Cardiovascular disease remains the first cause of mortality in Western countries. New strategies for prevention and control of cardiovascular disease are needed. At the same time, the incidence of risk factors that lead to the development of this disease, such as obesity, hypertension and diabetes, continues to rise. Therefore, the search for new markers or mediators is a priority in most cardiovascular prevention programs. The study of the intestinal microbiota is emerging because it is known that intestinal microorganisms act collectively as an integrated organ, regulating multiple biological functions that can modulate cardiovascular risk factors and the pathogenic mechanisms of this process. This review considers the current situation regarding the influence of gut microbiota on cardiovascular disease and particularly, its influence on the main traditional risk factors that lead to cardiovascular disease, such as obesity, diabetes, hypertension and lipids.
La enfermedad cardiovascular sigue siendo la primera causa de mortalidad en los países occidentales. Se necesitan nuevas estrategias para la prevención y el control de esta enfermedad. Al mismo tiempo, la incidencia de factores de riesgo que conducen al desarrollo de esta afección, como la obesidad, la hipertensión y la diabetes, sigue aumentando. Por lo tanto, la búsqueda de nuevos marcadores o mediadores es una prioridad en la mayoría de los programas de prevención cardiovascular. El estudio de la microbiota intestinal está surgiendo porque se sabe que los microorganismos intestinales actúan colectivamente como un órgano integrado, regulando múltiples funciones biológicas que pueden modular los factores de riesgo cardiovascular y los mecanismos patógenos de este proceso. Esta revisión considera la situación actual con respecto a la influencia de la microbiota intestinal en la enfermedad cardiovascular y, en particular, su influencia en los principales factores de riesgo tradicionales que conducen a la enfermedad cardiovascular, como la obesidad, la diabetes, la hipertensión y los lípidos.
