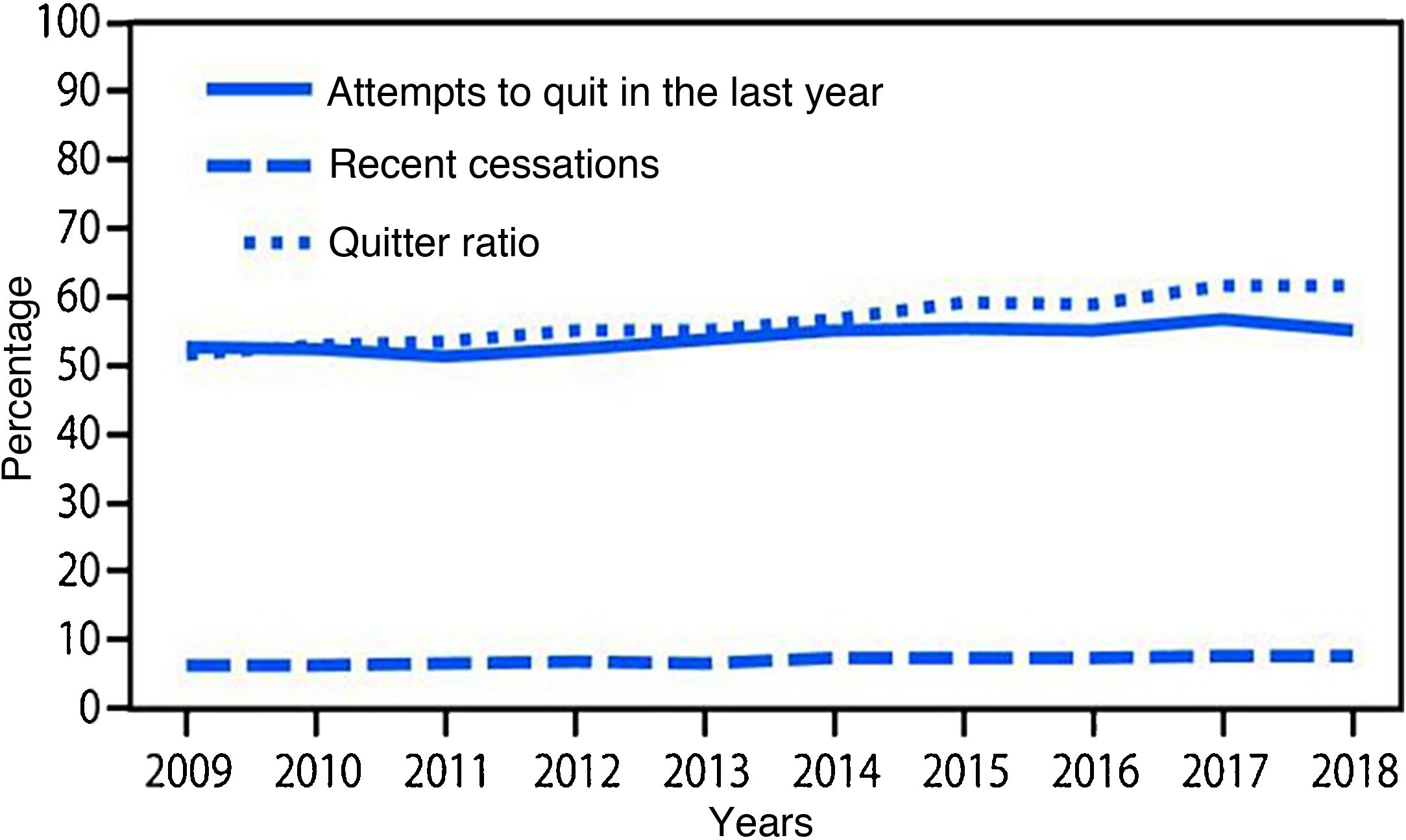
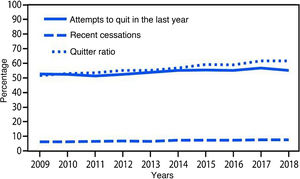
Smoking cessation rates are primarily based on three indicators: 1) cessation attempts in the past year, 2) recent cessation attempts, and 3) cessation ratio (or successful quitters). There was a statistically significant increase in all these indices (p < .001) from 2009 to 2018. The number of quit attempts in the past year increased from 52.8% to 55.1% during the study period, and recent cessation attempts by smokers with at least 2 years of smoking increased from 6.3% to 7.5%. Finally, the cessation rate in smokers who had smoked more than 100 cigarettes increased from 51.7% to 61.7% over those years (Fig. 2).10
Variations in cessation indicators. Adapted from Creamer et al. 2018.10
Many smokers try to quit relying on their own willpower or by using the various resources available to smokers and health professionals.11 However, success rates in quitting without subsequent relapse indicate that quitting is not a smooth process. Data from the USA indicate that, although the number of ex-smokers is higher than the number of new smokers since 2002, less than 10% succeed in quitting for good.12
Tobacco use affects various systems of the human body, such as the cardiovascular and respiratory systems, making it the most aggravating factor in many diseases and clinical biomarkers. Other interventions need to be explored for patients seen in internal medicine departments, with profiles that include advanced age, multi-pathology (arterial hypertension, diabetes, COPD…), and at high cardiovascular risk,13 who have not managed to quit smoking. Through these alternative approaches, we try to reduce the harm caused by smoking, to give these patients a better prognosis and a better quality of life in living with their diseases.
Emerging strategies in the approach to smoking cessationThe process of smoking cessation usually involves a combination of pharmacological and non-pharmacological approaches. The problem arises when these tools do not achieve their intended function, as there is no additional therapeutic step. This is when we resort to harm reduction strategies, used in patients who are unable or unwilling to quit smoking.
These strategies are based on interventions aimed at reducing the negative effects of smoking without the need for complete cessation.14 This reduction is based on the absence of combustion, responsible for most of the production of unhealthy components. There are different products that do not produce these toxic substances, which are grouped into three different categories: smokeless or oral tobacco products (snus being the best known and most widely used), e-cigarettes, and heated tobacco products.
