The sodium-glucose co-transporter 2 inhibitors (SGLT2i) were first conceived to treat type 2 diabetes due to their hypoglycaemic effect. However, due to an increasing number of studies, SGLT2i are changing the way we treat, and understand, diabetes, and cardiovascular risk, in general.
The EMPA-REG OUTCOME clinical trial, in 2015, showed for the first time that empagliflozine-a glucose lowering agent-lowers the risk of death from cardiovascular causes and death from any cause. Also, this SGLT2i lowered hospital admission for heart failure and delayed renal function worsening. From then on, other clinical trials with SGLT2i such as CANVAS (Canagliflozin) and DECLARE-TIMI 58 (Dapagliflozin) confirmed these positive effects.
With a proven and non-related glucose-lowering effect on heart failure, overall death, cardiovascular death, and renal function, SGLT2i stands out among the rest of anti-diabetic drugs. Since its role in treating patients with heart failure and type 2 diabetes has been undoubtedly established, new studies are paving the way for non-diabetic patients as well. A potential paradigm shift is being witnessed and, probably, the dawn of a new field, cardio-endocrinology, which involves new and far-reaching pharmacological agents.
Los inhibidores del cotransportador de sodio-glucosa tipo 2 (iSGLT2) fueron incialmente desarrollados para el tratamiento de la diabetes por su actividad hipoglucemiante. Sin embargo, a la luz de los estudios clínicos más recientes, están revolucionando el abordaje de la enfermedad cardiovascular (CV) en el paciente diabético.
En el año 2015, el ensayo clínico EMPA-REG OUTCOME nos demuestra por primera vez que la empagliflozina –un fármaco considerado «antidiabético»—reduce la mortalidad CV y por cualquier causa, además de eventos CV mayores, hospitalización por IC y progresión de enfermedad renal. Posteriormente, otros estudios clínicos con agentes del mismo grupo farmacológico, CANVAS con canagliflozina y DECLARE-TIMI-58 con dapagliflozina, corroboran la exitencia de los beneficios CV asociados a la inhibición del receptor SGLT2.
Los beneficios observados los sitúan más allá de simples agentes hipoglucemiantes, con un demostrado efecto cardionefroprotector en la enfermedad aterosclerótica, insuficiencia cardíaca, mortalidad total, mortalidad cardiovascular y progresión de insuficiencia renal. Actualmente ya son una realidad en pacientes diabéticos de alto y muy alto riesgo cardiovascular, mientras su evidencia en el paciente no diabético es cada vez mayor. Asistimos por tanto a un cambio de paradigma y posiblemente al nacimiento de una nueva especialidad, la cardio-endocrinología, con la implicación de nuevos tratamientos que deben ser considerados más que sólo fármacos antidiabéticos.
The sodium-glucose cotransporter 2 (SGLT2) inhibitors (SGLT2i) were initially developed for the treatment of diabetes due to their hypoglycaemic activity. However, the most recent clinical studies are showing them to be revolutionising the approach to cardiovascular (CV) disease in the diabetic patient.
Type 2 diabetes mellitus (T2DM) is currently one of the most prevalent health problems in the western world due to its high incidence and impact on cardiovascular disease. It is estimated that by 2050, one in three American citizens will be diabetic. In Spain, the figures are fairly similar; 20% of participants in the recent IBERICAN1 study were diabetic. Cardiovascular complications are the leading cause of death in diabetic patients, and up to 35% of patients with established CV disease also have T2DM,2 with a mortality risk at least three times higher than those without T2DM.3 Moreover, around 40% of heart failure (HF) patients have T2DM,4 which confers a significantly higher risk of hospitalisation, CV death and death from any cause.
To date, strategies to reduce CV morbidity and mortality in patients with DM have focused on strict glycaemic control and control of accompanying risk factors (especially blood pressure and dyslipaemia). Classical oral antidiabetics and insulin therapy can control elevated glucose levels continuously and effectively, thus reducing chronic microcirculation complications (retinopathy, nephropathy, and neuropathy) by about 25%. However, they do not significantly reduce the chronic complications of macrocirculation (CV disease and HF), as demonstrated by the ACCORD,5 ADVANCE6 and VADT7 studies.