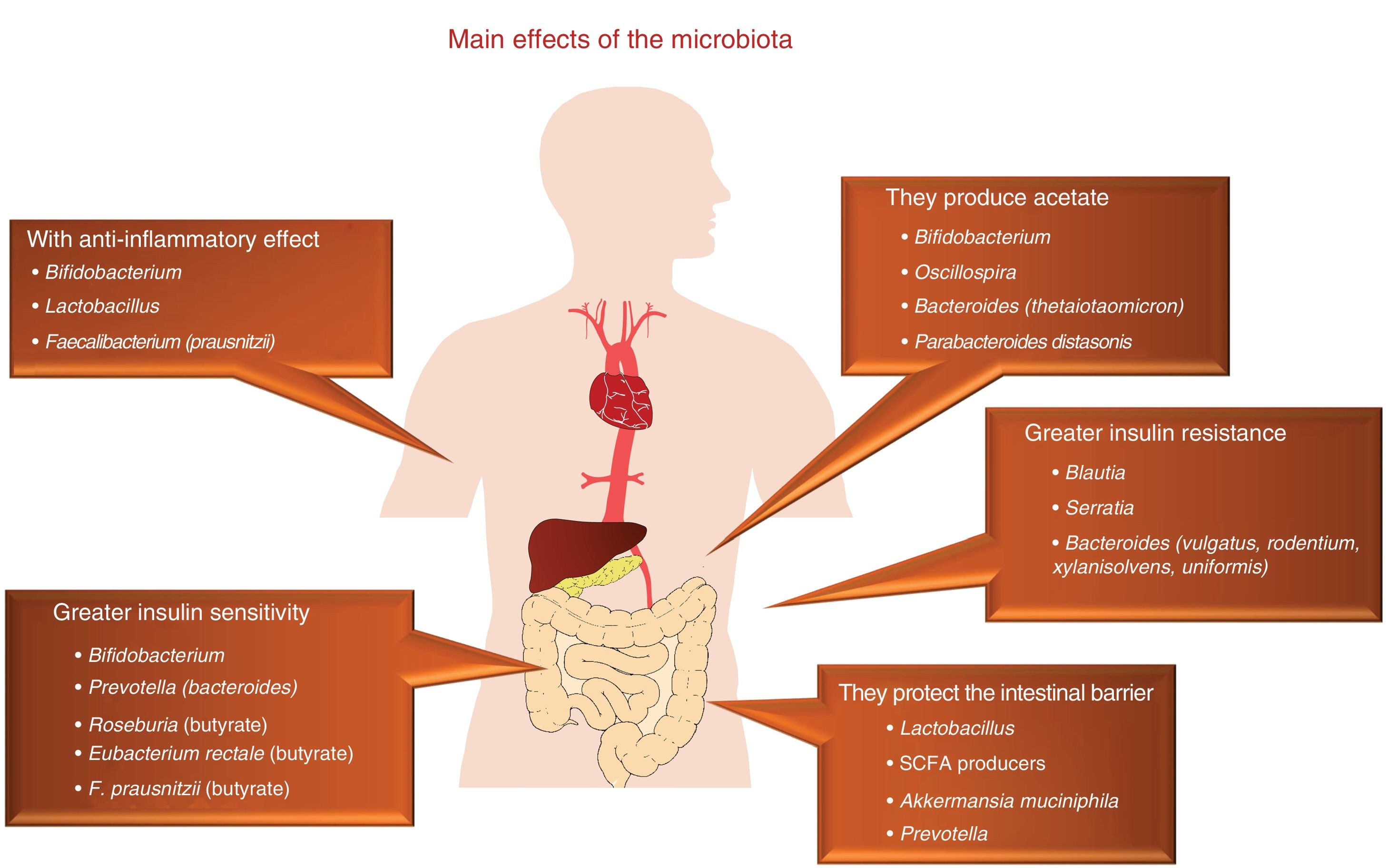
At the end of the 1980s, the infectious aetiology of atherosclerosis, in relation to the infection by several specific pathogenic agents, was revealed with great significance and anticipation.1 This hypothesis was abandoned in light of the negative result of several clinical trials with antibiotics, which we now consider to not always be of a successful design. In this context, this pathogenesis is currently being reconsidered, although from a very different point of view, as now it does not focus on specific microorganisms, but on the relevance of the rich saprophytic flora present in humans, including the extensive commensal bacteria population that resides in our intestines.2 In this sense, the human gastrointestinal tract has traditionally been considered as a passive organ that receives components of the diet, where they are absorbed or metabolised. However, today we know that our intestine is home to billions of microorganisms, named collectively as gut microbiota, which play a key role as endocrine, metabolic and immunological organ, generating multiple signals which act locally on multiple organ systems, due to their interaction with components of the diet (Fig. 1).
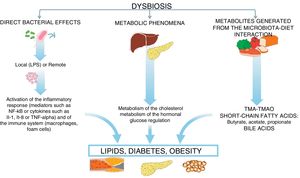
In this microbiota-intestine-systemic organs interaction, mechanisms can be activated, which can have an impact on the expression of the main risk factors, such as diabetes, hypertension, obesity and dyslipidaemia, as well as having a direct impact on different biological mechanisms which participate in atherosclerosis,3–5 which we will now see (Fig. 2).