SnusSnus is a tobacco product unique to the Nordic countries. Consumption is higher than European averages in males and similar in females.15 It is a tobacco preparation that is placed next to the gums inside the oral cavity where the nicotine it contains is absorbed by diffusion.16 Snus is considered a less toxic product compared to conventional cigarettes (CC) in certain aspects, which are summarised in Table 1.15–19
The risks of cancer or circulatory disease from snus use are no more than 1%. Switching from CC to this product improves the health prospects of users, as there is no scientific evidence that it encourages smoking initiation or discourages smoking cessation.18 Likewise, no association with colorectal or pancreatic cancer has been established, and its effect on the incidence of oral cancer is much smaller than that associated with smoking in general.19
The nicotine release generated by snus consumption could theoretically contribute to haemodynamic effects and various cardiovascular events, such as ischaemic heart disease or acute myocardial infarction (AMI). However, the study by Hansson et al.20 found no association between consumption and the risk of cardiovascular disease. Likewise, no association between snus use and effects on blood pressure or elevation of any of the relevant risk factors for these conditions has been demonstrated.21
In conclusion, the data indicate that switching from conventional tobacco use to snus causes a very similar risk of vascular disease and cancer in quitters, with minimal impact on the health of users.22 Therefore, in October 2019, the FDA approved snus as a modified risk tobacco product because switching to snus may carry a lower risk of mouth cancer, heart disease, lung cancer, stroke, emphysema, and chronic bronchitis.23
Electronic cigarettesE-cigarettes (ECs) or vapes are electronic devices that deliver nicotine by inhaling the vapour produced after heating the liquid they contain, generating an aerosol.24 The liquid’s main components are flavourings such as menthol or sweeteners; propylene glycol; vegetable glycerine; a variable amount of ethanol; nicotine concentrations of around 28% (1.6−19 mg/cartridge)25; and a specific pH that allows the bioavailability of nicotine, given that its non-ionised form is much easier and faster to absorb.26 There are also e-cigarettes that do not contain nicotine.