In 2015, the EMPA-REG OUTCOME clinical trial showed for the first time that empagliflozin-a drug considered “antidiabetic”-reduces CV and all-cause mortality, as well as major CV events, hospitalisation due to HF and progression of kidney disease.8 Subsequently, other clinical studies with agents from the same pharmacological group, CANVAS with canaglyphlozin9 and DECLARE-TIMI-5810 with dapaglyphlozin, corroborate CV benefits associated with inhibition of the SGLT2 receptor. Dapagliflozin also recently showed a robust benefit in patients with heart failure and reduced left ventricular ejection fraction (LVEF), regardless of the presence or otherwise of diabetes.11 The cardiovascular benefit associated with different SGLT2i supports the idea of a mechanistic class effect rather than a drug-specific effect.
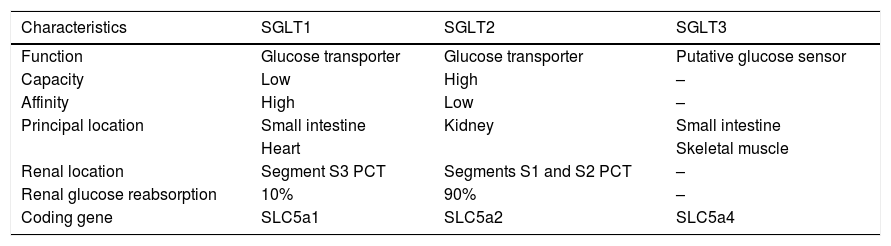
SGLT receptorsSGLT receptors form a family of membrane transporters (Table 1) for different substrates (mainly glucose, but also other sugars, inositol, or urea). They use the electrochemical sodium (Na+) gradient generated by the Na+/K+ pump to transport glucose into the cells against their own gradient.
SGLT receptors.
| Characteristics | SGLT1 | SGLT2 | SGLT3 |
|---|---|---|---|
| Function | Glucose transporter | Glucose transporter | Putative glucose sensor |
| Capacity | Low | High | – |
| Affinity | High | Low | – |
| Principal location | Small intestine | Kidney | Small intestine |
| Heart | Skeletal muscle | ||
| Renal location | Segment S3 PCT | Segments S1 and S2 PCT | – |
| Renal glucose reabsorption | 10% | 90% | – |
| Coding gene | SLC5a1 | SLC5a2 | SLC5a4 |
PCT: proximal contoured tubule.
The first of these transporters to be identified and cloned was SGLT1, a low capacity, high affinity transporter. Its tissue expression is quite broad, found in different organs such as the lung, liver, pancreatic alpha cells, skeletal muscle, heart,12 intestine and kidney (S3 segment of the proximal contoured tubule). It performs its main function in the intestine, playing a more secondary role in the kidney (10% of urinary glucose reabsorption).
The SGLT1 receptor is the only subtype of this receptor family that is expressed in heart cells. Various preclinical studies suggest that it plays a key role in energy supply, especially in patients with diabetes or myocardial ischaemia, where increased expression has been observed. Whether its overexpression is a defensive mechanism, or a consequence of heart damage is yet to be determined.
SGLT2, on the other hand, has an almost exclusively renal location, specifically in the S1 and S2 segment of the proximal contoured tubule. It is a low affinity and high-capacity transporter and reabsorbs almost 90% of filtered glucose. It is usually associated with the Na+ NHE3 transporters.
The hypoglycaemic effect in diabetics is conditioned by the elimination of excess glucose in urine, which has been calculated at around 80–100 mg per day. Its inhibition induces glycosuria and osmotic natriuresis. From a pharmacological perspective, its effect could be considered systemic since its hypoglycaemic activity depends directly on the amount of glucose in the urine. This mechanism of action also gives it a high safety profile: it is not related to endogenous insulin production and reduces the risk of hypoglycaemia and drug interactions with the polypharmacy associated with the diabetic population.
The three FDA-approved SGLT2is, canaglyphlozin, dapaglyphlozin and empaglyphlozin, also have some theoretical inhibition on the SGLT1 receptor. The highest SGLT2/SGLT1 selectivity corresponds to empaglyphlozin (>2500), as opposed to canaglyphlozin or dapaglyphlozin (>250, >1200, respectively). In any case, the plasma levels used in the clinic do not seem to be sufficient to achieve real inhibition of SGLT1.
Within this group of transporters, we also find SGLT3, which in contrast to the above, seems to act more like a biochemical glucose sensor than a transporter as such. Initially, its expression was described in enterocytes, in the enteric nervous system, hypothalamic neurons and kidney.13 However, the lack of specific antibodies and in vivo studies makes it difficult to confirm its tissue expression and function. They complete the SGLT4, SGLT5 and SGLT6 family, still under study, whose function remains unknown.