Biological mechanisms by which the microflora influences the development of atherosclerosisThe mechanisms by which the microflora influences the development of atherosclerosis include:
- •
Certain products derived from the interaction of the diet with the intestinal flora may alter the inflammatory and immune response which influences the atherosclerotic process,6–8 leading to the production of circulating proatherogenic factors which may have an impact on cardiovascular risk.9,10 It is well known that the microbiota produces trimethylamine, using phosphatidylcholine from red meat, shellfish and eggs, or l-carnitine, also abundant in red meat but which is also found in energy drinks or in certain nutraceuticals. This trimethylamine is oxidised in the liver, by flavin-containing monooxygenase enzymes, producing trimethylamine N-oxide (TMAO), which acts as an atherogenic product, from this reaction.11 This evidence, which puts the role of the microbiota as a focal point, was confirmed through a study which measured the levels of TMAO after the marked consumption of phosphatidylcholine, with and without antibiotics, revealing that antibiotics would suppress the increase in plasma TMAO.12 In this regard, Wang et al. also demonstrated that the microbial metabolism of phosphatidylcholine promotes the development of cardiovascular disease (CVD). In this study, the authors identified TMAO as a possible modifiable risk factor for CVD.6 Subsequently, a clinical study with more than 4000 participants confirmed that serum levels of TMAO preceded the incidence of cardiovascular risk in a dose-dependent manner.7 Very recent studies have confirmed that the microbiota influences plasma levels of TMAO and, at the same time, reveal that this event involves platelet activation.13 However, the debate currently persists on whether the increase in TMAO concentrations is the consequence or the result of the development of CVD.14
- •
Another possible mechanism is mediated by short-chain fatty acids (SCFAs), such as acetic, butyric, propionic and valeric acid, which are metabolites derived from gut microbiota with hormonal properties and that are derived from the fermentation of fibre through a subset of bacteria with saccharolytic activity.15–17 These SCFAs have a direct impact on the risk of developing CVD as they modulate the action of insulin, immune response and energy metabolism.18–20 Specifically, SCFAs derived from bacterial fermentation stimulate the release of peptide similar to glucagon type 1 by L-cells of the intestine, which leads to an increase in the secretion of pancreatic insulin, a suppression of glucagon and an increase in the sensitivity of the same.21 At the same time, it has been demonstrated that SCFAs stimulate the secretion of peptide YY, a hormone involved in appetite regulation, specifically reducing it, and that, at the same time, inhibits intestinal motility and reduces the obtainment of energy from the diet.22 Finally, in vitro studies have demonstrated that SCFAs can influence the development of dyslipidaemia as they inhibit lipolysis and the final concentration of free fatty acids through the activation of the free fatty acid receptor 2.21
- •
Furthermore, gut microbiota can exercise a modulator role of lipid metabolism, influencing the development of atherosclerosis through the metabolism of bile acids.23,24 Primary bile acids, colic acid and chenodeoxycholic acid are synthesised in the liver from cholesterol and are essential for the absorption of this and fat-soluble vitamins. Gut microbiota has the ability to deconjugate these bile acids and transform them into secondary acids (mainly deoxycholic acid), thereby influencing the metabolism of cholesterol since, as is well-known, this synthesis of bile acids is the main cholesterol catabolic pathway. Simultaneously, certain gut bacteria are able to metabolise the primary bile acids and these have multiple functions due to their ability to bind to receptors such as Farnesoid X (mainly activated by primary bile acids) or the bile acid receptor bound to protein G-5, mainly the secondary bile acids.25,26 In summary, the gut microbiota intervene and modulate the metabolism of bile acids, which are key elements in the lipid homeostasis.
- •
Another potential atherogenic mechanism of the microbiota is through the role that it plays in the maintenance of the integrity of the intestinal barrier, which avoids the absorption of proinflammatory bacterial components such as components of the bacterial cell wall, mainly lipopolysaccharides (LPS). These LPS are recognised by toll-like receptors (TLRs), which is why they have the potential to activate the inflammatory pathway through the activation in turn of NF-κB, which has demonstrated that it produces inflammatory cytokines such as IL-1, IL-8 or TNF-α.27
- •
Finally, it is well known that gut microbiota can influence the endocrine system of the host by altering the functional metabolism of important hormones such as leptin, ghrelin and cortisol.28–30 This points to the fact that there is a dialogue between our intestinal system and our brain in terms of hormone production, where the microbiota play a key role in the regulation of this axis.