It is the smoke resulting from the combustion of CC that contains most of the toxic products. However, in this case, the production of the aerosol avoids combustion, reducing harm by not having so many toxic particles in suspension. Table 2 lists the main advantages of EC over CC use.
The study by Polosa et al.27 showed that the use of EC resulted in a decrease in tobacco smoking, although no changes in lung function were observed after 3 years of follow-up. In addition, significant improvements were seen in lung disease exacerbations, COPD Assessment Test (CAT) score, and 6-minute walk test (6 MWD). These improvements were also observed in dual smokers. Finally, this study demonstrated that long-term use of EC reverses some tobacco-related harm.
In longer follow-ups, no changes in spirometric indices or markers of lung inflammation in exhaled air were observed. Neither did subjects develop respiratory symptoms and no findings of early lung damage were reported. Exposure to this device did not generate significant changes in terms of lung function or lung inflammation.28 In fact, improvements in respiratory symptoms were perceived in patients with asthma or COPD when switching from CC to EC,29 resulting in an absence of changes in blood pressure and cardiac activity.28
In asthma patients, with a smoking prevalence similar to the general population, switching from CC to EC increases forced expiratory flow and airway hyperresponsiveness, improving and stabilising control of the disease and corticosteroid tolerance. Although there is no significant change in the number of disease exacerbations, the EC has been shown to be an alternative that achieves a reduction in harm and a marked improvement in lung function. However, as these are preliminary results, studies with larger cohorts are needed to confirm these observations.30
Previous studies have found that smoking affects the cardiovascular system, causing changes in the profile of oxidative stress biomarkers, platelet activation, endothelial function, inflammatory response, and lipid modifications, and an increase in proatherogenic adhesion molecules.31 EC use partially reverses these alterations, making it an option to reduce the damage caused by CC.30 In addition, unlike snus, the relationship between EC use and the development of AMI varies according to the patient's smoking history. Whereas in smokers the onset of AMI may be associated with smoking, there is no scientific evidence that in EC users there is a relationship between the two.32
The data show that, although not risk-free, EC use is not more dangerous than CC. Likewise, despite the lack of regulations around EC, switching from CC to this product is beneficial, especially in smokers who have not managed to quit.33 Because the EC may be associated with an increased risk of smoking initiation, regulation should be strict with the aim of curbing its use among non-smokers and young people. The aim would be to reduce CC use at the population level, thereby reducing the number of smokers.34
In any event, substituting CC for EC could be an alternative in smoking cessation.24 Studies have indicated that quit rates are higher in EC users than in users of nicotine replacement therapy (relative risk 1.69, 95% confidence interval 1.25–2.27). Furthermore, there was no clear evidence that EC affected users. Therefore, Canada has approved a programme to use EC as an effective and lower-risk alternative for smokers of conventional cigarettes.35 However, although the FDA allows the sale of EC, some American organisations such as the American Heart Association are in favour of banning them. In the case of Europe, both the National Health Service and the British Heart Foundation in the UK support the use of ECs as a tool to encourage smoking cessation. However, the European Society of Cardiology has not expressed an official position.33 In conclusion, there is currently no consensus among the various authorities and organisations.
Finally, it should be stressed that these types of devices and products are not risk-free. Although in smaller quantities compared to CC, the EC still produces toxic substances. This fact, together with the need for long-term studies to assess the health effect of these components, are factors that cannot be overlooked when assessing these devices as an alternative to traditional tobacco. However, although these products are not harmless, they are a good option in smokers who have not been able to quit.
Heat-not-burn tobacco productsFinally, alternatives to CC include heated tobacco products (HTP). These release nicotine by heating the tobacco preparation they contain, and are an option for smokers who have been unable or unwilling to quit.36 As well as providing higher user satisfaction than EC,37 the harmful effects of HTP on health are lower.