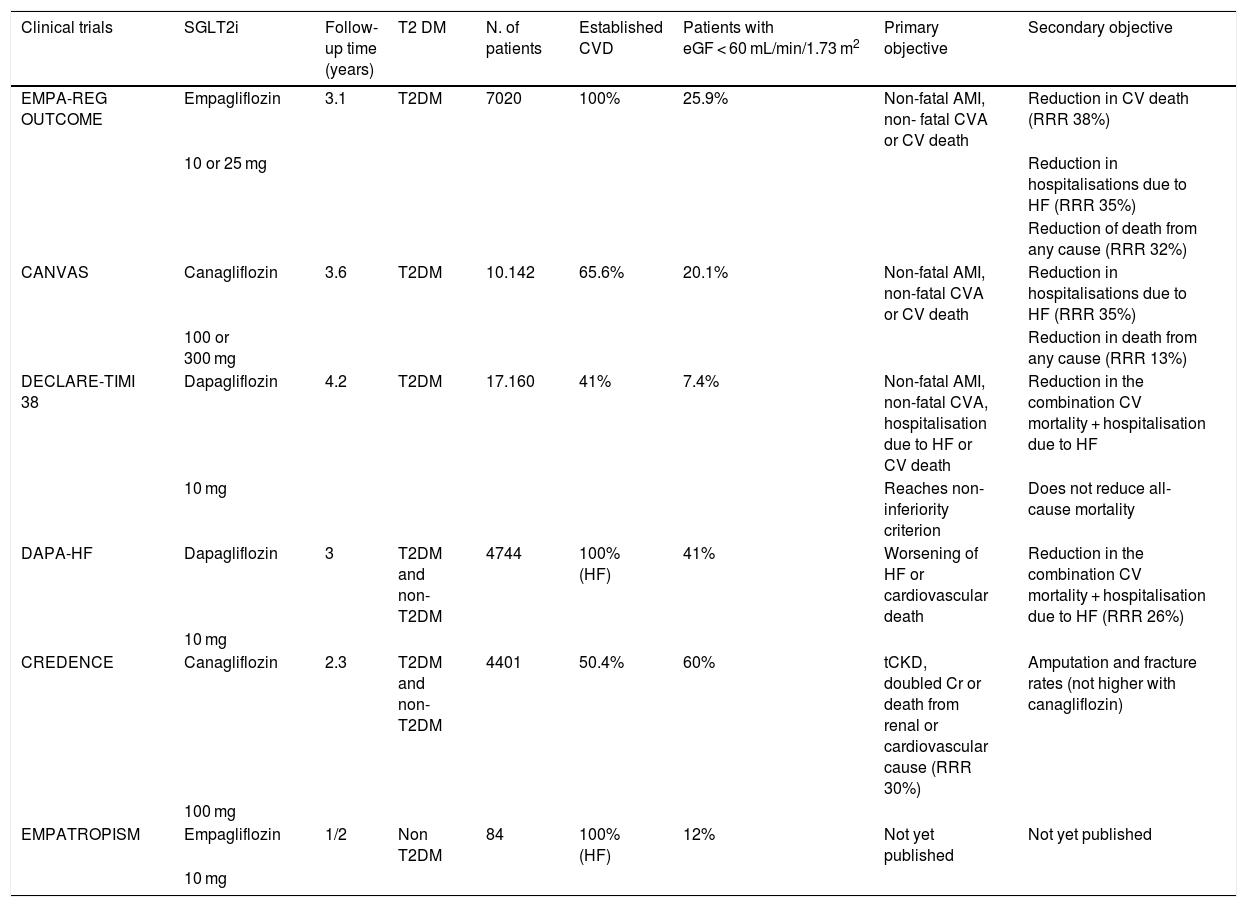
Cardiovascular benefitsCardiovascular safety studies with SGLT2iEMPA-REG OUTCOME (Empagliflozin, Cardiovascular Outcomes, and EMPA-REG OUTCOME (Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes)8 was the first study to evaluate the safety and effectiveness of an SGLT2i (Table 2). Patients with T2DM (n = 7028) and established CV disease (myocardial infarction, coronary heart disease, unstable angina, stroke, or peripheral arterial disease) were randomly assigned to receive either empagliflozin or placebo over a three-year follow-up period. Glomerular filtration was at least 30 mL-min-1.73 m2, empaglyphlozin, added to standard antidiabetic treatment, reduced the composite risk of cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke (3-point MACE) by 14% (hazard ratio .86; 95.02% CI, .74–.99; p = .04 for superiority), at the expense mainly of a reduction in cardiovascular death of 38%. It also reduced all-cause death by 32% and hospitalisation due to heart failure by 35%, compared with placebo. Interestingly, in the empaglyphlozin group there was no effect on the incidence of atherothrombotic events (non-fatal AMI or CVA). In terms of adverse effects, an increase in genital infections (6.4% vs. 1.8%) was mentioned, possibly related to glycosuria.