The prevalence and incidence of obesity are continually growing, just as happens with other cardiovascular risk factors, probably thanks to inappropriate lifestyles in which poor dietary habits and sedentary lifestyles play a relevant role.31 The pathogenesis of obesity is very complex and it has been demonstrated that gut microbiota is also one of the most important metabolic regulation mechanisms32,33 (Table 1). It has been demonstrated in animal studies that the obesity phenotype is accompanied by a reduction in bacterial diversity and an increase in the Firmicutes to Bacteroidetes ratio, two of the main bacterial phyla.34 Furthermore, it has been observed that changes in the feed of mice, subjected to obesogenic diets, induced modifications in the microbiota that were independent of weight gain,35 and that the faecal transplant from obese mice to thin mice induces weight gain in the latter.36
It has been possible to partially transfer observations carried out in experimental animals to studies in humans, where the results are more inconsistent and variable. This fact is probably due to the greater complexity of humans, population heterogeneity, as well as the difficulty in controlling the environment in which we move around. In obese humans, some studies have confirmed the increase in the Firmicutes to Bacteroidetes ratio, while in others the results have been more inaccurate or an increase in Actinobacteria has been detected.37,38 In addition, it has been observed that genera which belong to the same families may have similar functionalities, which gives a limited value to the fact that there are changes in the proportion of certain families, due to the functional heterogeneity of their components. One example consists of lactobacilli and bifidobacteria, which are very common genera in the gut population and very often used as probiotics, that may have variable characteristics according to the specific species. Therefore, Lactobacillus plantarum and Lactobacillusparacasei are associated with normal weight, while Lactobacillus reuteri has been reported to be associated with obesity.39
We still do not have enough clinical studies which have investigated the influence of the microbiota on obesity in human beings. In one of them, our group showed that 31% of the interindividual variability in the body mass index (BMI) depends on the composition of gut flora. In the study, 39 men and 36 postmenopausal women were analysed, demonstrating that the abundance of the genus Bacteroides was lower in men than in women when the BMI was greater than 33kg/m2, but not in lower obesity levels. On the contrary, this finding was not confirmed in women in different BMI ranges. Furthermore, other differences were found in terms of gender, such as the greater presence of the genera Veillonella and Methanobrevibacter in men, which exemplifies that this is one of the factors that prevents extrapolating evidence in this field to other populations.40 Moreover, our group has demonstrated that the chronic consumption of a heart-healthy diet is a powerful tool to improve intestinal dysbiosis observed in obese patients with CVD.41,42
In terms of the pathogenesis of obesity, although specifically it is unknown, we know that there is low-grade inflammation which undoubtedly influences its development and in which the microbiota plays a fundamental role. Therefore, it has been demonstrated that certain components generated by Gram-negative bacteria have a proinflammatory effect.43 Similarly, it has been observed in experimental animals that the chronic infusion of LPS produces a chronic endotoxemia state which induces inflammation, accompanied by weight gain. Furthermore, in mice fed with LPS for four weeks, adipose tissue inflammation was increased and resistance to insulin was induced.44 Finally, it is well known that the microbiota status will determine the permeability of the intestinal barrier and this, in turn, will determine the passage to it through toxins, antigens and bacteria present in the intestinal lumen, initiating inflammation and leading to the development of obesity.45
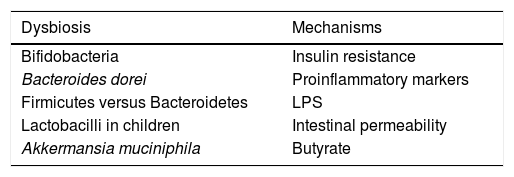
Influence of the microbiota on diabetesWe live in a time in which the therapeutic measures to combat this disease are increasing, although its control is still far from being reached and research into its pathogenesis will need to be continued. In recent years, the evidence that gut microbiota has an impact on diabetes has been on the rise46 (Table 2). One example that shows this connecting link is that children born by caesarean section have a greater risk of developing type 1 diabetes mellitus than children born through vaginal delivery and that gut colonisation is different in both. Thus, it has been demonstrated that the presence of Bacteroides dorei in children genetically predisposed to suffering from type 1 diabetes mellitus may be useful for predicting the development of the disease.47 A lower presence of Clostridia and of Bifidobacteria has also been reported, with greater presence of lactobacilli in children with type 1 diabetes, with the interesting finding that the abundance of bacteria involved in the maintenance of the integrity of the intestinal wall was lower compared to the healthy controls.48 This has an enormous scientific relevance, given that it has been demonstrated that the increase in abundance of Bifidobacteria in the gut of mice is accompanied by an anti-inflammatory effect, through the production of glucagon-like peptide 2, with reduced intestinal permeability.49