Table 3 shows a comparison of the emission of toxins in HTP and conventional cigarettes, demonstrating a reduction in the emission of various carcinogens by HTP. This is largely due to the difference between the temperature to which HTP and CC are subjected, which reduces the risk of exposure to carbonyls, as their generation is much lower. These substances include important carcinogens and toxins such as benzene, acetaldehyde, formaldehyde, acrylonitrile, and 1,3-butadiene.38 In the study by Rodrigo et al.,39 these markers of exposure were evaluated together with others such as N-nitrosonornicotine, cadmium, arsenic, benzopyrene, etc. Although they should not be considered safe products, HTP showed a reduction by several orders of magnitude in biomarkers of cancer and other diseases.
Variations in toxin release in heated tobacco products vs. traditional tobacco.
| Toxin | Conventional tobacco (μg/mL) | HTP (μg/mL) |
|---|---|---|
| Benzene38 | 1.57 × 10−1 | 9.32 × 10−4 |
| Acetaldehyde38 | 2.55 × 10−0 | 3.33 × 10−1 |
| Formaldehyde38 | 1.54 × 10−1 | 1.06 × 10−2 |
| Acrylonitrile38 | 4.59 × 10−2 | 2.96 × 10−4 |
| 1,3-Butadiene38 | 1.83 × 10−1 | 3.94 × 10−4 |
| N-nitrosonornicotine39 | 1.40 × 10–1 | 3.92 × 10–3 |
| Cadmium39 | 5.01 × 10–2 | 3.64 × 10–5 |
| Arsenic39 | 3.21 × 10–3 | 1.82 × 10–4 |
| Benzopyrene39 | 5.69 × 10–3 | 1.42 × 10–4 |
HTP use has been observed to cause changes in the respiratory system. COPD is the most important respiratory disease in terms of mortality and impact on quality of life. In patients with COPD, studies show that switching from conventional cigarettes to HTP leads to a significant improvement in CAT (COPD Assessment Test) score in 40% of users. Also, better FEV1 (forced expiratory flow) values were observed before and after the use of bronchodilators with the preparation. Regarding the effect of HTP on patients with metabolic syndromes, a cohort study with 801 CC smokers and 400 HTP users showed that the use of HTP reduced metabolic syndrome in 29.3% of participants, obesity in 18.8%, triglycerides in 14.4%, and blood pressure in 16.1%. Additionally, mean reductions of 17.92 and 9.62 m in the 6MWT were observed in HTP and conventional tobacco users, respectively. Finally, an increase in HDL-cholesterol of 63.3% over CC was calculated.40
A retrospective study of COPD outpatients from 4 Italian hospitals concluded that there was a reduction in the number of exacerbations per year from 2.2 to 1.3 with the use of these new products after a 3-year follow-up. A statistically significant decrease in the CAT score and an improvement in the walk test (6 MWD) were also observed, with a mean increase of 69m. About 60% of the patients analysed in the study who used HTPs abstained completely from CC use, with a reduction in daily cigarette consumption of 70% for dual users.41 The reduction in exacerbations was observed in a real-world evidence study in Japan, showing that after the nationwide market launch of HTPs, the rate of hospitalisations for exacerbations in COPD patients decreased significantly.42
HTPs are also presented as an option for harm reduction in cardiovascular conditions. It has been previously reported that exposure markers for benzene, 1,3-butadiene, and formaldehyde are significantly reduced with HTP consumption compared to CC. The reduction of these cardiovascular risk contributors would theoretically lead to a decrease in markers of cardiovascular toxicity. However, further studies are needed to confirm this.43 On the other hand, with regard to biomarkers linked to cardiovascular risk, switching from CC to HTP use leads to changes in levels of Nox-2-derived peptide, nitric oxide, H2O2, 8-iso- prostaglandin F2α, sCD40L, P-selectin, blood pressure, and cotinines (Table 4).37
Differences in biomarker levels with heated tobacco products (HTP) vs. conventional cigarettes.37.