Principal clinical trials with SGLT2i.
| Clinical trials | SGLT2i | Follow-up time (years) | T2 DM | N. of patients | Established CVD | Patients with eGF < 60 mL/min/1.73 m2 | Primary objective | Secondary objective |
|---|---|---|---|---|---|---|---|---|
| EMPA-REG OUTCOME | Empagliflozin | 3.1 | T2DM | 7020 | 100% | 25.9% | Non-fatal AMI, non- fatal CVA or CV death | Reduction in CV death (RRR 38%) |
| 10 or 25 mg | Reduction in hospitalisations due to HF (RRR 35%) | |||||||
| Reduction of death from any cause (RRR 32%) | ||||||||
| CANVAS | Canagliflozin | 3.6 | T2DM | 10.142 | 65.6% | 20.1% | Non-fatal AMI, non-fatal CVA or CV death | Reduction in hospitalisations due to HF (RRR 35%) |
| 100 or 300 mg | Reduction in death from any cause (RRR 13%) | |||||||
| DECLARE-TIMI 38 | Dapagliflozin | 4.2 | T2DM | 17.160 | 41% | 7.4% | Non-fatal AMI, non-fatal CVA, hospitalisation due to HF or CV death | Reduction in the combination CV mortality + hospitalisation due to HF |
| 10 mg | Reaches non-inferiority criterion | Does not reduce all-cause mortality | ||||||
| DAPA-HF | Dapagliflozin | 3 | T2DM and non-T2DM | 4744 | 100% (HF) | 41% | Worsening of HF or cardiovascular death | Reduction in the combination CV mortality + hospitalisation due to HF (RRR 26%) |
| 10 mg | ||||||||
| CREDENCE | Canagliflozin | 2.3 | T2DM and non-T2DM | 4401 | 50.4% | 60% | tCKD, doubled Cr or death from renal or cardiovascular cause (RRR 30%) | Amputation and fracture rates (not higher with canagliflozin) |
| 100 mg | ||||||||
| EMPATROPISM | Empagliflozin | 1/2 | Non T2DM | 84 | 100% (HF) | 12% | Not yet published | Not yet published |
| 10 mg |
CVD: Cardiovascular disease; eGF: Estimated glomerular filtration; tCKD: Terminal chronic kidney disease (dialysis, renal transplantation, or eGF lower than 15 mL/1.73 m2 for 30 days).
The CANVAS programme (Canagliflozin Cardiovascular Assessment Study)9 integrates the results of two clinical trials (CANVAS and CANVAS-R), that compare canagliflozin vs. placebo in diabetic patients at high cardiovascular risk. Patients in primary prevention (34.4%) with no history of established CVD were included.
It reached 3-point MACE for both inferiority and superiority, occurring in 26.9 vs. 31.5 individuals in the placebo group per 1000 patients per year (hazard ratio, .86; 95% CI, .75–.97; p < .001 for non-inferiority; p = .02 for superiority). The individual objectives (AMI, CVA, and CVM) did not reach statistical significance separately, but showed a marked favourable trend. There was also benefit in the secondary objective (re-hospitalization due to heart failure, reduced glomerular filtration of more than 40%, or development of end-stage kidney failure with need for dialysis or death from renal cause). In terms of safety, the frequency of genital infections in men was higher with canagliflozin (3.5% vs. 1.1%) and of fungal genital infections in women (6.9% vs. 1.8%). In this study, an increased risk of amputation was observed (.6% vs. .3%). Even so, it is not clear whether this last point would be just a statistical effect since it has no clear mechanistic explanation and was not subsequently confirmed in the CREDENCE study or with any other SGLT2i.