Compared to type 2 diabetes, dysbiosis with an increase in the phylum Firmicutes and a decrease in Bacteroidetes has been observed. It is necessary to point out that the functionality of bacteria is critical in its relationship with the presence of diabetes, apart from its phylum or the family it belongs to, as we discussed above. This is the case of the bacteria called Roseburia and Faecalibacterium prausnitzii, which produce butyrate and their abundance is lower in the microbiota of type 2 diabetic patients, while other clostridial organisms that do not produce butyrate increase. Given that butyrate is capable of increasing sensitivity to insulin, whether or not these types of bacteria are present may have pathogenic significance in the risk or severity of the metabolic alteration. At the same time, it is paradoxical that species of clostridial organisms do not correlate with overweight indicators, such as BMI or waist circumference.50
Some of the protective mechanisms of the microbiota on the development of diabetes are related to their anti-inflammatory action and due to their ability to modify insulin resistance. One example can be found in the fermentation of non-digestible polysaccharides of dietary fibre, which leads to the production of SCFAs, with a relevant role in the action of insulin, the regulation of energy intake and inflammation. In fact, it is well known that SCFAs act by regulating hormone secretion, activating the adipocyte receptors, suppressing insulin signalling and preventing the accumulation of fat. As we have mentioned previously, they can even increase insulin sensitivity by stimulating the secretion of glucagon-like peptide 1.51,52 Furthermore, the microbiota can be involved in the inflammation typical of diabetes, through the intestinal translocation of LPS and peptidoglycans. It is well known that plasma levels of LPS in patients with type 2 diabetes are elevated,53 which could lead to the activation of TLRs that induce the transcription of NF-κB, which regulates the genes involved in the synthesis of inflammatory mediators, including the cytokines TNF-α, IL-6 and IL-1β. Although these studies are based fundamentally on experimental animals, it may be that their role is also relevant in the human species.
Finally, another clinical example of the microbiota-diabetes connection is the faecal transplant from healthy donors to patients with metabolic syndrome, with an improvement in insulin sensitivity, weight reduction, increased microbial diversity, increased faecal butyrate and increased butyrate-producing bacteria, Roseburia intestinalis, although the effects were discrete and temporary.54 These results lead us to think that we would have to look for tools or therapeutic alternatives that will help to colonise the colon with “healthy” bacteria from the metabolic point of view, which are integrated in a stable and long-lasting way in the individual's microbial community.
Influence of the microbiota on hypertensionThe World Health Organisation describes hypertension as one of the most significant causes of premature death worldwide. Controlling blood pressure in routine clinical practice is a difficult challenge to tackle, probably due to the fact that it is the result of the alteration of multiple systems such as the cardiovascular, nervous, renal and endocrine systems. In this sense, there is no doubt that diet plays a key role in its control. Therefore, given that the intestine interacts with the diet, it does not seem implausible that the microbiota may participate in its regulation. This connection has been demonstrated in studies carried out in experimental animals with hypertension in whom an increase in the Firmicutes to Bacteroidetes ratio and a reduction in bacterial richness and diversity has been revealed.55 In a recent meta-analysis, the consumption of probiotics was related to reduced hypertension, although the effect obtained was very modest and particularly more relevant when the patients started off with high blood pressure.56 Despite the promising effects of probiotics in this field, the level of evidence is low and more evidence is necessary in order to confirm whether or not they are useful.