| Biomarker | Conventional tobacco | HTP |
|---|---|---|
| Peptide Nox-2, pg/mL | 44.1 ± 17.1 | 29.9 ± 5.0 |
| 8-iso-prostaglandin F2α, pmol/L | 276 ± 29 | 207 ± 36 |
| sCD40L, pg/mL | 5.26 ± 1.97 | 4.18 ± 1.56 |
| P-selectin, ng/mL | 11.58 ± 3.56 | 8.03 ± 1.40 |
| Nitric oxide, μmol/L | 12.7 ± 6.6 | 19.8 ± 6.6 |
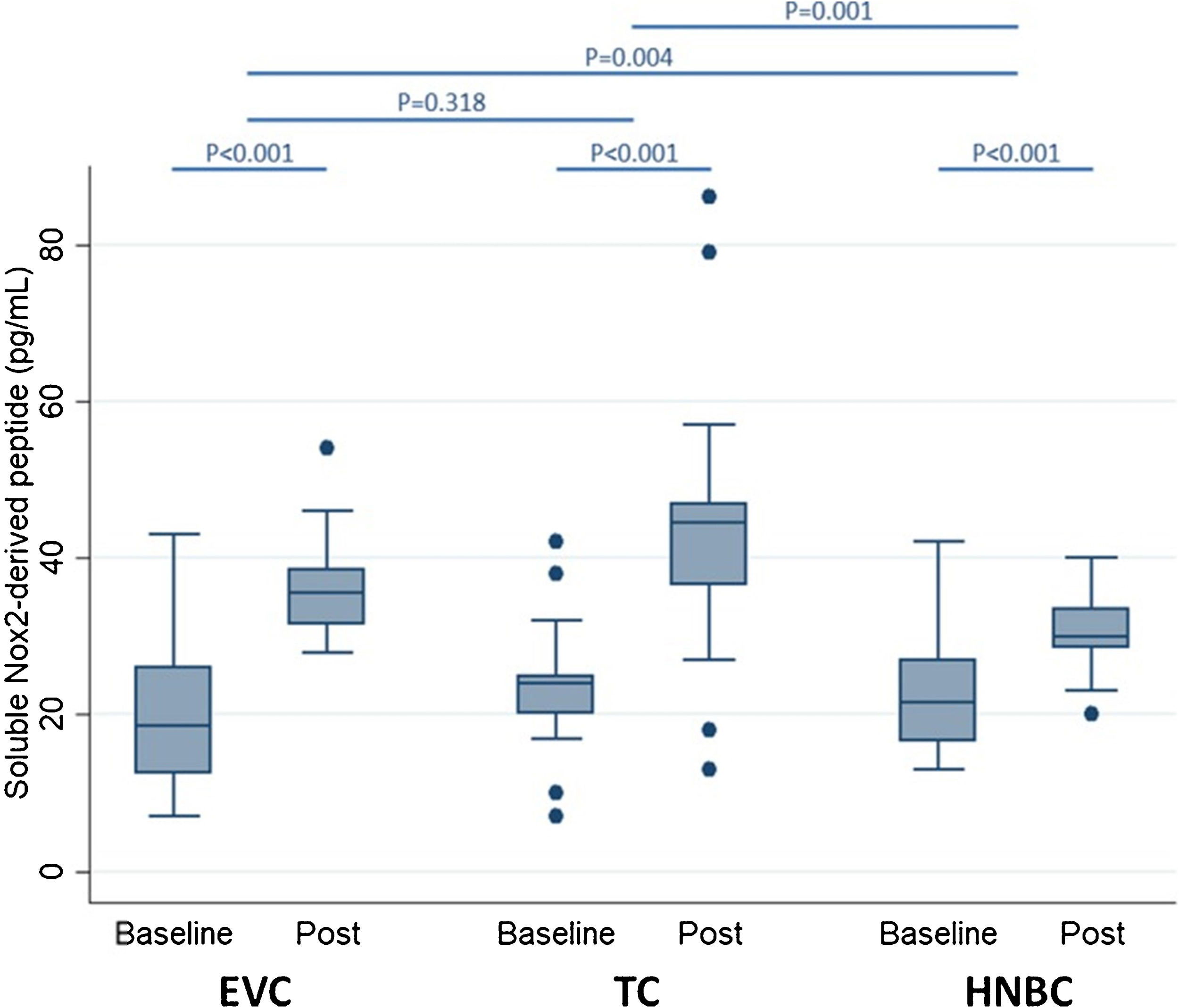
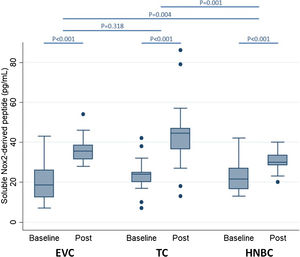
Preliminary comparative studies, such as the SURVAPES2 survey, demonstrate a lower impact of HTP and EC on oxidative stress, platelet function, and blood pressure. The study obtained better results for HTP for some of the variables analysed, such as soluble Nox2-derived peptide (a marker of NADPH enzyme activity; Fig. 3), 8-iso-PGF2a-III (an isoprostane), and vitamin E.37
Impact of electronic vaping cigarettes (EVC), traditional tobacco combustion cigarettes (TC), and heat-not-burn cigarettes (HNBC) on blood levels of soluble Nox2-derived peptide.37
It is evident, therefore, that switching from conventional cigarettes to HTP results in harm reduction in important aspects of users' health. Switching from CC to HTP leads to a reduction in the production of carcinogenic and toxic substances such as benzene, acetaldehyde, and formaldehyde; a reduction in CAT, obesity and triglycerides, an increase in HDL cholesterol and an improvement in FEV1; and a decrease in oxidative stress, platelet activation, and improvement in endothelial dysfunction. As a result, scientific societies such as the American College of Cardiology (ACC) have included this product in treatments for smoking cessation in cases where the patient does not wish to adhere to pharmacological therapy.44 The FDA considers this strategy an alternative that could lead to a reduction in the risks associated with smoking, and has therefore authorised it to be marketed as a tool for modifying exposure to toxic substances.45
However, it is important to bear in mind that long-term studies with HTPs are needed to confirm that the reduction in toxicant exposure translates into benefits for users.