The DECLARE study (Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes)10 evaluated the effect of dapagliflozin against placebo. It included over 17000 patients with T2DM, with CV risk factors (59%) and established cardiovascular disease. Dapagliflozin did not meet the criterion of superiority versus placebo (8.8% in the dapagliflozin group versus 9.4% in the placebo group; HR, .93; 95% CI, .84–1.03; p = .17). It does, however, meet the criterion of non-inferiority. The combined objective of cardiovascular death or hospitalisation due to heart failure was reduced (4.9 vs. 5.8), mainly due to a reduction in admissions for heart failure. There was no significant difference in cardiovascular mortality. There was some significant but rare increase in diabetic ketoacidosis (.3% vs. .1%; p = .02) and genital infections (.9% vs. .1%; p < .001).
A recent meta-analysis14 compared the three main clinical trials (EMPA-REG OUTCOME, CANVAS and DECLARE), with a total of 34,322 diabetic patients. The percentage of patients with established cardiovascular disease (not only CVRF) differs between the three: 100% EMPA-REG (secondary prevention), more than 60% CANVAS and 41% DECLARE. According to these differences, the CV benefit seems to become more evident the higher the disease burden, so that in the DECLARE study the results only border on positivity and do not reach the criterion of superiority, unlike EMPA-REG. The percentage of patients diagnosed with heart failure, at the beginning of the study, was similar, ranging from 10% to 15%. Consistently, the most striking point of the SGLT2is as a whole is the reduction in the risk of hospitalization due to heart failure (30%) and the prevention of nephropathy (45%).
Effect at the metabolic levelFrom a pharmacological perspective there are several factors to be highlighted:
- 1
CV benefits are obtained without a reduction in the rate of atherothrombotic events (ACS or CVA) normally increased in diabetic patients. In parallel, antidiabetic drugs that have been shown to reduce atherothrombotic events (GLP1-agonists) have no effect on heart failure outcome.15
- 2
The differences start to be significant after two to three months of treatment with SGLT2i.
- 3
The additional hypoglycaemic effect of SGLT2i in clinical trials is around a .3% additional reduction in HbA1c levels. An effect also obtained in trials with DPP4 inhibitors (DPP4i), but without associated CV benefits.
- 4
The effect on CV risk factors such as reduced body weight (1–2 kg), blood pressure (3–5 mmHg), or lipid profile, would not explain the magnitude or speed of the benefits obtained, as analysed in a recent post-hoc analysis.16
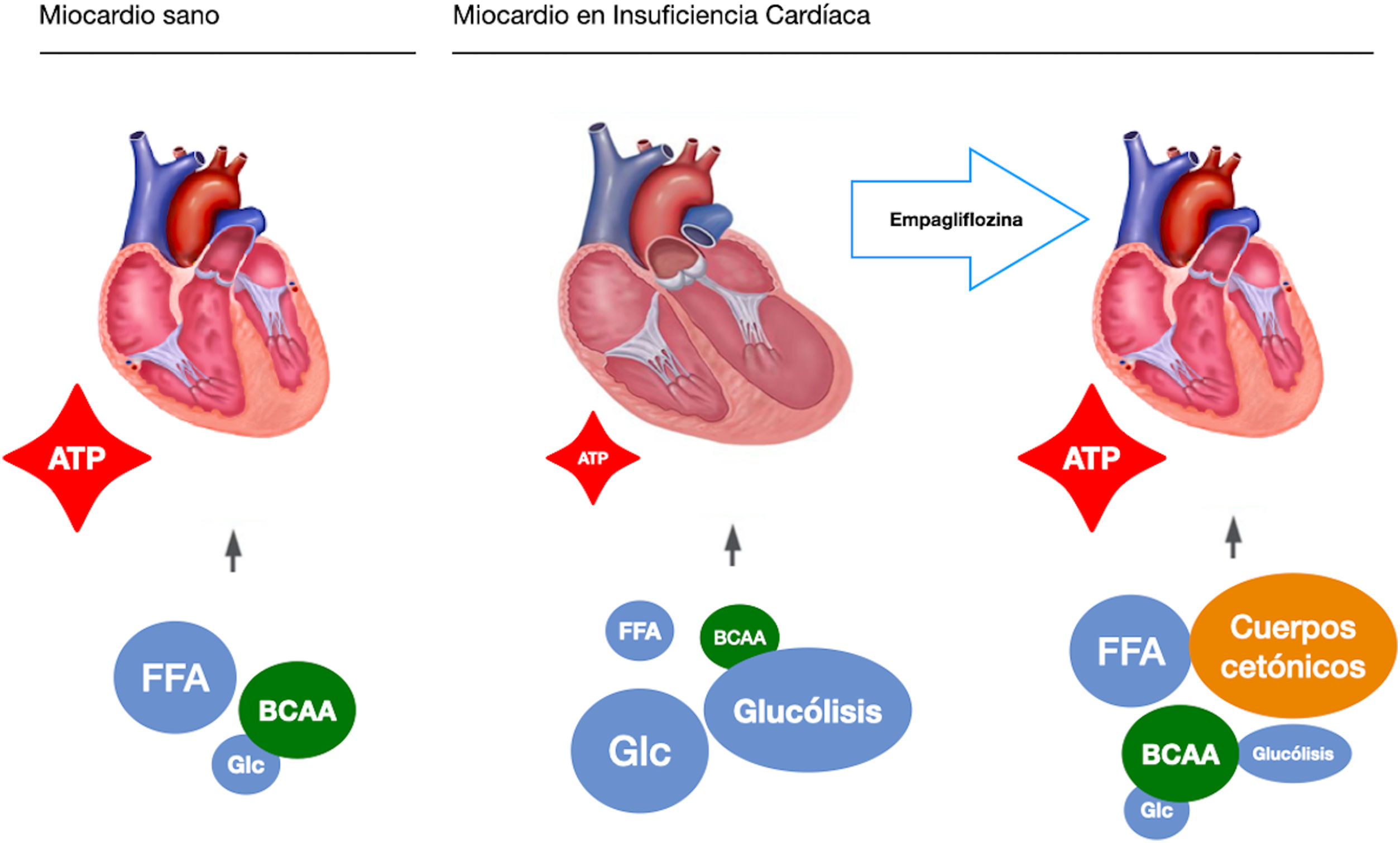
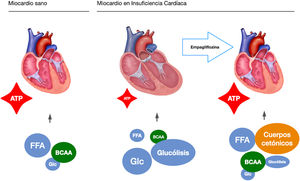