There is still a long way to go to demonstrate with regard to the possible mechanisms involved in explaining this link, although it is suggested that one of them could be that the increase in the sympathetic activity in the intestine is associated with dysbiosis, which would lead to an increase in the permeability of the wall and, in turn, to the implementation of inflammatory mechanisms which contribute to the development of hypertension.57,58 Further studies will be necessary to confirm the relevance of these facts.
Influence of the microbiota on lipid metabolismAs with the other cardiovascular risk factors discussed above, it has been demonstrated that the gut microbiome influences lipid metabolism, in particular due to its importance in the metabolism of bile acids and of its Farnesoid X receptor, involved in the production of VLDLs and in the protection from hepatic steatosis.59 Nevertheless, this theory is peculiar, as it is well known that the same receptors act in the gut in a contrary way, that is promoting hepatic steatosis and obesity.60 Our group has demonstrated that in overweight and obese patients there are 66 taxa which explain 29% of the interindividual variability in triglycerides, 33% in that of HDL cholesterol and 46% in that of LDL cholesterol.40 Although with less quantitative influence, Fu et al. demonstrated that gut microbiota can explain up to 4.5% of the variations found in BMI, up to 6% in triglycerides and up to 4% in HDL cholesterol, regardless of age, gender and genetic factors.61
The relationship between microbiota and lipid metabolism is very complex. There are various interrelation mechanisms that we will discuss briefly. On the one hand, TLR signalling modulates the metabolism of cholesterol62 and, through this mechanism, the gut microbiota may be an important factor to modify the plasma lipid levels.63 Similarly, liver X receptor nuclear receptors control the metabolism of cholesterol and are activated by means of endogenous oxysterols. In this way, it has been demonstrated that agonists of liver X receptor nuclear receptors increase the expression of ABCA1 and ABCG1 transporters, which gives as a result an increase in reverse cholesterol transport.64 Furthermore, TLR4 inhibits the activation of liver X receptor nuclear receptors and, therefore, has a significant influence on reverse cholesterol transport (RCT), as well as on inflammation and on glucose metabolism.62 Another potential mechanism would be through molecules such as acetic acid, butyric acid, propionic acid and valeric acid, metabolites with a local action, that serve as an energy source, but that are also absorbed and stimulate gluconeogenesis19 or influence insulin sensitivity.65 Finally, it has been demonstrated that high levels of TMAO modify lipid metabolism through changes in RCT, through the metabolism of sterols and by modifying the quality and quantity of bile acids.66,67
ConclusionsGut microbiota is an important determiner of many mechanisms related to atherosclerosis, although the majority of studies have an experimental nature and its real dimension in human disease is still to be demonstrated. These mechanisms are established through the production of proatherogenic factors; through short-chain fatty acids that participate in the regulation of insulin action, immune response and energy metabolism; through bile acids, which are key in the metabolism of cholesterol; or by modulating the permeability of the intestinal barrier and, therefore, the absorption of components of the bacterial cell wall, mainly lipopolysaccharides and peptidoglycans, which are essential in the inflammatory response and in glucose metabolism. It is now time to consolidate this evidence by looking into these mechanisms with the idea of identifying the risk subgroups and applying preventive measures or personalised and adequate interventions.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We would like to thank Mr Esteban Tarradas Merino, of the Servicio de Imagen de Apoyo a la Docencia e Investigación [Supporting Teaching and Research Imaging Department] (SIADI) of the Faculty of Medicine and Nursing of the Universidad de Córdoba, for his collaboration in the graphic design of the images.
Please cite this article as: García-Ríos A, Camargo Garcia A, Perez-Jimenez F, Perez-Martinez P. Microbiota intestinal: ¿un nuevo protagonista en el riesgo de enfermedad cardiovascular? Clín Investig Arterioscler. 2019;31:178–185.