ConclusionSmoking is the primary cause of morbidity and mortality in Spain and worldwide, and is one of the main risk factors for both respiratory and cardiovascular diseases. The new damage-reduction products are emerging strategies that, without being completely harmless, achieve a reduction in the toxins generated compared to CC use. This reduction is based on the absence of combustion of the organic matter, or the liquid in the device in the case of HTP and CE respectively. This avoids the formation of smoke and instead produces an aerosol containing a significantly lower level of toxic substances compared to CC smoke.
As the scientific evidence presented in this article shows, these alternatives to tobacco use could be a valid option for the reduction of harm to the health of patients who are unable or unwilling to give up CC use. These tools are shown as an alternative that could prevent a worsening of individual and collective health. These tools could potentially reduce costs to the health care system through the reduction of smoking-related diseases. It is important that future regulations to be implemented in the area of smoking take the existing scientific evidence into account. This would provide smokers with alternatives to reduce their health risk (as is the case for internal medicine patients) and prevent non-smokers, especially young people, from starting to use these devices.
Long-term evidence is still lacking, but the available evidence provides a sufficient basis for these tools to be considered in patients who are unable or unwilling to quit smoking. Especially given that the risk of smokers who continue to use conventional cigarettes is greater than the uncertainty generated by the long-term use of these alternatives.
FundingThis research study did not receive specific support from public sector agencies, the commercial sector, or non-profit organisations.
Authors’ contributionsAll authors were equally involved in the conception and design of the manuscript, analysis, and interpretation of the data, and drafting, revising, and approving the submitted manuscript.
Conflict of interestsNone.