These observations highlight a possible mechanism of action, independent of their hypoglycaemic action, which is a starting point for present and future research. In this sense, our study group raises the possibility of an independent mechanism in a pre-clinical study on a pig model of heart failure of ischaemic origin.17 To extract the glycaemic component of the equation, all the pigs were non-diabetic. Like EMPA-REG OUTCOME, after only two months of treatment we observed a significant reduction in adverse left ventricular remodelling, improvement in cardiac function and reduction in neuro-hormonal remodelling. We postulated that the mechanism of action responsible for the observed benefits was a change in the myocardial energy source of the ischaemic heart. Under normal conditions, the myocardium consumes fatty acids and glucose as an energy source. In heart failure it switches to glucose consumption as the main resource, which requires more oxygen molecules and generates fewer ATP molecules. Pigs treated with empaglyphlozin used ketone bodies and branched-chain amino acids (BBCA) as a main energy source. These energy sources not only required less oxygen, but also generated more ATP energy molecules (Fig. 1). Therefore, a possible metabolic switch from glucose to ketone bodies as a new energy source could potentially explain some improvement in myocardial efficiency induced by SGLT2i.
SGLT2i in the non-diabetic patient with heart failureTo date, the reduction in the risk of hospitalisation due to HF observed in different clinical trials appears to be independent of the presence of a known history of HF and is greater the worse the baseline kidney function. In the DECLARE-TIMI-58 study, the reduction in HF hospitalisation was similar in patients with reduced and preserved EF. None of these studies, however, was designed to evaluate efficacy in HF. The proportion of HF patients was therefore low.
DAPA-HF11 is the first study to evaluate the effects of an SGLT2i drug on HF patients. It included both diabetic and non-diabetic patients, all of whom had symptomatic HF and LVEF of less than 40%. They were randomised to dapagliflozin or placebo, added to standard medical treatment. The primary objective was a composite of CV death, HF hospitalisation or emergency visit requiring intravenous treatment. Dapagliflozin reduced the primary objective by 26% with respect to the placebo group (HR .74, 95% CI .65–.85). The independent analysis of the components of the primary objective remained significant. The benefit of dapaglifozin was positive irrespective of the presence or absence of diabetes. In patients with heart failure with preserved ejection fraction (greater than 40%), we found the DELIVER study focused on searching for cardiovascular events.
Based on the observations from our pre-clinical non-diabetic pig model, we designed the EMPA-TROPISM18 clinical study (Are the “Cardiac Benefits” of Empagliflozin Independent of Its Hypoglycaemic Activity?; NC***T 03485222) within the Mount Sinai (USA) hospital network. EMPA-TROPISM is a double-blind, randomised, placebo-controlled mechanistic study with the aim of demonstrating the cardiovascular benefits of empagliflozin administration on cardiac function, cardiopulmonary activity, and quality of life in HF patients with reduced ejection fraction. The main characteristic of this study is that all the patients are non-diabetic.
Other ongoing clinical trials have started to include both diabetic and non-diabetic patients. A major trial is the EMPEROR-HF programme (EMPEROR-REDUCED for reduced ejection fraction and EMPEROR-PRESERVED for preserved ejection fraction), which focuses on the detection of long-term morbidity and mortality criteria in HF patients. Specifically, pending publication, the results of EMPEROR-REDUCED have been preliminarily reported. Empagliflozin would meet the primary endpoint of reducing the risk of cardiovascular death or hospitalisation due to heart failure in patients with and without diabetes.
Other possible CV mechanisms of actionSGLT2is moderately improve glycaemic control, weight, and slightly improve blood pressure. The reduction in HbA1c appears to be greater the poorer the glycaemic control (in direct relation to the glycaemic load to be filtered), around .79% in monotherapy and .61% added to standard antidiabetic treatment.11 Their hypoglycaemic effect requires relatively preserved renal function (clearance above 30 mL/min/1.73 m3). In contrast, the effect on blood pressure, without an increase in heart rate, is maintained even in patients with reduced glomerular filtration, which could translate into a possible inhibitory effect on the activation of the sympathetic nervous system in heart failure. Values around a mean 2.46 mmHg for systolic pressure and 1.46 for diastolic pressure have been reported.19
A prospective study of 76 patients treated with empagliflozin showed reductions not only in blood pressure but also in central aortic pressure and pulse pressure in diabetic patients.20 Dapagliflozin showed similar results in a randomised, placebo-controlled study on T2DM.21 Central aortic pressure is mainly determined by arterial stiffness in the large arteries and is considered an important afterload variable, intricately linked to cardiovascular prognosis. Reducing aortic stiffness relieves cardiac afterload and reduces energy demand, which is beneficial in HF.
SGLT2is also inhibit NHE1 receptors, transporters of Na+ and H+ located at the myocardial level.22 Their expression is increased in heart failure, increasing intracytoplasmic levels of Na+ and indirectly of Ca2+. All this eventually leads to cellular apoptosis and myocardial damage. Their inhibition would therefore be related to an improvement in ionic balance, mitochondrial function, and consequently, to the energy supply of the myocyte.21
Their prolonged natriuretic and glycosuric effect is also interesting. In addition, by reducing glucose absorption in the S1 segment of the loop of Henle, generating osmotic glycosuria, it also acts on the NHE3 transporters, which are responsible for a large part of Na+ reabsorption at the tubular level. It is precisely a different ionic profile compared to other diuretic agents that has been highlighted by some authors as responsible for a greater affinity for interstitial fluid,23 which could partly explain the CV benefits.
Improvements at a metabolic level and an increase in tubular reabsorption of potassium and magnesium24 have also been proposed by some authors as responsible for a possible antiarrhythmic effect and lower sudden death rates. Additional mechanisms of action include a decrease in adiposity, weight, an increase in lipogenesis and specific reduction in epicardial adipose tissue.25
Renal benefitRenal involvement in cardiovascular safety studiesThe same cardiovascular safety trials with SGLT2i (CANVAS study, EMPA-REG OUTCOME and DECLARE-TIMI-58) suggested for the first time an improvement in renal prognosis in T2DM as well.
The abovementioned meta-analysis14 encompassing these three clinical trials shows a 45% decrease in the triple endpoint of deterioration of kidney function, end-stage kidney disease and death from renal cause. Interestingly, the nephroprotective effect and the reduction in admissions due to heart failure showed an inverse distribution. The former is greater the better the kidney function, with reductions in composite objective of 33%, 44% and 56% for GF of <60 mL/ min, 60–90 mL/min and >90 mL/min, respectively. The reduction in hospital admission due to heart failure is greater in patients with poorer kidney function: 40%, 31% and 12% for GF <60 mL/min, 60–90 mL/min and >90 mL/min, respectively.
However, the primary objectives of these clinical trials were not renal and included few patients with advanced kidney disease (eGFR <60 mL/min/1.73 m2): 26% in the EMPA-REG OUTCOME study, 20% in the CANVAS study and 9% in the DECLARE-TIMI-58 study (Table 2). The first clinical trial with SGLT2i with a cardio-renal primary objective is the CREDENCE26 study (Canafliglozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation). It included patients with T2DM and chronic kidney disease with albuminuria, assigned to receive canagliflozin (100 mg per day) or placebo.
The eGFR was 30–90 mL/min/1.73 m2 (60% eGFR <60), the urine albumin/creatinine ratio was 300–5000 mg/g and treatment included renin-angiotensin-aldosterone system inhibitor. The primary objective was chronic end-stage renal disease (dialysis, transplantation or a sustained eGFR of <15 mL/min/1.73 m2), doubling of the serum creatinine level or death from renal or cardiovascular causes. It had an average follow-up of 2.26 years and at the time of its discontinuation 4401 patients had been recruited.
The relative risk of the primary objective was 30% lower in the canaglyphlozin group, compared to the placebo group, with event rates of 43.2 and 61.2 per 1000 patients/year (p = .00001). The relative risk of the composite objective of CKD, doubling of creatinine level and death from renal causes was reduced by 34% (p < .001) and the relative risk of CKD was reduced by 32% (p = .002). There was also a lower risk of cardiovascular death, acute myocardial infarction, and stroke in the canagliflozin group (p = .001), and a reduction in hospitalization due to heart failure (HR .61, 95% CI .47–.80, p < .001). There was no significant difference in the risk of cardiovascular death or all-cause death. In terms of safety, there was no increased rate of adverse events in the canaglyphlozin group (except for fungal infections, mainly in men) and a modest increase in diabetic ketoacidosis. No increase in the rate of amputations or fractures was confirmed with canagliflozin.
Mechanisms of actionAs with the cardiovascular aspect, the benefit at kidney level appears to be not only a result of simply optimising glycaemic control. Tubuloglomerular feedback, reduction of blood pressure, reduction of renal ischaemia, and the anti-inflammatory and anti-fibrotic effect could play a key role in the benefit observed with SGLT2is.27
Diabetes, chronic kidney disease, and very possibly heart failure, are associated with a state of hyperfiltration at the glomerular level. The reduction of hyperfiltration and intraglomerular hypertension has been precisely indicated as a potential mechanism of action. As a result, in the first weeks of treatment with SGLT2i, eGFR decreases in a range of 3–5 mL/min/1.73 m2 and then stabilizes. Furthermore, progression to more advanced or severe stages of kidney disease is also slowed.
Kidokoro et al.,28 for the first time, recently directly visualized in vivo the haemodynamic changes at the glomerular level induced by an iSGLT2. In a murine model, they demonstrate how empaglyphlozin reduces glomerular hyperfiltration by an adenosine-dependent mechanism, independent of glucose elimination and mediated by vasoconstriction of the afferent arteriole. Vasoconstriction of the efferent arteriole and reduction of hyperfiltration were already detected a few hours after administration of empaglyphlozin. By highlighting natriuresis over glycosuria as a fundamental mechanism, they suggest that its effect could be equally beneficial in patients with non-diabetic kidney disease, a context where renal elimination of glucose would not be so relevant.
ConclusionResearch with SGLT2i is helping not only to clarify the underlying mechanisms of heart failure, kidney disease and diabetes mellitus, but to position this pharmacological group as a new therapeutic weapon within the cardiovascular pharmacopoeia.
The benefits observed place them beyond simple hypoglycaemic agents, with a proven cardioprotective effect on atherosclerotic disease, heart failure, total mortality, cardiovascular mortality, and progression of kidney failure. They are already a reality in high and very high cardiovascular risk diabetic patients, while evidence in their favour is growing for non-diabetic patients.
We are therefore witnessing a paradigm shift and possibly the birth of a new speciality, cardio-endocrinology, involving new treatments that should be considered to be more than simply anti-diabetic drugs.
FundingJ.A. Requena Ibáñez receives funding from the Alfonso Martin Escudero Foundation (research grant in universities or centres abroad 2019) to carry out research in the Mount Sinai Heart Atherothrombosis Unit.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Vargas Delgado AP, Requena Ibañez JA, Santos-Gallego CG, Badimon JJ. ¿Son los inhibidores del receptor SGLT2 fármacos antidiabéticos o cardiovasculares? Clin Investig Arterioscler. 2021;33:33–40.